Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Slaughter Procedure, Carcass Measurements, Tissue Sampling, and Dual-Energy X-ray Absorptiometry (DXA) Measurements
2.3. Meat Quality Traits
2.4. Feed and Meat Analysis
2.5. Analysis of Boar Taint in Fat and Muscle
2.6. RNA Isolation, Primer Design, and Quantitative Real-Time PCR
2.7. DNA Extraction and Sequencing
2.8. NGS Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Growth Performance, Feeding Behaviour, Carcass Characteristics, and Organ Weights
3.2. Meat Quality, Boar Taint Compounds, and Cytochrome Isoenzyme Gene Expression
3.3. Fatty Acid Composition of the Intramuscular Fat and Adipose Tissue
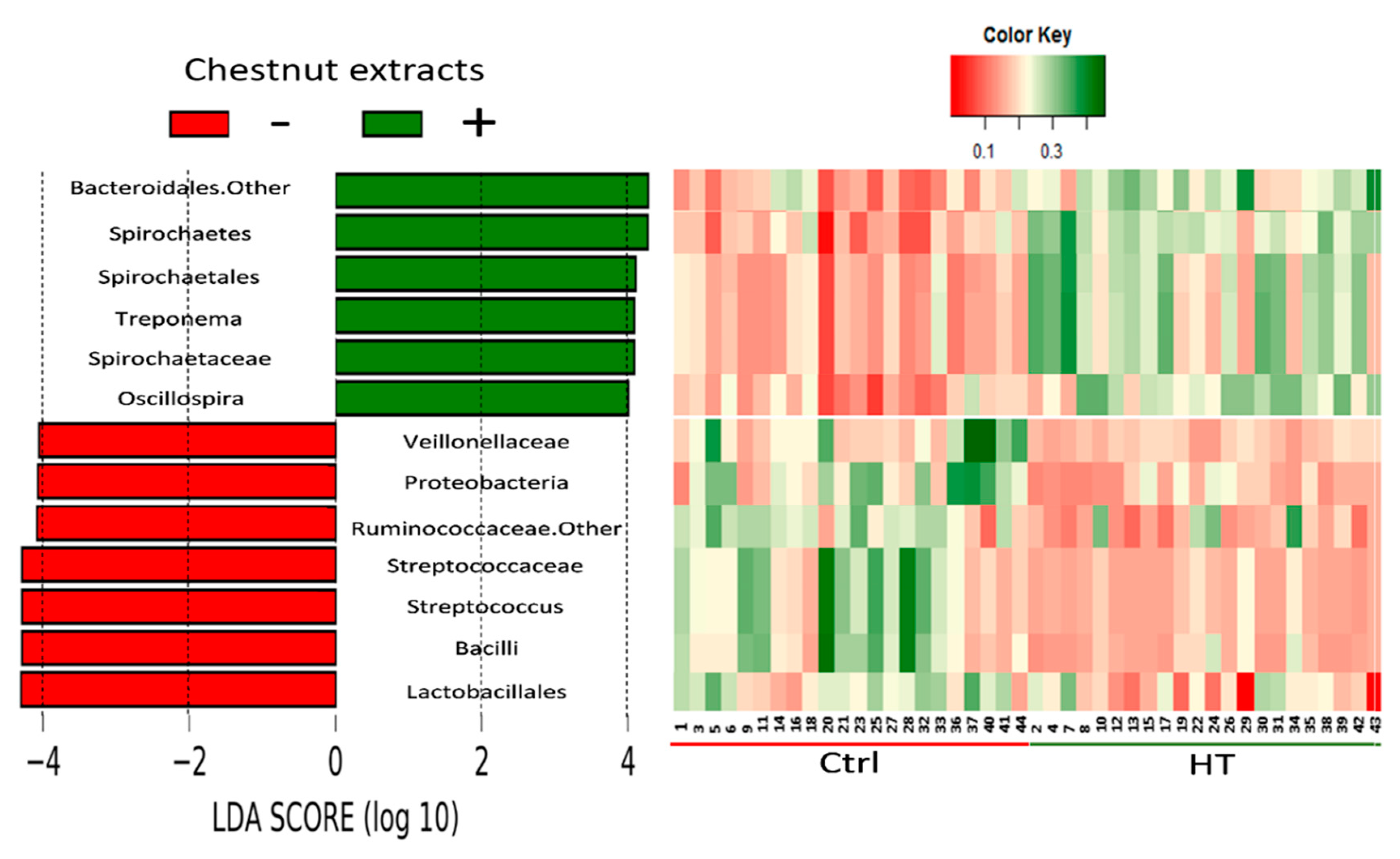
3.4. Effects of HTs and PUFA on Gut Microbiota
4. Discussion
4.1. Dietary Effects on Growth Performance
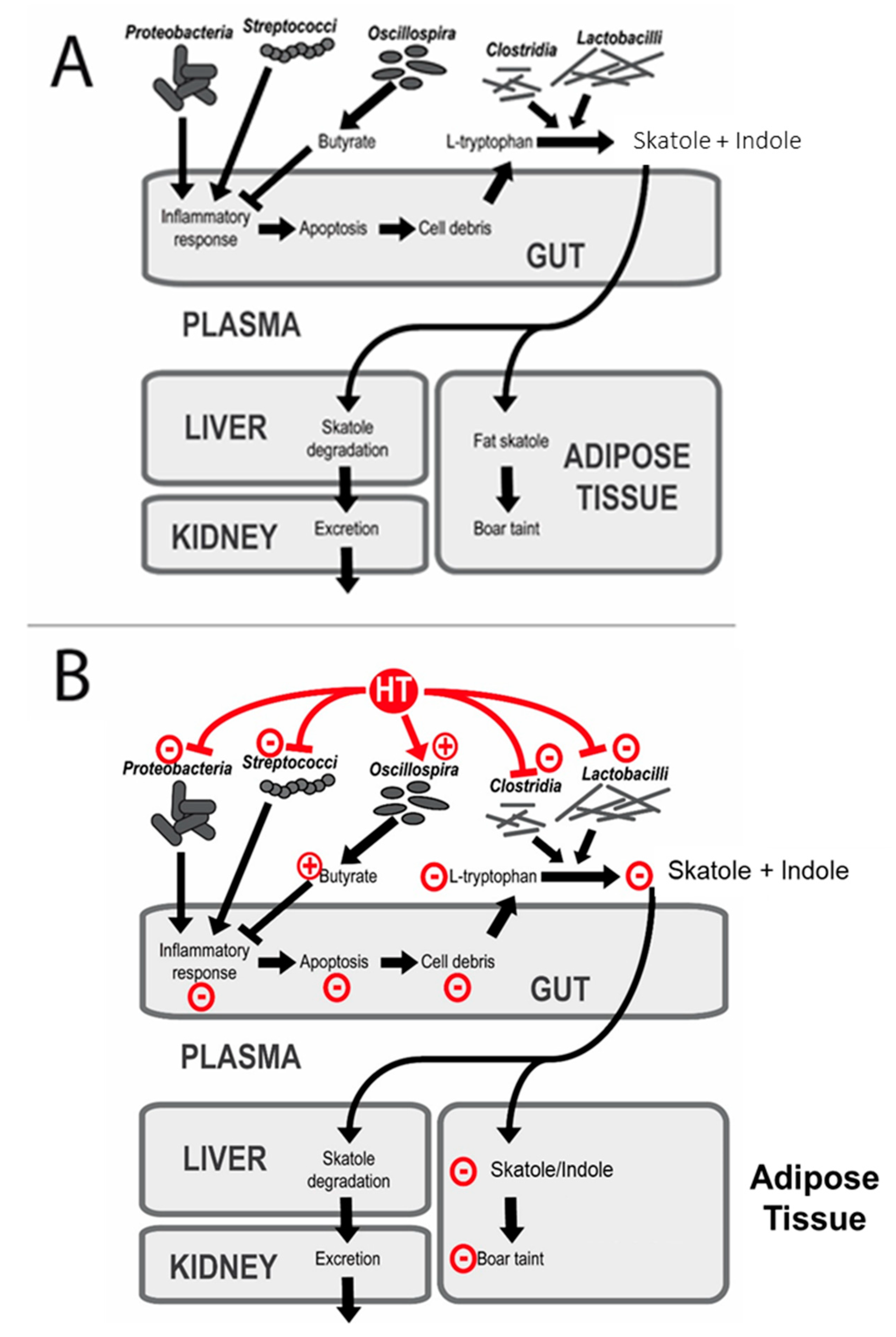
4.2. Dietary Effects on Skatole, Indole, and Androstenone Levels in Adipose Tissue
4.3. Dietary Effects on the Fatty Acid Profile of the Adipose Tissue
4.4. Dietary Effects on the Faecal Microbial Composition
4.5. Effects of HTs on the Gut Microbial Structure and Boar Taint
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bee, G.; Chevillon, P.; Bonneau, M. Entire male pig production in Europe. Anim. Prod. Sci. 2015, 55, 1347–1359. [Google Scholar] [CrossRef]
- Font-i-Furnols, F. Consumer studies on sensory acceptability of boar taint: A review. Meat Sci. 2012, 92, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Čandek-Potokar, M.; Škrlep, M.; Lukač, N.; Zamaratskaia, G.; Povše, M.; Bolta, Š.; Bee, G. Hydrolysable tannin fed to entire male pigs affects intestinal production, tissue deposition and hepatic clearance of skatole. Vet. J. 2015, 204, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bee, G.; Silacci, P.; Ampuero-Kragten, S.; Čandek-Potokar, M.; Wealleans, A.; Litten-Brown, J.; Mueller-Harvey, I. Hydrolysable tannin-based diet rich in gallotannins has a minimal impact on pig performance but significantly reduces salivary and bulbourethral gland size. Animal 2017, 2, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Bilić-Šobot, D.; Kubale, V.; Škrlep, M.; Čandek-Potokar, M.; Prevolnik Povše, M.; Fazarinc, G.; Škorjanc, D. Effect of hydrolysable tannins on intestinal morphology, proliferation and apoptosis in entire male pigs. Arch. Anim. Nutr. 2016, 2, 378–388. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Squires, E. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 2, 1508–1521. [Google Scholar] [CrossRef]
- Jen, K.; Squires, E. Efficacy of non-nutritive sorbent materials as intestinal-binding agents for the control of boar taint. Animal 2011, 5, 1814–1820. [Google Scholar] [CrossRef]
- Ruoff, W.; Dziuk, P. Absorption and metabolism of estrogens from the stomach and duodenum of pigs. Domest. Anim. Endocrin. 1994, 11, 197–208. [Google Scholar] [CrossRef]
- Mörlein, D.; Tholen, E. Fatty acid composition of subcutaneous adipose tissue from entire male pigs with extremely divergent levels of boar taint compounds—An exploratory study. Meat Sci. 2015, 99, 1–7. [Google Scholar] [CrossRef]
- Bee, G.; Jacot, S.; Guex, G.; Biolley, C. Effects of two supplementation levels of linseed combined with CLA or tallow on meat quality traits and fatty acid profile of adipose and different muscle tissues in slaughter pigs. Animal 2008, 2, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.; Kreuzer, M.; Bee, G. Effect of average litter weight in pigs on growth performance, carcass characteristics and meat quality of the offspring as depending on birth weight. Animal 2013, 7, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Kragten, S.; Collomb, M.; Dubois, S.; Stoll, P. Composition des acides gras dans l’alimentation animale–méthodes d’analyse. Rech. Agron. Suisse. 2014, 5, 330–337. [Google Scholar]
- Johnson, M.; Ives, A.; Ahern, J.; Salminen, J. Macroevolution of plant defenses against herbivores in the evening primroses. New Phytol. 2014, 203, 267–279. [Google Scholar] [CrossRef]
- Kragten, S.; Verkuylen, B.; Dahlmans, H.; Hortos, M.; Garcia-Regueiro, J.; Dahl, E.; Harlizius, B. Inter-laboratory comparison of methods to measure androstenone in pork fat. Animal 2011, 5, 1634–1642. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, C.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Rasmussen, M.; Zamaratskaia, G.; Ekstrand, B. Gender-related Differences in Cytochrome P450 in Porcine Liver–Implication for Activity, Expression and Inhibition by Testicular Steroids. Reprod. Domest. Anim. 2011, 46, 616–623. [Google Scholar] [CrossRef]
- Edgar, R.; Haas, B.; Clemente, J.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Cole, J.; Tiedje, J. History and impact of RDP: A legacy from Carl Woese to microbiology. RNA Biol. 2014, 11, 239–243. [Google Scholar] [CrossRef]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Huttley, G. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef]
- Bokulich, N.; Subramanian, S.; Faith, J.; Gevers, D.; Gordon, J.; Knight, R.; Caporaso, J. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.; Goodrich, J.; Nawrocki, E.; DeSantis, T.; Probst, A.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Boil. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Bee, G.; Gebert, S.; Messikommer, R. Effect of dietary energy supply and fat source on the fatty acid pattern of adipose and lean tissues and lipogenesis in the pig. J. Anim. Sci. 2002, 80, 1564–1574. [Google Scholar] [CrossRef]
- Kim, J.; Ingale, S.; Lee, S.; Choi, Y.; Kim, E.; Lee, D.; Chae, B. Impact of dietary fat sources and feeding level on adipose tissue fatty acids composition and lipid metabolism related gene expression in finisher pigs. Anim. Feed Sci. Technol. 2014, 196, 60–67. [Google Scholar] [CrossRef]
- Kato, C.; Gonçalves, G.; Peralta, R.; Seixas, F.; de Sá-Nakanishi, A.; Bracht, L.; Peralta, R. Inhibition of α-amylases by condensed and hydrolysable tannins: Focus on kinetics and hypoglycemic actions. Enzyme Res. 2017, 2017. [Google Scholar] [CrossRef]
- Rezar, V.; Salobir, J.; Levart, A.; Tomažin, U.; Škrlep, M.; Lukač, N.; Čandek-Potokar, M. Supplementing entire male pig diet with hydrolysable tannins: Effect on carcass traits, meat quality and oxidative stability. Meat Sci. 2017, 133, 95–102. [Google Scholar] [CrossRef]
- Wood, J.; Enser, M.; Fisher, A.; Nute, G.; Sheard, P.; Richardson, R.; Whittington, F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Corino, C.; Magni, S.; Pastorelli, G.; Rossi, R.; Mourot, J. Effect of conjugated linoleic acid on meat quality, lipid metabolism, and sensory characteristics of dry-cured hams from heavy pigs. J. Anim. Sci. 2003, 81, 2219–2229. [Google Scholar] [CrossRef]
- Babol, J.; Squires, E. Quality of meat from entire male pigs. Food Res. Int. 1995, 28, 201–212. [Google Scholar] [CrossRef]
- Warnants, N.; Van Oeckel, M.; Boucqué, C. Incorporation of dietary polyunsaturated fatty acids in pork tissues and its implications for the quality of the end products. Meat Sci. 1996, 44, 125–144. [Google Scholar] [CrossRef]
- Bryhni, E.; Kjos, N.; Ofstad, R.; Hunt, M. Polyunsaturated fat and fish oil in diets for growing-finishing pigs: Effects on fatty acid composition and meat, fat, and sausage quality. Meat Sci. 2002, 62, 1–8. [Google Scholar] [CrossRef]
- Stoll, P.; Zihlmann, U.; Hofstetter, P. Produktionssysteme mit saisonaler Freilandhaltung von Schweinen. Agrarforschung 2008, 15, 64–69. [Google Scholar]
- Lima, V.; Oliveira-Tintino, C.; Santos, E.; Morais, L.; Tintino, S.; Freitas, T.; Coutinho, H. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Girard, M.; Thanner, S.; Pradervand, N.; Hu, D.; Ollagnier, C.; Bee, G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: Efficacy on an experimental ETEC F4 model. PLoS ONE. 2018, 13, e0197878. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.; Stanton, T. Altered egos: Antibiotic effects on food animal microbiomes. Annu. Rev. Microbiol. 2014, 68, 297–315. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 2015, 36, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Tretola, M.; Luciano, A.; Ottoboni, M.; Baldi, A.; Pinotti, L. Influence of Traditional vs Alternative Dietary Carbohydrates Sources on the Large Intestinal Microbiota in Post-Weaning Piglets. Animals 2019, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Cox, L.; Blaser, M. Pathways in microbe-induced obesity. Cell Metab. 2013, 17, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, D.; Hughes, R.; Geier, M.; Moore, R. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Konikoff, T.; Gophna, U. Oscillospira: A central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Marchandin, H.; Jumas-Bilak, E. The family veillonellaceae. In The Prokaryotes Firmicutes and Tenericutes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 433–453. [Google Scholar]
- Evivie, S.; Huo, G.; Igene, J.; Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. J. Food Nutr. Res. 2017, 61, 1318034. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Raab, S. Influences on skatole formation from tryptophan in the pig colon. In Tryptophan, Serotonin, and Melatonin; Springer: Boston, MA, USA, 1999; pp. 679–684. [Google Scholar]
- Raab, S.; Leiser, R.; Kemmer, H.; Claus, R. Effects of energy and purines in the diet on proliferation, differentiation, and apoptosis in the small intestine of the pig. Metabolism 1998, 47, 1105–1111. [Google Scholar] [CrossRef]
- Huang, C.; Lee, T.; Shih, Y.; Yu, B. Effects of dietary supplementation of Chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J. Anim. Physiol. Anim. Nutr. 2012, 96, 285–294. [Google Scholar] [CrossRef]
- Walters, W.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Baker, S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.; Gill, S. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Yokoyama, M.; Carlson, J. Production of skatole and para-cresol by a rumen Lactobacillus sp. Appl. Environ. Microbiol. 1981, 41, 71–76. [Google Scholar]

| Item | Grower Diet (from 73 to 122 d of Age) | Finisher Diet (from 122 to 170 d of Age) | ||||||
|---|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | H− | L− | H+ | L+ | |
| Wheat | 49.75 | 49.75 | 49.75 | 49.75 | 42.59 | 42.59 | 42.59 | 42.59 |
| Barley | 10.56 | 10.56 | 10.56 | 10.56 | 27.7 | 27.7 | 27.7 | 27.7 |
| Corn | 2.43 | 2.43 | 2.43 | 2.43 | ||||
| Wheat starch | 7 | 7 | 7 | 7 | 9.09 | 9.09 | 9.09 | 9.09 |
| Soy extraction meal | 12.71 | 12.71 | 12.71 | 12.71 | 9.7 | 9.7 | 9.7 | 9.7 |
| Potato protein | 2.47 | 2.47 | 2.47 | 2.47 | 0.59 | 0.59 | 0.59 | 0.59 |
| Wheat bran | 5 | 5 | 5 | 5 | 1.14 | 1.14 | 1.14 | 1.14 |
| Soy oil | 2 | - | 2 | - | 2 | - | 2 | - |
| Tallow | - | 2 | - | 2 | - | 2 | - | 2 |
| Arbocel | 3 | 3 | - | - | 3 | 3 | - | - |
| Hydrolysable tannins 2 | - | - | 3 | 3 | - | - | 3 | 3 |
| Dicalcium phosphate | 1.45 | 1.45 | 1.45 | 1.45 | 1.05 | 1.05 | 1.05 | 1.05 |
| Mono-sodium phosphate | 0.4 | 0.4 | 0.4 | 0.4 | - | - | - | - |
| Calcium carbonate | 1.38 | 1.38 | 1.38 | 1.38 | 0.88 | 0.88 | 0.88 | 0.88 |
| NaCl | 0.08 | 0.08 | 0.08 | 0.08 | 0.46 | 0.46 | 0.46 | 0.46 |
| Natuphos 5000 G | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| L-lysine-HCl | 0.34 | 0.34 | 0.34 | 0.34 | 0.36 | 0.36 | 0.36 | 0.36 |
| DL-methionine | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| L-threonine | 0.07 | 0.07 | 0.07 | 0.07 | 0.1 | 0.1 | 0.1 | 0.1 |
| Mikrogrit | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Pellan 3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Mineral-vitamin premix 4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Analyzed nutrient and tannin composition, g/kg DM | ||||||||
| Total ash | 59 | 59.9 | 59.6 | 59.3 | 48.6 | 48.1 | 49.3 | 49 |
| Crude fiber | 46.2 | 44.7 | 27.7 | 27 | 45.1 | 44.9 | 32.6 | 31.5 |
| Crude protein | 177.1 | 180.7 | 180.1 | 180.5 | 146.4 | 146.9 | 147.3 | 145.4 |
| Crude fat | 42.3 | 44.5 | 42.3 | 45.6 | 41.3 | 42.9 | 42.3 | 45 |
| SFA (g/100 total fatty acid) | 38.46 | 42.91 | 38.46 | 42.91 | 37.31 | 42.45 | 37.31 | 42.45 |
| MUFA (g/100 total fatty acid) | 32.69 | 44.98 | 32.69 | 44.98 | 32.34 | 45.32 | 32.34 | 45.32 |
| PUFA (g/100 total fatty acid) | 28.85 | 12.11 | 28.85 | 12.11 | 30.35 | 12.23 | 30.35 | 12.23 |
| Total hydrolysable tannin | - | - | 14.81 | 14.81 | - | - | 14.81 | 14.81 |
| Calculated DE content, MJ/kg DM 5 | 13.54 | 13.54 | 13.54 | 13.54 | 13.54 | 13.54 | 13.54 | 13.54 |
| Item | Dietary Treatments | SEM | p-Values 2 | |||||
|---|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | T | P | T × P | ||
| Body weight, kg | ||||||||
| At birth | 1.68 | 1.64 | 1.75 | 1.71 | 0.109 | 0.36 | 0.64 | 0.98 |
| At start of grower period | 25.60 | 25.42 | 26.51 | 26.64 | 1.553 | 0.42 | 0.98 | 0.91 |
| At start of finisher period | 66.45 | 62.60 | 65.86 | 66.83 | 2.377 | 0.44 | 0.54 | 0.31 |
| At slaughter | 112.76 | 109.00 | 108.49 | 110.40 | 3.791 | 0.63 | 0.76 | 0.34 |
| Average daily gain, kg/d | ||||||||
| Grower period | 0.83 | 0.76 | 0.80 | 0.82 | 0.029 | 0.58 | 0.46 | 0.14 |
| Finisher period | 0.94 | 0.94 | 0.87 | 0.88 | 0.037 | 0.01 | 0.94 | 0.71 |
| Grower–finisher period | 0.89 | 0.85 | 0.83 | 0.85 | 0.029 | 0.27 | 0.69 | 0.27 |
| Total feed intake, kg | ||||||||
| Grower period | 83.8 | 77.9 | 84.6 | 86.5 | 2.831 | 0.10 | 0.48 | 0.17 |
| Finisher period | 124.1 | 119.4 | 118.2 | 118.4 | 6.571 | 0.34 | 0.53 | 0.49 |
| Grower–finisher period | 207.7 | 197.5 | 202.9 | 205.3 | 8.535 | 0.80 | 0.50 | 0.28 |
| Average feed intake, kg/d | ||||||||
| Grower period | 1.71 | 1.59 | 1.73 | 1.77 | 0.058 | 0.10 | 0.48 | 0.17 |
| Finisher period | 2.51 | 2.43 | 2.40 | 2.40 | 0.093 | 0.33 | 0.56 | 0.56 |
| Grower–finisher period | 2.11 | 2.01 | 2.06 | 2.09 | 0.071 | 0.82 | 0.51 | 0.31 |
| Gain-to-feed, kg/kg | ||||||||
| Grower period | 0.49 | 0.48 | 0.47 | 0.47 | 0.008 | 0.01 | 0.34 | 0.34 |
| Finisher period | 0.38 | 0.39 | 0.36 | 0.37 | 0.008 | <0.01 | 0.19 | 0.63 |
| Grower–finisher period | 0.42 | 0.42 | 0.41 | 0.41 | 0.006 | <0.01 | 0.56 | 0.78 |
| Item | Dietary Treatments | SEM | p-Values 2 | |||||
|---|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | T | P | T × P | ||
| Boar taint compounds, µg/g adipose tissue | ||||||||
| Androstenone | 0.51 | 0.41 | 0.37 | 0.39 | 0.085 | 0.32 | 0.62 | 0.40 |
| Skatole | 0.13 y | 0.05 x | 0.09 x,y | 0.12 x,y | 0.026 | 0.48 | 0.25 | <0.05 |
| Indole | 0.05 | 0.03 | 0.02 | 0.02 | 0.007 | 0.08 | 0.16 | 0.21 |
| Item | Dietary Treatments | SEM | p-Values 2 | |||||
|---|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | T | P | T × P | ||
| LIVER | ||||||||
| CYP1A1 * | 1.11 | 1.19 | 0.93 | 1.22 | 0.285 | 0.83 | 0.69 | 0.53 |
| CYP1A2 | 1.59 | 1.24 | 0.93 | 1.03 | 0.200 | 0.04 | 0.52 | 0.28 |
| CYP2A19 * | 2.08 | 1.62 | 2.26 | 1.08 | 0.812 | 0.21 | 0.13 | 0.23 |
| CYP2E1 | 1.15 x,y | 1.45 y | 1.13 x,y | 1.12 x | 0.166 | 0.07 | 0.22 | 0.11 |
| CYP3A29 | 1.43 | 1.24 | 1.25 | 1.02 | 0.109 | 0.40 | 0.36 | 1.00 |
| Colon | ||||||||
| CYP1A1 | 0.10 | 0.79 | 1.25 | 0.18 | 0.566 | 0.59 | 0.71 | 0.09 |
| CYP3A29 * | 1.14 | 1.55 | 0.10 | 1.17 | 0.075 | 0.14 | 0.06 | 0.42 |
| Item | Dietary Treatments | SEM | P-Values 2 | |||||
|---|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | T | P | T × P | ||
| Intramuscular fat (g/kg) | 22.30 | 19.02 | 21.12 | 20.59 | 1.882 | 0.88 | 0.25 | 0.43 |
| Fatty acid profile (g/100 g total fatty acid) | ||||||||
| 14:0 | 1.03 | 0.91 | 0.98 | 0.99 | 0.074 | 0.81 | 0.22 | 0.13 |
| 16:0 | 22.9 | 22.7 | 22.8 | 23.0 | 0.389 | 0.79 | 0.94 | 0.24 |
| 17:0 | 0.20 | 0.20 | 0.20 | 0.20 | 0.031 | 0.92 | 0.98 | 0.80 |
| 18:0 | 11.8 x | 12.4 y | 12.2 x,y | 12.5 y | 0.288 | 0.31 | 0.04 | 0.46 |
| 20:0 | 0.07 | 0.06 | 0.07 | 0.07 | 0.025 | 0.82 | 0.86 | 0.68 |
| 16:1n-7 | 3.29 y | 3.27 y | 3.02 x | 3.30 y | 0.125 | 0.12 | <0.01 | 0.05 |
| 17:1n-7 | 0.20 | 0.20 | 0.18 | 0.22 | 0.030 | 0.94 | 0.33 | 0.34 |
| 18:1n-9 | 45.3 | 45.9 | 45.0 | 46.3 | 0.539 | 0.96 | 0.08 | 0.47 |
| 20:1n-9 | 0.67 | 0.58 | 0.65 | 0.68 | 0.040 | 0.27 | 0.46 | 0.12 |
| 18:2n-6 | 10.9 | 10.3 | 11.4 | 9.65 | 0.661 | 0.80 | 0.02 | 0.26 |
| 18:3n-3 | 0.74 | 0.40 | 0.70 | 0.43 | 0.041 | 0.89 | <0.01 | 0.19 |
| 20:2n-6 | 0.28 | 0.17 | 0.25 | 0.21 | 0.052 | 0.83 | 0.01 | 0.15 |
| 20:4n-6 | 2.04 | 2.52 | 2.16 | 2.11 | 0.203 | 0.40 | 0.23 | 0.14 |
| 22:4n-6 | 0.18 | 0.17 | 0.13 | 0.17 | 0.058 | 0.51 | 0.56 | 0.54 |
| 22:5n-3 | 0.21 | 0.14 | 0.18 | 0.17 | 0.059 | 0.95 | 0.31 | 0.54 |
| 16:1n-7/16:0 | 0.14 y | 0.14 y | 0.13 x | 0.14 y | 0.005 | 0.08 | 0.07 | 0.09 |
| 18:1n-9/18:0 | 3.86 | 3.72 | 3.71 | 3.74 | 0.094 | 0.40 | 0.43 | 0.29 |
| 20:4n-6/18:2n-6 | 0.19 | 0.24 | 0.19 | 0.22 | 0.012 | 0.27 | <0.01 | 0.22 |
| 22:5n-3/18:3n-3 | 0.29 | 0.32 | 0.25 | 0.35 | 0.101 | 0.96 | 0.35 | 0.63 |
| Sum of n-6 fatty acids | 13.45 | 13.21 | 13.94 | 13.15 | 0.860 | 0.66 | 0.13 | 0.24 |
| Sum of n-3 fatty acids | 0.96 | 0.54 | 0.88 | 0.60 | 0.083 | 0.76 | <0.01 | 0.13 |
| SFA | 36.1 | 36.3 | 36.2 | 36.7 | 0.592 | 0.48 | 0.33 | 0.75 |
| MUFA | 49.5 | 49.9 | 48.9 | 50.5 | 0.586 | 0.93 | 0.07 | 0.29 |
| PUFA | 14.4 | 13.8 | 14.8 | 12.7 | 0.899 | 0.66 | 0.05 | 0.29 |
| n-6/n-3 fatty acid ratio | 14.6 x | 24.0z | 17.8 y | 23.1 z | 2.022 | 0.31 | <0.01 | 0.07 |
| 18:2n-6/18:3n-3 ratio | 14.9 x | 23.2 z | 17.1 y | 22.8 z | 1.125 | 0.18 | <0.01 | 0.07 |
| Adipose tissue | ||||||||
| Fatty acid profile (g/100 g total fatty acid) | ||||||||
| 14:0 | 1.19 | 1.30 | 1.16 | 1.31 | 0.025 | 0.61 | <0.01 | 0.43 |
| 16:0 | 22.5 | 24.0 | 22.2 | 24.0 | 0.294 | 0.57 | <0.01 | 0.58 |
| 18:0 | 12.2 | 13.2 | 11.9 | 13.1 | 0.413 | 0.54 | <0.01 | 0.75 |
| 20:0 | 0.19 | 0.17 | 0.17 | 10.1 | 0.006 | 0.10 | 0.01 | 0.91 |
| 16:1n-7 | 1.98 | 2.43 | 1.90 | 2.48 | 0.074 | 0.75 | <0.01 | 0.21 |
| 18:1n-9 | 41.3 | 43.6 | 41.4 | 43.1 | 0.404 | 0.51 | <0.01 | 0.44 |
| 20:1n-9 | 0.85 | 0.95 | 0.83 | 0.88 | 0.039 | 0.22 | 0.05 | 0.42 |
| 18:2n-6 | 15.9 | 11.5 | 16.5 | 11.9 | 0.568 | 0.24 | <0.01 | 0.92 |
| 20:2n-6 | 0.64 | 0.50 | 0.66 | 0.49 | 0.025 | 0.61 | <0.01 | 0.39 |
| 18:3n-3 | 1.80 | 0.86 | 1.87 | 0.94 | 0.053 | 0.15 | <0.01 | 0.90 |
| 20:3n-3 | 0.25 | 0.15 | 0.26 | 0.16 | 0.010 | 0.37 | <0.01 | 0.95 |
| 20:4n-6 | 0.24 | 0.22 | 0.22 | 0.21 | 0.011 | 0.21 | 0.08 | 0.98 |
| 22:4n-3 | 0.07 | 0.07 | 0.06 | 0.06 | 0.005 | 0.08 | 0.82 | 0.34 |
| 22:5n-3 | 0.10 | 0.07 | 0.10 | 0.07 | 0.006 | 0.88 | <0.01 | 0.93 |
| 16:1n-7/16:0 | 0.09 | 0.10 | 0.09 | 0.10 | 0.003 | 0.91 | <0.01 | 0.42 |
| 18:1n-9/18:0 | 3.44 | 3.33 | 3.54 | 3.31 | 0.127 | 0.71 | 0.14 | 0.60 |
| 20:4n-6/18:2n-6 | 0.01 | 0.02 | 0.01 | 0.02 | 0.001 | <0.01 | <0.01 | 0.82 |
| 22:5n-3/18:3n-3 | 0.06 | 0.09 | 0.05 | 0.08 | 0.004 | 0.24 | <0.01 | 0.491 |
| SFA | 36.5 | 39.2 | 35.9 | 39.2 | 0.647 | 0.55 | <0.01 | 0.62 |
| MUFA | 44.5 | 47.4 | 44.4 | 46.9 | 0.428 | 0.46 | <0.01 | 0.54 |
| PUFA | 18.9 | 13.3 | 19.6 | 13.9 | 0.661 | 0.26 | <0.01 | 0.92 |
| n-6/n-3 fatty acid ratio | 7.81 x | 11.4 z | 7.81 x | 10.9 y | 0.132 | 0.07 | <0.01 | 0.06 |
| 18:2n-6/18:3n-3 ratio | 8.81 x | 13.4 z | 8.81 x | 12.8 y | 0.174 | 0.05 | <0.01 | 0.05 |
| Iodine value | 72.1 | 64.4 | 73.2 | 64.9 | 1.100 | 0.37 | <0.01 | 0.77 |
| Item | Dietary Treatments | p-Values 2 | |||||
|---|---|---|---|---|---|---|---|
| H− | L− | H+ | L+ | T | P | T × P | |
| Chao1 3 | 1002 ± 94.7 | 1017 ± 120 | 900 ± 83.7 | 912 ± 63.9 | <0.01 | 0.64 | 0.96 |
| OTUs 4 | 916 ± 93.8 | 944 ± 118 | 831 ± 74.8 | 840 ± 54.8 | <0.01 | 0.47 | 0.75 |
| Shannon 5 | 4.89 ± 0.41 x | 5.17 ± 0.23 y | 5.07 ± 0.12 x,y | 5.02 ± 0.11 x,y | 0.86 | 0.15 | 0.03 |
| PD 6 | 55.5 ± 3.21 | 57.1 ± 4.66 | 52.3 ± 3.27 | 52.8 ± 2.31 | <0.01 | 0.31 | 0.63 |
| Taxonomic Rank | HT | p-Value | ||
|---|---|---|---|---|
| − | + | |||
| Phylum | Bacteroidetes | |||
| Family | Unclassified Bacteroidales | 0.106 | 0.182 | <0.001 |
| Phylum | Spirochaetes | 0.058 | 0.096 | <0.001 |
| Order | Spirochaetales | 0.026 | 0.051 | <0.001 |
| Family | Spirochaetaceae | 0.025 | 0.050 | <0.001 |
| Genus | Treponema | 0.025 | 0.049 | <0.001 |
| Phylum | Firmicutes | |||
| Class | Bacilli | 0.053 | 0.014 | <0.001 |
| Family | Veillonellaceae | 0.047 | 0.025 | <0.001 |
| Genus | Unclassified Ruminococcaceae | 0.077 | 0.052 | 0.002 |
| Genus | Oscillospira | 0.022 | 0.042 | <0.001 |
| Order | Lactobacillales | 0.051 | 0.011 | <0.001 |
| Family | Streptococcaceae | 0.045 | 0.007 | <0.001 |
| Genus | Streptococcus | 0.045 | 0.007 | <0.001 |
| Phylum | Proteobacteria | 0.051 | 0.028 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretola, M.; Maghin, F.; Silacci, P.; Ampuero, S.; Bee, G. Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males. Animals 2019, 9, 1063. https://doi.org/10.3390/ani9121063
Tretola M, Maghin F, Silacci P, Ampuero S, Bee G. Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males. Animals. 2019; 9(12):1063. https://doi.org/10.3390/ani9121063
Chicago/Turabian StyleTretola, Marco, Federica Maghin, Paolo Silacci, Silvia Ampuero, and Giuseppe Bee. 2019. "Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males" Animals 9, no. 12: 1063. https://doi.org/10.3390/ani9121063
APA StyleTretola, M., Maghin, F., Silacci, P., Ampuero, S., & Bee, G. (2019). Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males. Animals, 9(12), 1063. https://doi.org/10.3390/ani9121063





