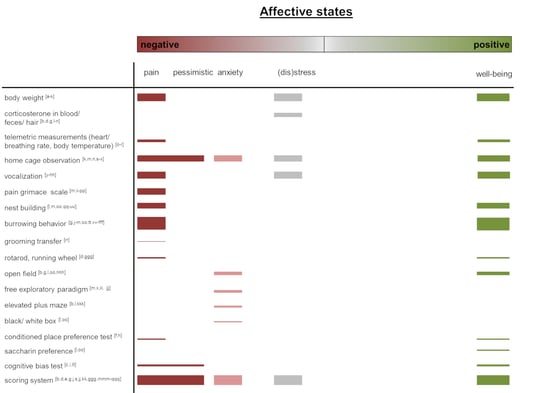
Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Affective State Assessment in Laboratory Rodents
2.1. Physiological and Clinical Measurements
2.2. Behavioral Measurements
2.2.1. Spontaneous Behavior
2.2.2. Play Behavior
2.2.3. Vocalization
2.2.4. Facial Expression
2.2.5. Nest Building
2.2.6. Burrowing
2.2.7. Grooming
2.3. Apparatus Based Behavioral Test Paradigms
2.3.1. Anxiety Related Tests
2.3.2. Preference Tests
2.3.3. Strengths of Preferences
2.3.4. Cognitive Judgment Bias
2.3.5. Drug Self-Administration
2.4. Scoring Systems, Score Sheets, Composite Scores
2.5. Automation of Assessment
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fraser, D. Science, Values and Animal Welfare: Exploring the ‘Inextricable Connection’. Anim. Welf. 1995, 4, 103–117. [Google Scholar]
- Stafleu, F.; Grommers, F.; Vorstenbosch, J. Animal welfare: Evolution and erosion of a moral concept. Anim. Welf. 1996, 5, 225–234. [Google Scholar]
- Fisher, M.W. Defining animal welfare—Does consistency matter? N. Z. Vet. J. 2009, 57, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.; van der Staay, F.J. Animal welfare: At the interface between science and society. Vet. J. 2012, 192, 13–19. [Google Scholar] [CrossRef]
- Mellor, D.J. Moving beyond the “Five Freedoms” by Updating the “Five Provisions” and Introducing Aligned “Animal Welfare Aims”. Animals 2016, 6. [Google Scholar] [CrossRef]
- Lewejohann, L.; Schwabe, K.; Hager, C.; Jirkof, P. Impulse for animal welfare outside the experiment. Lab. Anim. in press.
- Broom, D.M. Quality of life means welfare: How is it related to other concepts and assessed? Anim. Welf. 2007, 16, 45–53. [Google Scholar]
- Green, T.C.; Mellor, D.J. Extending ideas about animal welfare assessment to include ‘quality of life’ and related concepts. N. Z. Vet. J. 2011, 59, 263–271. [Google Scholar] [CrossRef]
- Dawkins, M.S. From an animal’s point of view: Motivation, fitness, and animal welfare. Behav. Brain Sci. 1990, 13, 1–9. [Google Scholar] [CrossRef]
- Desire, L.; Boissy, A.; Veissier, I. Emotions in farm animals: A new approach to animal welfare in applied ethology. Behav. Process. 2002, 60, 165–180. [Google Scholar] [CrossRef]
- Spinka, M. Social dimension of emotions and its implication for animal welfare. Appl. Anim. Behav. Sci. 2012, 138, 170–181. [Google Scholar] [CrossRef]
- McDougall, M. An Introduction to Social Psychology. Supplementary Chapter 1: Theories of Action; John W. Luce & Co.: Boston, MA, USA, 1926; pp. 359–392. [Google Scholar]
- Fraser, D.; Duncan, I.J.H. ‘Pleasures’, ’pains’ and animal welfare: Toward a natural history of affect. Anim. Welf. 1998, 7, 383–396. [Google Scholar]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. Rev. 2011, 35, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J. Positive animal welfare states and reference standards for welfare assessment. N. Z. Vet. J. 2015, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Bethell, E.J.; Holmes, A.; MacLarnon, A.; Semple, S. Cognitive bias in a non-human primate: Husbandry procedures influence cognitive indicators of psychological well-being in captive rhesus macaques. Anim. Welf. 2012, 21, 185–195. [Google Scholar] [CrossRef]
- Kloke, V.; Schreiber, R.S.; Bodden, C.; Möllers, J.; Ruhmann, H.; Kaiser, S.; Lesch, K.P.; Sachser, N.; Lewejohann, L. Hope for the best or prepare for the worst? Towards a spatial cognitive bias test for mice. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Gygax, L. Wanting, liking and welfare: The role of affective states in proximate control of behaviour in vertebrates. Ethology 2017, 123, 689–704. [Google Scholar] [CrossRef]
- Makowska, I.J.; Weary, D.M. Assessing the emotions of laboratory rats. Appl. Anim. Behav. Sci. 2013, 148, 1–12. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Howarth, G.S. Use of spontaneous behaviour measures to assess pain in laboratory rats and mice: How are we progressing? Appl. Anim. Behav. Sci. 2014, 151, 1–12. [Google Scholar] [CrossRef]
- Jirkof, P.; Tourvieille, A.; Cinelli, P.; Arras, M. Buprenorphine for pain relief in mice: Repeated injections vs sustained-release depot formulation. Lab. Anim. 2015, 49, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Hager, C.; Keubler, L.M.; Talbot, S.R.; Biernot, S.; Weegh, N.; Buchheister, S.; Buettner, M.; Glage, S.; Bleich, A. Running in the wheel: Defining individual severity levels in mice. PLoS Biol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Spani, D.; Arras, M.; Konig, B.; Rulicke, T. Higher heart rate of laboratory mice housed individually vs. in pairs. Lab. Anim. 2003, 37, 54–62. [Google Scholar] [CrossRef]
- Van Loo, P.L.P.; Kuin, N.; Sommer, R.; Avsaroglu, H.; Pham, T.; Baumans, V. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab. Anim. 2007, 41, 441–455. [Google Scholar] [CrossRef]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. 2004, 43, 42–51. [Google Scholar]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Bracke, M.B.M.; Hopster, H. Assessing the importance of natural behavior for animal welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Burn, C.C. Bestial boredom: A biological perspective on animal boredom and suggestions for its scientific investigation. Anim. Behav. 2017, 130, 141–151. [Google Scholar] [CrossRef]
- Ahloy-Dallaire, J.; Espinosa, J.; Mason, G. Play and optimal welfare: Does play indicate the presence of positive affective states? Behav. Process. 2018, 156, 3–15. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Achterberg, E.J.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Morley-Fletcher, S.; Rea, M.; Maccari, S.; Laviola, G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci. 2003, 18, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Klein, Z.A.; Padow, V.A.; Romeo, R.D. The effects of stress on play and home cage behaviors in adolescent male rats. Dev. Psychobiol. 2010, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.S.; Sanguinetti-Scheck, J.I.; Hartmann, K.; Brecht, M. Behavioral and neural correlates of hide-and-seek in rats. Science (New York, NY, USA) 2019, 365, 1180–1183. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J. “Laughing” rats and the evolutionary antecedents of human joy? Physiol. Behav. 2003, 79, 533–547. [Google Scholar] [CrossRef]
- Richter, S.H.; Kastner, N.; Kriwet, M.; Kaiser, S.; Sachser, N. Play matters: The surprising relationship between juvenile playfulness and anxiety in later life. Anim. Behav. 2016, 114, 261–271. [Google Scholar] [CrossRef]
- Freund, J.; Brandmaier, A.M.; Lewejohann, L.; Kirste, I.; Kritzler, M.; Krüger, A.; Sachser, N.; Lindenberger, U.; Kempermann, G. Association between exploratory activity and social individuality in genetically identical mice living in the same enriched environment. Neuroscience 2015, 309, 140–152. [Google Scholar] [CrossRef] [Green Version]
- Burgdorf, J.; Panksepp, J.; Moskal, J.R. Frequency-modulated 50 kHz ultrasonic vocalizations: A tool for uncovering the molecular substrates of positive affect. Neurosci. Biobehav. Rev. 2011, 35, 1831–1836. [Google Scholar] [CrossRef]
- Bobrovskaya, L.; Beard, D.; Bondarenko, E.; Beig, M.I.; Jobling, P.; Walker, F.R.; Day, T.A.; Nalivaiko, E. Does exposure to chronic stress influence blood pressure in rats? Auton. Neurosci. Basic 2013, 177, 217–223. [Google Scholar] [CrossRef]
- Portfors, C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. 2007, 46, 28–34. [Google Scholar]
- Niel, L.; Weary, D.M. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl. Anim. Behav. Sci. 2006, 100, 295–308. [Google Scholar] [CrossRef]
- Finlayson, K.; Lampe, J.F.; Hintze, S.; Wurbel, H.; Melotti, L. Facial Indicators of Positive Emotions in Rats. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFollette, M.R.; O’Haire, M.E.; Cloutier, S.; Blankenberger, W.B.; Gaskill, B.N. Rat tickling: A systematic review of applications, outcomes, and moderators. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquardt, N.; Feja, M.; Hunigen, H.; Plendl, J.; Menken, L.; Fink, H.; Bert, B. Euthanasia of laboratory mice: Are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidinger, C.; Herrmann, F.; Thone-Reineke, C.; Baumgart, N.; Baumgart, J. Introducing Clicker Training as a Cognitive Enrichment for Laboratory Mice. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Jourdan, D.; Ardid, D.; Chapuy, E.; Eschalier, A.; Le Bars, D. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain 1995, 63, 237–249. [Google Scholar] [CrossRef]
- Dinh, H.K.; Larkin, A.; Gatlin, L.; Piepmeier, E. Rat ultrasound model for measuring pain resulting from intramuscularly injected antimicrobials. PDA J. Pharm. Sci Technol. 1999, 53, 40–43. [Google Scholar]
- Colpaert, F.C.; Meert, T.; Dewitte, P.; Schmitt, P. Further Evidence Validating Adjuvant Arthritis as an Experimental-Model of Chronic Pain in the Rat. Life Sci. 1982, 31, 67–75. [Google Scholar] [CrossRef]
- Jourdan, D.; Ardid, D.; Eschalier, A. Analysis of ultrasonic vocalisation does not allow chronic pain to be evaluated in rats. Pain 2002, 95, 165–173. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Jonas, B.M.; Lindsay, T.H.; Halvorson, K.G.; Ghilardi, J.R.; Kuskowski, M.A.; Mukherjee, P.; Maggio, J.E.; Mantyh, P.W. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology 2006, 131, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Kurejova, M.; Nattenmuller, U.; Hildebrandt, U.; Selvaraj, D.; Stosser, S.; Kuner, R. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol. Pain 2010, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, W.O.; Riskin, D.K.; Mott, A.K. Ultrasonic sound as an indicator of acute pain in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 8–10. [Google Scholar] [PubMed]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faller, K.M.E.; McAndrew, D.J.; Schneider, J.E.; Lygate, C.A. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the Mouse Grimace Scale. Exp. Physiol. 2015, 100, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.C.; Klaus, K.; Miller, A.L.; di Perrotolo, M.S.; Sotocinal, S.G.; Flecknell, P.A. The Assessment of Post-Vasectomy Pain in Mice Using Behaviour and the Mouse Grimace Scale. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Miller, A.L.; Leach, M.C. Using the mouse grimace scale to assess pain associated with routine ear notching and the effect of analgesia in laboratory mice. Lab. Anim. 2015, 49, 117–120. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thone-Reineke, C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-Assessing the degree of distress. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Moller, C.; Wolf, F.; van Dijk, R.M.; Di Liberto, V.; Russmann, V.; Keck, M.; Palme, R.; Hellweg, R.; Gass, P.; Otzdorff, C.; et al. Toward evidence-based severity assessment in rat models with repeated seizures: I. Electrical kindling. Epilepsia 2018, 59, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Hager, C.; Keubler, L.M.; Biernot, S.; Dietrich, J.; Buchheister, S.; Buettner, M.; Bleich, A. Time to Integrate to Nest Test Evaluation in a Mouse DSS-Colitis Model. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P.; Durst, M.; Klopfleisch, R.; Palme, R.; Thone-Reineke, C.; Buttgereit, F.; Schmidt-Bleek, K.; Lang, A. Administration of Tramadol or Buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beninson, J.A.; Lofgren, J.L.; Lester, P.A.; Hileman, M.M.; Berkowitz, D.J.; Myers, D.D. Analgesic Efficacy and Hematologic Effects of Robenacoxib in Mice. J. Am. Assoc. Lab. Anim. 2018, 57, 258–267. [Google Scholar]
- Oliver, V.L.; Thurston, S.E.; Lofgren, J.L. Using Cageside Measures to Evaluate Analgesic Efficacy in Mice (Mus musculus) after Surgery. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 186–201. [Google Scholar]
- Deacon, R.M.J. Burrowing in rodents: A sensitive method for detecting behavioral dysfunction. Nat. Protoc. 2006, 1, 118–121. [Google Scholar] [CrossRef]
- Rutten, K.; Robens, A.; Read, S.J.; Christoph, T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. Eur. J. Pain 2014, 18, 213–222. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Lymn, K.A.; Nicholson, A.; Howarth, G.S. The assessment of general well-being using spontaneous burrowing behaviour in a short-term model of chemotherapy-induced mucositis in the rat. Lab. Anim. 2015, 49, 30–39. [Google Scholar] [CrossRef]
- Gould, S.A.; Doods, H.; Lamla, T.; Pekcec, A. Pharmacological characterization of intraplantar Complete Freund’s Adjuvant-induced burrowing deficits. Behav. Brain Res. 2016, 301, 142–151. [Google Scholar] [CrossRef]
- Jirkof, P.; Cesarovic, N.; Rettich, A.; Nicholls, F.; Seifert, B.; Arras, M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front. Behav. Neurosci. 2010, 4. [Google Scholar] [CrossRef] [Green Version]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thone-Reineke, C. Systematic Assessment of Well-Being in Mice for Procedures Using General Anesthesia. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Gjendal, K.; Ottesen, J.L.; Olsson, I.A.S.; Sorensen, D.B. Burrowing and nest building activity in mice after exposure to grid floor, isoflurane or ip injections. Physiol. Behav. 2019, 206, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, U.; Yau, T.; Fink, D.; Tichy, A.; Palme, R.; Egerbacher, M.; Rulicke, T. Assessment and refinement of intra-bone marrow transplantation in mice. Lab. Anim. 2015, 49, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P.; Cesarovic, N.; Rettich, A.; Fleischmann, T.; Arras, M. Individual housing of female mice: Influence on postsurgical behaviour and recovery. Lab. Anim. 2012, 46, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirkof, P.; Cesarovic, N.; Rettich, A.; Arras, M. Housing of female mice in a new environment and its influence on post-surgical behaviour and recovery. Appl. Anim. Behav. Sci. 2013, 148, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Baumann, A.; Moreira, C.G.; Morawska, M.M.; Masneuf, S.; Baumann, C.R.; Noain, D. Preliminary Evidence of Apathetic-Like Behavior in Aged Vesicular Monoamine Transporter 2 Deficient Mice. Front. Hum. Neurosci. 2016, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Strekalova, T.; Steinbusch, H.W. Measuring behavior in mice with chronic stress depression paradigm. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Jorgensen, B.P.; Hansen, J.T.; Krych, L.; Larsen, C.; Klein, A.B.; Nielsen, D.S.; Josefsen, K.; Hansen, A.K.; Sorensen, D.B. A Possible Link between Food and Mood: Dietary Impact on Gut Microbiota and Behavior in BALB/c Mice. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Crawley, J.N. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Kloke, V.; Heiming, R.S.; Bölting, S.; Kaiser, S.; Lewejohann, L.; Lesch, K.P.; Sachser, N. Unexpected effects of early-life adversity and social enrichment on the anxiety profile of mice varying in serotonin transporter genotype. Behav. Brain Res. 2013, 247. [Google Scholar] [CrossRef]
- Bert, B.; Schmidt, N.; Voigt, J.P.; Fink, H.; Rex, A. Evaluation of cage leaving behaviour in rats as a free choice paradigm. J. Pharmacol. Toxicol. Methods 2013, 68, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Clement, Y.; Joubert, C.; Kopp, C.; Lepicard, E.M.; Venault, P.; Misslin, R.; Cadot, M.; Chapouthier, G. Anxiety in mice: A principal component analysis study. Neural Plast. 2007, 2007, 35457. [Google Scholar] [CrossRef] [PubMed]
- Beuzen, A.; Belzung, C. Link between emotional memory and anxiety states: A study by principal component analysis. Physiol. Behav. 1995, 58, 111–118. [Google Scholar] [CrossRef]
- Habedank, A.; Kahnau, P.; Diederich, K.; Lewejohann, L. Severity assessment from an animal’s point of view. Berl. Und Münch. Tierärztl. Wochenschr. 2018. [Google Scholar] [CrossRef]
- Franks, B. What do animals want? Anim. Welf. 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Nicol, K. Changes in meal patterning by mice measure the cost imposed by natural obstacles. Appl. Anim. Behav. Sci. 1995, 43, 291–300. [Google Scholar] [CrossRef]
- Lewejohann, L.; Sachser, N. Präferenztests zur Beurteilung unterschiedlicher Haltungsbedingungen von männlichen Labormäusen. Aktuelle Arbeiten zur artgemäßen Tierhaltung 1999. KTBL Schr. 2000, 391, 170–177. [Google Scholar]
- Jensen, G.D. Preference for bar pressing over “freeloading” as a function of number of rewarded presses. J. Exp. Psychol. 1963, 65, 451–454. [Google Scholar] [CrossRef]
- Harding, E.J.; Paul, E.S.; Mendl, M. Animal behaviour: Cognitive bias and affective state. Nature 2004, 427, 312. [Google Scholar] [CrossRef]
- Papciak, J.; Popik, P.; Fuchs, E.; Rygula, R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 2013, 256, 305–310. [Google Scholar] [CrossRef]
- Brydges, N.M.; Leach, M.; Nicol, K.; Wright, R.; Bateson, M. Environmental enrichment induces optimistic cognitive bias in rats. Anim. Behav. 2011, 81, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, R.R.; Swan, M.P.; Hickman, D.L. Effect of multilevel laboratory rat caging system on the well-being of the singly-housed Sprague Dawley rat. Lab. Anim. 2015, 49, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rygula, R.; Pluta, H.; Popik, P. Laughing rats are optimistic. PLoS ONE 2012, 7, e51959. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Ewan, E. Chronic Pain Alters Drug Self-Administration: Implications for Addiction and Pain Mechanisms. Exp. Clin. Psychopharmacol. 2008, 16, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Michalidis, V.; Lecker, I.; Collymore, C.; Hanwell, D.; Loka, M.; Danesh, M.; Pham, C.; Urban, P.; Bonin, R.P.; et al. Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci. Rep. UK 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Abelson, K.S.; Jacobsen, K.R.; Sundbom, R.; Kalliokoski, O.; Hau, J. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab. Anim. 2012, 46, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Hovard, A.M.B.; Teilmann, A.C.; Hau, J.; Abelson, K.S.P. The applicability of a gel delivery system for self-administration of buprenorphine to laboratory mice. Lab. Anim. 2015, 49, 40–45. [Google Scholar] [CrossRef]
- Molina-Cimadevila, M.J.; Segura, S.; Merino, C.; Ruiz-Reig, N.; Andres, B.; de Madaria, E. Oral self-administration of buprenorphine in the diet for analgesia in mice. Lab. Anim. 2014, 48, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Woller, S.A.; Moreno, G.L.; Hart, N.; Wellman, P.J.; Grau, J.W.; Hook, M.A. Analgesia or Addiction?: Implications for Morphine Use after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1650–1662. [Google Scholar] [CrossRef] [Green Version]
- Golledge, H.; Jirkof, P. Score sheets and analgesia. Lab. Anim. 2016, 50, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, K.; Jourdan, T.; Kock, M.; Unger, J.; Schulz, A.; Thone-Reineke, C.; Abramjuk, C. Recommendations for the development and use of Score Sheets as a tool for applied refinement. Berl. Und Munch. Tierarztl. Wochenschr. 2018, 131, 292–298. [Google Scholar] [CrossRef]
- Dunbar, M.L.; David, E.M.; Aline, M.R.; Lofgren, J.L. Validation of a Behavioral Ethogram for Assessing Postoperative Pain in Guinea Pigs (Cavia porcellus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 29–34. [Google Scholar] [PubMed]
- Koch, A.; Gulani, J.; King, G.; Hieber, K.; Chappell, M.; Ossetrova, N. Establishment of Early Endpoints in Mouse Total-Body Irradiation Model. PLoS ONE 2016, 11, e0161079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paster, E.V.; Villines, K.A.; Hickman, D.L. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009, 59, 234–241. [Google Scholar] [PubMed]
- Roughan, J.V.; Flecknell, P.A. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 2001, 90, 65–74. [Google Scholar] [CrossRef]
- Hong, W.; Kennedy, A.; Burgos-Artizzu, X.P.; Zelikowsky, M.; Navonne, S.G.; Perona, P.; Anderson, D.J. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl. Acad. Sci. USA 2015, 112, E5351–E5360. [Google Scholar] [CrossRef] [Green Version]
- Silasi, G.; Boyd, J.D.; Bolanos, F.; LeDue, J.M.; Scott, S.H.; Murphy, T.H. Individualized tracking of self-directed motor learning in group-housed mice performing a skilled lever positioning task in the home cage. J. Neurophysiol. 2018, 119, 337–346. [Google Scholar] [CrossRef]
- Andresen, N.; Wöllhaf, M.; Hohlbaum, K.; Lewejohann, L.; Hellwich, O.; Thöne-Reineke, C.; Belik, V. Towards a fully automated surveillance of well-being status in laboratory mice using deep learning. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ernst, L.; Kopaczka, M.; Schulz, M.; Talbot, S.R.; Zieglowski, L.; Meyer, M.; Bruch, S.; Merhof, D.; Tolba, R.H. Improvement of the Mouse Grimace Scale set-up for implementing a semi-automated Mouse Grimace Scale scoring (Part 1). Lab. Anim. 2019. [Google Scholar] [CrossRef]
- Loos, M.; Koopmans, B.; Aarts, E.; Maroteaux, G.; van der Sluis, S.; Verhage, M.; Smit, A.B. Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLoS ONE 2014, 9, e108563. [Google Scholar] [CrossRef] [Green Version]
- Pernold, K.; Iannello, F.; Low, B.E.; Rigamonti, M.; Rosati, G.; Scavizzi, F.; Wang, J.; Raspa, M.; Wiles, M.V.; Ulfhake, B. Towards large scale automated cage monitoring—Diurnal rhythm and impact of interventions on in-cage activity of C57BL/6J mice recorded 24/7 with a non-disrupting capacitive-based technique. PLoS ONE 2019, 14, e0211063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiers, J.G.; Chen, H.C.; Steyn, F.J.; Lavidis, N.A.; Woodruff, T.M.; Lee, J.D. Noninvasive assessment of altered activity following restraint in mice using an automated physiological monitoring system. Stress 2017, 20, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shieh, K.R.; Chen, R.J.; Yang, S.C. Circadian Patterns of Rats in Their Home Cages Detected Using a Video Tracking System. Chin. J. Physiol. 2017, 60, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Redfern, W.S.; Tse, K.; Grant, C.; Keerie, A.; Simpson, D.J.; Pedersen, J.C.; Rimmer, V.; Leslie, L.; Klein, S.K.; Karp, N.A.; et al. Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. PLoS ONE 2017, 12, e0181068. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Olechnowicz, S.W.Z.; Kratschmer, P.; Jepson, J.E.C.; Edwards, C.M.; Edwards, J.R. Small Animal Video Tracking for Activity and Path Analysis Using a Novel Open-Source Multi-Platform Application (AnimApp). Sci. Rep. 2019, 9, 12343. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.L.; Fleming, K.E.; Zhang, L.; Scimemi, A. M-Track: A New Software for Automated Detection of Grooming Trajectories in Mice. PLoS Comput. Biol. 2016, 12, e1005115. [Google Scholar] [CrossRef]
- Roughan, J.V.; Wright-Williams, S.L.; Flecknell, P.A. Automated analysis of postoperative behaviour: Assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab. Anim. 2009, 43, 17–26. [Google Scholar] [CrossRef]
- Wiltschko, A.B.; Johnson, M.J.; Iurilli, G.; Peterson, R.E.; Katon, J.M.; Pashkovski, S.L.; Abraira, V.E.; Adams, R.P.; Datta, S.R. Mapping Sub-Second Structure in Mouse Behavior. Neuron 2015, 88, 1121–1135. [Google Scholar] [CrossRef] [Green Version]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, A.H.; Molinaro, M.J.; Jethwa, J.F.; Sotocinal, S.G.; Prieto, J.C.; Styner, M.A.; Mogil, J.S.; Zylka, M.J. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 2018, 14. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Pascuzzo, R.; Leach, M.C.; Dai, F.; Lebelt, D.; Vantini, S.; Minero, M. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS ONE 2018, 13, e0200339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, V.; Zhang, E.; Pang, D.S.J. Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci. Rep. UK 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.B.; Vigors, B.; Sandoe, P. What Is so Positive about Positive Animal Welfare?—A Critical Review of the Literature. Animals 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor, D.J. Affective States and the Assessment of Laboratory-Induced Animal Welfare Impacts. Altex Proc. 2012, 1, 445–449. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirkof, P.; Rudeck, J.; Lewejohann, L. Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research? Animals 2019, 9, 1026. https://doi.org/10.3390/ani9121026
Jirkof P, Rudeck J, Lewejohann L. Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research? Animals. 2019; 9(12):1026. https://doi.org/10.3390/ani9121026
Chicago/Turabian StyleJirkof, Paulin, Juliane Rudeck, and Lars Lewejohann. 2019. "Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research?" Animals 9, no. 12: 1026. https://doi.org/10.3390/ani9121026
APA StyleJirkof, P., Rudeck, J., & Lewejohann, L. (2019). Assessing Affective State in Laboratory Rodents to Promote Animal Welfare—What Is the Progress in Applied Refinement Research? Animals, 9(12), 1026. https://doi.org/10.3390/ani9121026