Early Diagnostics of Freemartinism in Polish Holstein-Friesian Female Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cytogenetic Diagnostics
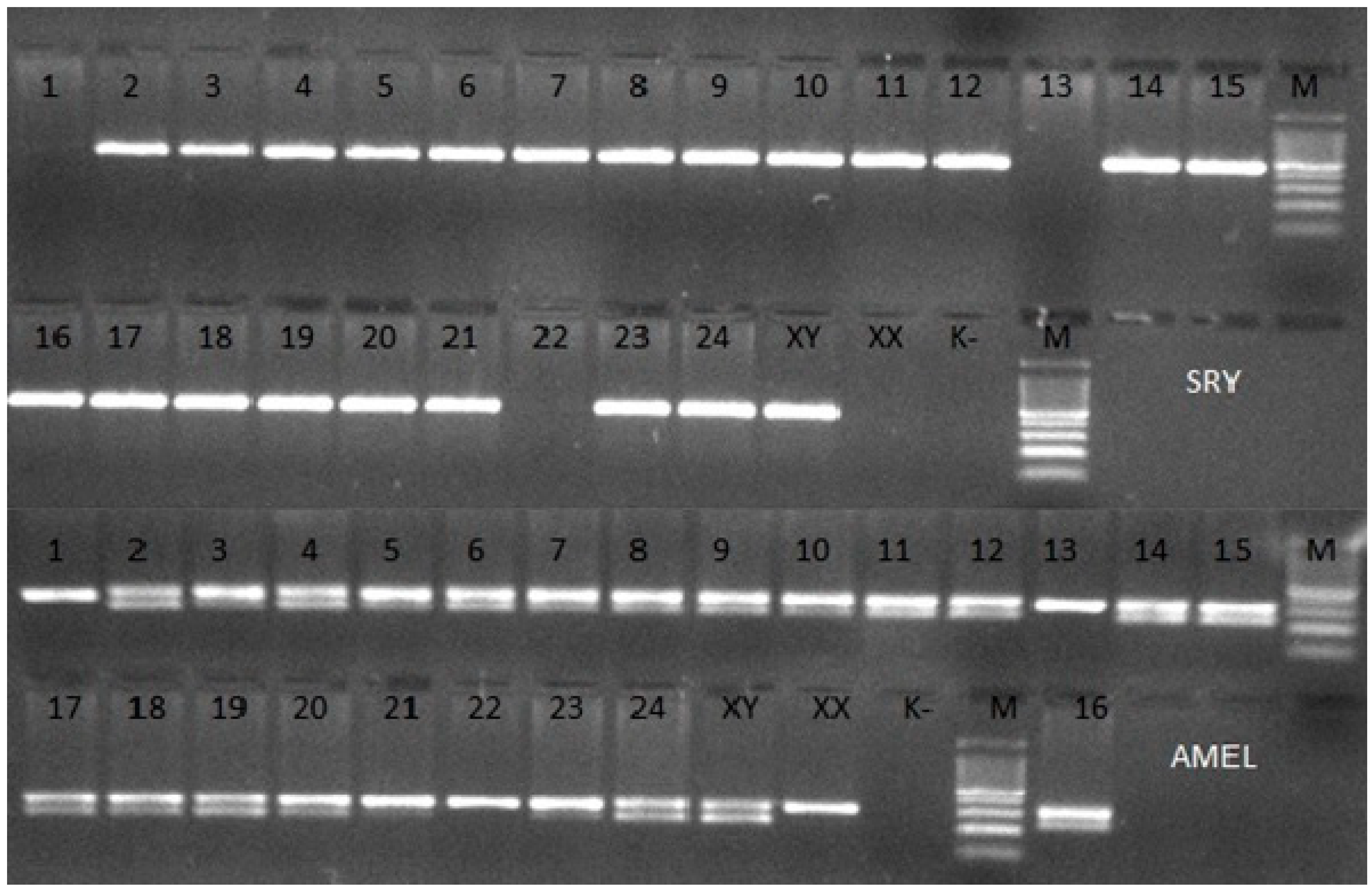
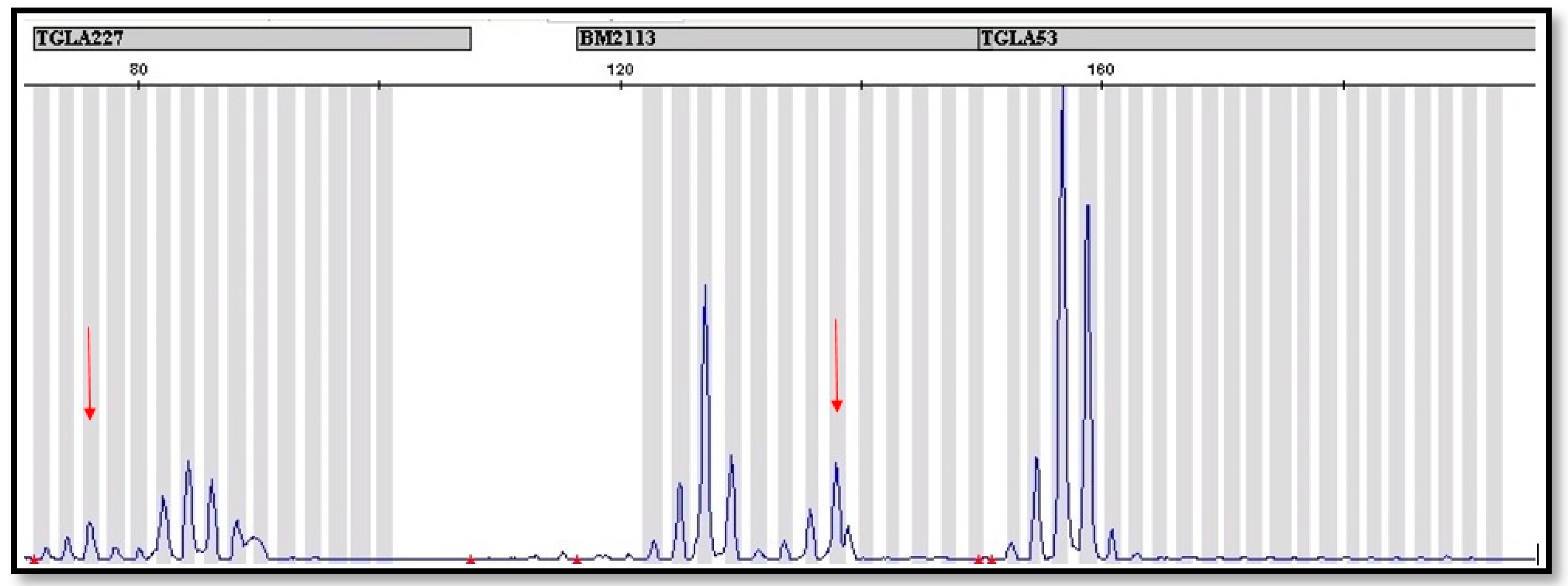
2.3. Molecular Genetic Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Animal * | Age [Months] | Cytogenetic Analysis | Molecular Analysis | |||
|---|---|---|---|---|---|---|
| 60, XX (%) | 60, XY (%) | SRY | AMEL Y | STR | ||
| 1. | 5 | 98.4 100 | 1.6 0 | − | − | - |
| 2. | 8 | 67 | 37 | + | + | TGLA 122, TGLA 227 |
| 3. | 9.5 | 82 | 18 | + | + | TGLA 227 |
| 4. | 9 | 72 | 28 | + | + | TGLA227 |
| 5. | 11 | 56 | 44 | + | + | TGLA53, ETH3 |
| 6. | 9 | 81 | 19 | + | + | TGLA227, ETH10 |
| 7. | 3.5 | 56 | 44 | + | + | TGLA227, ETH10, INRA23 |
| 8. | 3 | 61 | 39 | + | + | TGLA227, ETH10, INRA23 |
| 9. | 2.5 | 65 | 35 | + | + | TGLA53, SPS115, ETH225, AGLA293 |
| 10. | 2 | 75 | 25 | + | + | ETH3, BM1824 |
| 11. | 2 | 71 | 39 | + | + | TGLA53, TGLA126, ETH3 |
| 12. | 0.5 | 64 | 36 | + | + | TGLA126, ETH225 |
| 13. | 12 | 99.2 100 † | 0.8 0 | − | − | |
| 14. | 1 | 34 | 66 | + | + | TGLA53, TGLA122, ETH10, CSSM66 |
| 15. | 1 | 88 | 12 | + | + | CSRM60 CSSM66, HUIJ177 |
| 16. | 0.5 | 70 | 30 | + | + | TGLA227, ETH225, ILSTS065 |
| 17. | 1 | 48 | 52 | + | + | TGLA 227, TGLA 53, INRA 23 |
| 18. | 2 | 72 | 28 | + | + | TGLA 227, TGLA 126, INRA 23 |
| 19. | 2 | 84 | 16 | + | + | ETH 10, TGLA 53, INRA 23 |
| 20. | 0.5 | 57 | 43 | + | + | TGLA 227, TGLA 122, INRA 23 |
| 21. | 12 | 96 | 4 | + | + | CSRM60 |
| 22. | 3 days | 100 | 0 | − | − | - |
| 23. | 8 | 86 | 14 | + | + | TGLA 53 |
| 24. | 3 | 80 | 20 | + | + | TGLA 53, ETH225 |
References
- Kozubska-Sobocinska, A.; Rejduch, B.; Slota, E.; Sysa, P. New Aspects of Degenerative Changes in Reproductive System of Freemartin Heifers. Ann. Anim. Sci. 2011, 11, 229–239. [Google Scholar]
- Kozubska-Sobocińska, A.; Danielak-Czech, B.; Rejduch, B. Cytogenetic and Molecular Diagnostics of XX/XY Chimerism in Cattle, Sheep, and Goats—A Review. Ann. Anim. Sci. 2016, 16, 989–1005. [Google Scholar] [CrossRef]
- Słota, E.; Kozubska-Sobocińska, A.; Danielak-Czech, B.; Rejduch, B.; Kowol, P.; Żyga, A. A Note on Cytogenetic Monitoring of Polish Red Cattle. J. Anim. Feed Sci. 2004, 13, 65–71. [Google Scholar] [CrossRef]
- Padula, A.M. The Freemartin Syndrome: An Update. Anim. Reprod. Sci. 2005, 87, 93–109. [Google Scholar] [CrossRef]
- Esteves, A.; Båge, R.; Payan Carreira, R. Freemartinism in Cattle. In Ruminants: Anatomy, Behavior and Diseases; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 99–120. [Google Scholar]
- Qiu, Q.; Shao, T.; He, Y.; Muhammad, A.-U.-R.; Cao, B.; Su, H. Applying Real-Time Quantitative PCR to Diagnosis of Freemartin in Holstein Cattle by Quantifying SRY Gene: A Comparison Experiment. PeerJ 2018, 6, e4616. [Google Scholar] [CrossRef]
- Bierman, C.D.; Kim, E.; Shi, X.-W.; Weigel, K.; Berger, P.J.; Kirkpatrick, B.W. Validation of Whole Genome Linkage-Linkage Disequilibrium and Association Results, and Identification of Markers to Predict Genetic Merit for Twinning. Anim. Genet. 2010, 41, 406–416. [Google Scholar] [CrossRef]
- Szczerbal, I.; Kociucka, B.; Nowacka-Woszuk, J.; Lach, Z.; Jaskowski, J.M.; Switonski, M. A High Incidence of Leukocyte Chimerism (60, XX/60, XY) in Single Born Heifers Culled Due to Underdevelopment of Internal Reproductive Tracts. Czech J. Anim. Sci. 2014, 59, 445–449. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Switonski, M.; Mackowski, M.; Slota, E.; Radko, A.; Zabek, T.; Urbaniak, K. The Ambiguity of Freemartinism Diagnosis in Cattle Revealed by Cytogenetic and Molecular Techniques. Czech J. Anim. Sci. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Harikae, K.; Tsunekawa, N.; Hiramatsu, R.; Toda, S.; Kurohmaru, M.; Kanai, Y. Evidence for Almost Complete Sex-Reversal in Bovine Freemartin Gonads: Formation of Seminiferous Tubule-like Structures and Transdifferentiation into Typical Testicular Cell Types. J. Reprod. Dev. 2012, 58, 654–660. [Google Scholar] [CrossRef]
- Vigier, B.; Watrin, F.; Magre, S.; Tran, D.; Garrigou, O.; Forest, M.G.; Josso, N. Anti-Müllerian Hormone and Freemartinism: Inhibition of Germ Cell Development and Induction of Seminiferous Cord-like Structures in Rat Fetal Ovaries Exposed in Vitro to Purified Bovine AMH. Reprod. Nutr. Dev. 1988, 28, 1113–1128. [Google Scholar] [CrossRef]
- She, Z.-Y.; Yang, W.-X. Molecular Mechanisms Involved in Mammalian Primary Sex Determination. J. Mol. Endocrinol. 2014, 53, R21–R37. [Google Scholar] [CrossRef] [PubMed]
- Komisarek, J.; Dorynek, Z. Genetic Aspects of Twinning in Cattle. J. Appl. Genet. 2002, 43, 55–68. [Google Scholar] [PubMed]
- Khan, M.Z.; Foley, G.L. Retrospective Studies on the Measurements, Karyotyping and Pathology of Reproductive Organs of Bovine Freemartins. J. Comp. Pathol. 1994, 110, 25–36. [Google Scholar] [CrossRef]
- Zhang, T.; Buoen, L.C.; Seguin, B.E.; Ruth, G.R.; Weber, A.F. Diagnosis of Freemartinism in Cattle: The Need for Clinical and Cytogenic Evaluation. J. Am. Vet. Med. Assoc. 1994, 204, 1672–1675. [Google Scholar]
- Peretti, V.; Ciotola, F.; Albarella, S.; Paciello, O.; Dario, C.; Barbieri, V.; Iannuzzi, L. XX/XY Chimerism in Cattle: Clinical and Cytogenetic Studies. Sex. Dev. 2008, 2, 24–30. [Google Scholar] [CrossRef]
- Villagómez, D.A.F.; Parma, P.; Radi, O.; Meo, G.D.; Pinton, A.; Iannuzzi, L.; King, W.A. Classical and Molecular Cytogenetics of Disorders of Sex Development in Domestic Animals. Cytogenet. Genome Res. 2009, 126, 110–131. [Google Scholar] [CrossRef]
- Slota, E.; Danielak-Czech, B.; Pietraszewska, J.; Kozubska-Sobocińska, A. Preliminary Identification of the Fragile X in Two Crossbred Cows. Vet. Med. (Praha) 2000, 45, 308–310. [Google Scholar]
- Rejduch, B.; Kozubska-Sobocińska, A.; Radko, A.; Rychlik, T.; Słota, E. The Application of Genetic Markers for Cell Chimerism Diagnosis in Lambs. J. Anim. Breed. Genet. 2004, 121, 197–203. [Google Scholar] [CrossRef]
- Rubes, J.; Pinton, A.; Bonnet-Garnier, A.; Fillon, V.; Musilova, P.; Michalova, K.; Kubickova, S.; Ducos, A.; Yerle, M. Fluorescence in Situ Hybridization Applied to Domestic Animal Cytogenetics. Cytogenet. Genome Res. 2009, 126, 34–48. [Google Scholar] [CrossRef]
- Rychlik, T.; Kozubska-Sobocinska, A.; Rejduch, B.; Sikora, J. The Phenomenon of Cell Chimerism in Goats. Veterinární Medicína 2012, 50, 311–314. [Google Scholar] [CrossRef]
- Sohn, S.H.; Cho, E.J.; Son, W.J.; Lee, C.Y. Diagnosis of Bovine Freemartinism by Fluorescence in Situ Hybridization on Interphase Nuclei Using a Bovine Y Chromosome-Specific DNA Probe. Theriogenology 2007, 68, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Demyda-Peyrás, S.; Anaya, G.; Bugno-Poniewierska, M.; Pawlina, K.; Membrillo, A.; Valera, M.; Moreno-Millán, M. The Use of a Novel Combination of Diagnostic Molecular and Cytogenetic Approaches in Horses with Sexual Karyotype Abnormalities: A Rare Case with an Abnormal Cellular Chimerism. Theriogenology 2014, 81, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- McNiel, E.A.; Madrill, N.J.; Treeful, A.E.; Buoen, L.C.; Weber, A.F. Comparison of Cytogenetics and Polymerase Chain Reaction Based Detection of the Amelogenin Gene Polymorphism for the Diagnosis of Freemartinism in Cattle. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2006, 18, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Royo, A.; Dervishi, E.; Alabart, J.L.; Jurado, J.J.; Folch, J.; Calvo, J.H. Freemartinism and FecXR Allele Determination in Replacement Ewes of the Rasa Aragonesa Sheep Breed by Duplex PCR. Theriogenology 2009, 72, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Porat, B.; Band, M.R.; Weller, J.I. Chimaerism Detection in Bovine Twins, Triplets and Quadruplets Using Sex Chromosome-Linked Markers. Anim. Genet. 2011, 42, 208–211. [Google Scholar] [CrossRef]
- Pourjafar, M.; Badiei, K.; Sharifiyazdi, H.; Naghib, S.; Chalmeh, A.; Divar, M.R. Application of Hormonal and Single Multiplex PCR Assays for Detection of Freemartinism in a Horned Goat. J. Fac. Vet. Med. Istanb. Univ. 2012, 38, 175–181. [Google Scholar]
- Vodicka, R.; Vrtel, R.; Scheinost, O.; Zapletalova, J.; Dusek, L.; Marie, G.; Santavy, J. Refined Quantitative Fluorescent PCR of Y-Chromosome DNA Sequences Mosaics in Turner’s Syndrome Patients—Alternative to Real-Time PCR. J. Biochem. Biophys. Methods 2004, 60, 151–162. [Google Scholar] [CrossRef]
- Donaghue, C.; Mann, K.; Docherty, Z.; Mazzaschi, R.; Fear, C.; Ogilvie, C. Combined QF-PCR and MLPA Molecular Analysis of Miscarriage Products: An Efficient and Robust Alternative to Karyotype Analysis. Prenat. Diagn. 2010, 30, 133–137. [Google Scholar] [CrossRef]
- Xu, A.-Q.; Xia, M.; Liu, J.-T.; Yao, X.-F.; Zhang, W.-M.; Hao, N.; Zhou, J.; Bian, X.-M. Validation of Quantitative Fluorescent-PCR for Rapid Prenatal Diagnosis of Common Aneuploidies in the Chinese Population. Genet. Mol. Res. GMR 2013, 12, 6379–6388. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bernard, M.; Danic, B.; Dauriac, C.; Birebent, B.; Lapart, C.; Lamy, T.; Le Prisé, P.-Y.; Beauplet, A.; Bories, D.; et al. Quantitative Assessment of Hematopoietic Chimerism after Bone Marrow Transplantation by Real-Time Quantitative Polymerase Chain Reaction. Blood 2002, 99, 4618–4625. [Google Scholar] [CrossRef]
- Craig, D.W.; Millis, M.P.; DiStefano, J.K. Genome-Wide SNP Genotyping Study Using Pooled DNA to Identify Candidate Markers Mediating Susceptibility to End-Stage Renal Disease Attributed to Type 1 Diabetes. Diabet. Med. 2009, 26, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, A.; Rubiś, D.; Bugno-Poniewierska, M. The Effect of Using DNA Obtained from Blood of Cattle with Genetic Chimerism on Illumina’s Beadchip Assay Performance. Ann. Anim. Sci. 2014, 14, 279–286. [Google Scholar] [CrossRef] [Green Version]
| Name | Sequence 5′–3′ |
|---|---|
| SRY F | AAGGGGAGAACATGTTAGGGAGAG |
| SRY R | TTTGCAGGAGTGAATTGGTTATGA |
| AMEL F | CAGCCAAACCTCCCTCTGC |
| AMEL R | CCCGCTTGGTCTTGTCTGTTGC |
| Name | Sequence 5′–3′ | Amplicon Size (bp) | TM (°C) |
|---|---|---|---|
| SRY F | GCCACAGAAATCGCTTCC | 229 | 60 |
| SRY R | CCGTGTAGCCAATGTTACCTT | ||
| GADPH F | GTGAGAGACGGAACAGGAAGAA | 110 | 60 |
| GADPH R | ATGAGGGAAGACAGGACAAAGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozubska-Sobocińska, A.; Smołucha, G.; Danielak-Czech, B. Early Diagnostics of Freemartinism in Polish Holstein-Friesian Female Calves. Animals 2019, 9, 971. https://doi.org/10.3390/ani9110971
Kozubska-Sobocińska A, Smołucha G, Danielak-Czech B. Early Diagnostics of Freemartinism in Polish Holstein-Friesian Female Calves. Animals. 2019; 9(11):971. https://doi.org/10.3390/ani9110971
Chicago/Turabian StyleKozubska-Sobocińska, Anna, Grzegorz Smołucha, and Barbara Danielak-Czech. 2019. "Early Diagnostics of Freemartinism in Polish Holstein-Friesian Female Calves" Animals 9, no. 11: 971. https://doi.org/10.3390/ani9110971
APA StyleKozubska-Sobocińska, A., Smołucha, G., & Danielak-Czech, B. (2019). Early Diagnostics of Freemartinism in Polish Holstein-Friesian Female Calves. Animals, 9(11), 971. https://doi.org/10.3390/ani9110971





