Evaluation of Collagen and Elastin Content in Skin of Multiparous Minks Receiving Feed Contaminated with Deoxynivalenol (DON, vomitoxin) with or without Bentonite Supplementation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
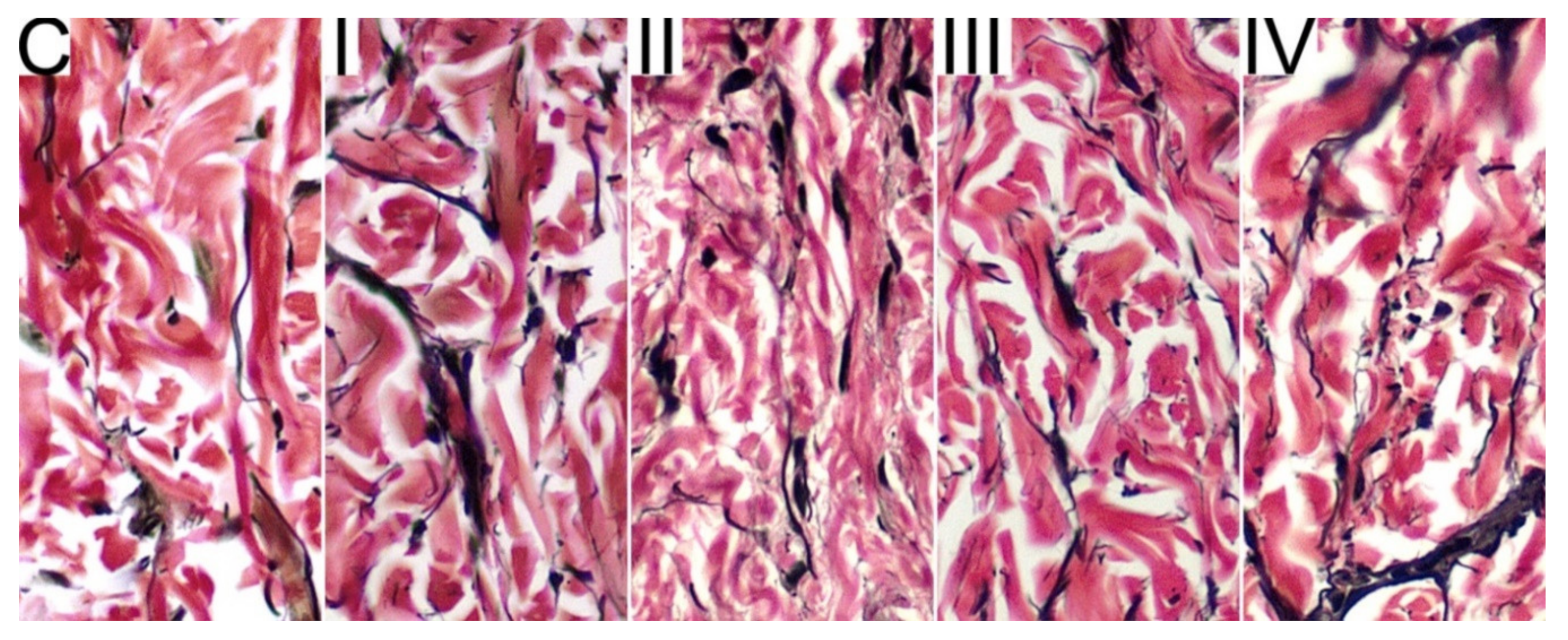
2.2. Histomophometrical Analysis of Skin
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thirstrup, J.P.; Jensen, J.; Lund, M.S. Genetic parameters for fur quality graded on live animals and dried pelts of American mink (Neovison vison). J. Anim. Breed. Genet. 2017, 134, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Piórkowska, M.; Natanek, A. Usefulness of histological examination in the evaluation of fur raw material quality. Wiad. Zootech. 2013, 51, 83–92. [Google Scholar]
- Szczepanik, M.P.; Wilkołek, P.M.; Pluta, M.; Adamek, Ł.; Gołyński, M.; Pomorski, Z.J.; Sitkowski, W. The examination of biophysical skin parameters (transepidermal water loss, skin hydration and pH value) in different body regions in Polish ponies. Pol. J. Vet. Sci. 2013, 16, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Momota, Y.; Shimada, K.; Noguchi, A.; Saito, A.; Nozawa, S.; Niina, A.; Tani, K.; Azakami, D.; Ishioka, K.; Sako, T. The modified corneocyte surface area measurement as an index of epidermal barrier properties: Inverse correlation with transepidermal water loss. Vet. Derm. 2016, 27, 67–e19. [Google Scholar] [CrossRef] [PubMed]
- Chełkowski, J. Mycotoxins, Toxin-forming Fungi and Mycotoxicoses. Cropnet. 2010. Available online: http://www.cropnet.pl/dbases/mycotoxins.pdf (accessed on 22 November 2019).
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Kuča, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: signaling pathways and human exposure risk assessment—An update. Arch. Toxicol. 2014, 88, 1915–1928. [Google Scholar] [CrossRef]
- Stanisławczyk, R.; Rudy, M.; Świątek, B. The occurrence of mycotoxins in cereals and cereal products present in retail outlets in the province of Podkarpacie (Poland). Żywność. Nauka. Technol. Jakość. 2010, 17, 58–66. [Google Scholar] [CrossRef]
- Solarska, E.; Marzec, M. Mycotoxins in cereal products from organic cultivation. J. Res. Appl. Agric. Eng. 2012, 57, 102–108. [Google Scholar]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: a focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Dobosz, B.; Król, K.; Lar, K.; Mroczek, A.; Zbrojkiewicz, E.; Złotkowska, R. Presence of mycotoxins in cereal preparations on the market in Silesia Province from 2013 to 2015. Environ. Med. 2017, 20, 34–40. [Google Scholar] [CrossRef]
- EC (European Commission). Opinion on Fusarium Toxins. Part 1: Deoxynivalenol (DON). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_fusarium_out44_en.pdf (accessed on 22 November 2019).
- CR (Commission Recommendation). Commission recommendation (CR) No 2006/583/EC of 17 August 2006 on the prevention and reduction of Fusarium toxins in cereals and cereal products. Off. J. Eur. Union 2006, 234, 35–40. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1464022623530&uri=CELEX:32006H0583 (accessed on 22 November 2019).
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Aguzey, H.A.; Zhenhua, G.; Haohao, W.; Guilan, C.; Zhengmin, W.; Junhong, C. The Effects of Deoxynivalenol (DON) on the Gut Microbiota, Morphology and Immune System of Chicken—A Review. Ann. Anim. Sci. 2019, 19, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef]
- Weaver, A.C.; Todd See, M.; Hansen, J.A.; Kim, Y.B.; De Souza, A.L.P.; Middleton, T.F.; Kim, S.W. The use of feed additives to reduce the effects of aflatoxin and deoxynivalenol on pig growth, organ health and immune status during chronic exposure. Toxins 2013, 5, 1261–1281. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Scientific Opinion on the safety and efficacy of bentonite (dioctahedral montmorillonite) as feed additive for all species. EFSA J. 2011, 9. [Google Scholar] [CrossRef] [Green Version]
- Nowakowicz-Dębek, B.; Wlazło, Ł. Effect of dietary sodium bentonite supplement on microbial contamination of mink feed. Pol. J. Env. Stud. 2011, 20, 1103–1106. [Google Scholar]
- Wlazło, Ł.; Nowakowicz-Dębek, B.; Fiołka, M.; Krukowski, H.; Zoń, A.; Trawińska, B.; Bryl, M. The effect of sodium bentonite supplementation in the diet of mink (Neovison vison) on the microbiological quality of feed and animal health parameters. Slovak. Vet. Res. 2015, 52, 165–171. [Google Scholar]
- Gibson, M.K.; Bursian, S.J.; Aulerich, R.J. Effects of deoxynivalenol on feed consumption and body weight gain in mink (Mustela vision). Bull. Env. Contam. Toxicol. 1993, 51, 6–11. [Google Scholar] [CrossRef]
- Rybińska, K.; Postupolski, J.; Ledzion, E.; Kurpińska-Jaworska, J.; Szczęsna, M. Determination of Fusarium Toxins—Deoxynivalenol (DON) in Cereals and Cereal Preparations by Means of High-Performance Liquid Chromatography Purification by Immunoaffinity Columns; Wyd. Metodyczne PZH: Warszawa, Poland, 2005. [Google Scholar]
- Park, S.H.; Kim, J.; Kim, D.; Moon, Y. Mycotoxin detoxifiers attenuate deoxynivalenol-induced pro-inflammatory barrier insult in porcine enterocytes as an in vitro evaluation model of feed mycotoxin reduction. Toxicol. In Vitro 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Council of European Union. Council Directive 98/58/EC of 20 July 1998 Concerning the Protection of Animals Kept for Farming Purposes. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0058 (accessed on 22 November 2019).
- Animal Legal & Historical Center. Polish Animal Protection Act. J. Laws Repub. Pol. 1997, 111, 724. [Google Scholar]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I. Postnatal administration of 2-oxoglutaric acid improves the intestinal barrier affected by the prenatal action of dexamethasone in pigs. Nutrition 2012, 28, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Kwiecień, M. Alterations in intestinal and liver histomorphology and basal hematological and biochemical parameters in relation to different sources of dietary copper in adult rats. Ann. Anim. Sci. 2017, 17, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.K.; Layton, C.D.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Varghese, B.; Verhagen, R.; Hussain, A.; Boudot, C.; Tai, Q.; Ding, S.; Holz, J.A.; Uzunbajakava, N.E. Quantitative assessment of birefringent skin structures in scattered light confocal imaging using radially polarized light. Sensors 2013, 13, 12527–12535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diana, A.; Guglielmini, C.; Fracassi, F.; Pietra, M.; Balletti, E.; Cipone, M. Use of high-frequency ultrasonography for evaluation of skin thickness in relations of hydration status and fluid distribution at various cutaneous sites in dogs. Am. J. Vet. Res. 2008, 69, 1148–1152. [Google Scholar] [CrossRef]
- Favero, G.; Woelflingseder, L.; Janker, L.; Neuditschko, B.; Seriani, S.; Gallina, P.; Sbaizero, O.; Gerner, C.; Marko, D. Deoxynivalenol induces structural alterations in epidermoid carcinoma cells A431 and impairs the response to biomechanical stimulation. Sci. Rep. 2018, 8, 11351. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillary topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Kędzia, P. Bentonite diminishes DON-induced changes in bone development in mink dams. J. Vet. Res. 2016, 60, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Muszyński, S.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Hułas-Stasiak, M. DON-induced changes in bone homeostasis in mink dams. J. Vet. Res. 2017, 61, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.C.; Schumann, L.; Rottkord, U.; Humpf, H.U.; Gekle, M.; Schwerdt, G. Synergistic action of the nephrotoxic mycotoxins ochratoxin A and citrinin at nanomolar concentrations in human proximal tubule-derived cells. Toxicol. Lett. 2018, 291, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, J.; Liu, F.; Li, S.; Chen, J.; Fu, Q.; Zhang, Z.; Liu, J.; Luo, M.; Wang, J.; et al. The effects of mycotoxins and selenium deficiency on tissue-engineered cartilage. Cell Tissues Organs 2012, 196, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Nowakowicz-Dębek, B.; Wlazło, Ł.; Tymczyna, L.; Chmielowiec-Korzeniowska, A.; Krukowski, H.; Bis-Wencel, H. Assessment the efficiency of sodium bentonite in limiting emission of gaseous inorganic pollutants emitted from mink farms. Przem. Chem. 2013, 95, 736–739. [Google Scholar]
- Wang, X.; Liu, Q.; Ihsan, A.; Huang, L.; Dai, M.; Hao, H.; Cheng, G.; Liu, Z.; Wang, Y.; Yuan, Z. JAK/STAT pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicol. Sci. 2012, 127, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Heimber, C.K.; Jespersen, A.; Moe, R.O. Tail tip lesions in mink (Neovison vison): Effects of an additional hammock in multilevel cages. Animals 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- WelFur. Welfare Assessment Protocol for Mink. Available online: https://www.sustainablefur.com/wp-content/uploads/2018/11/Mink_protocol_final_web_edition_light.pdf (accessed on 22 November 2019).

| Item | Control (C) | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| DON contamination (mg/kg) | 0 | 1.1 | 3.7 | 3.7 | 3.7 |
| Bentonite supplementation (%) | 0 | 0 | 0 | 0.05 | 0.2 |
| Skin Layer | Control (C) | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| Epidermis (µm) | 17.62 ± 4.78 | 20.09 ± 6.78 | 22.55 ± 6.79 A,b | 23.79 ± 7.62 a,b | 17.25 ± 4.35 C,d |
| Dermis (µm) | 507.25 ± 79.69 | 1307.46 ± 459.65 A | 1261.39 ± 316.25 A | 1210.85 ± 562.61 A | 1016.31 ± 320.62 A,c,D |
| Item | Control (C) | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| Elastin (%) | 5.90 ± 1.05 | 9.42 ± 2.91 A | 6.82 ± 1.32 b | 7.53 ± 1.94 a | 11.31 ± 1.84 A,b,C,D |
| Collagen/elastin | 10.13 ± 1.62 | 5.76 ± 1.62 A | 7.35 ± 1.73 A,b | 6.76 ± 1.67 A | 3.51 ± 0.69 A,B,C,D |
| Control (C) | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| 0.163 ± 0.055 | 0.113 ± 0.050 a | type III not observed 1 | type III not observed 1 | 0.021 ± 0.012 A,B |
| Control (C) | Group I | Group II | Group III | Group IV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Corr. 1 | p2 | Corr. | p | Corr. | p | Corr. | p | Corr. | p |
| −0.782 | <0.001 | −0.961 | <0.001 | −0.678 | 0.005 | −0.954 | <0.001 | −0.804 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taszkun, I.; Tomaszewska, E.; Dobrowolski, P.; Żmuda, A.; Sitkowski, W.; Muszyński, S. Evaluation of Collagen and Elastin Content in Skin of Multiparous Minks Receiving Feed Contaminated with Deoxynivalenol (DON, vomitoxin) with or without Bentonite Supplementation. Animals 2019, 9, 1081. https://doi.org/10.3390/ani9121081
Taszkun I, Tomaszewska E, Dobrowolski P, Żmuda A, Sitkowski W, Muszyński S. Evaluation of Collagen and Elastin Content in Skin of Multiparous Minks Receiving Feed Contaminated with Deoxynivalenol (DON, vomitoxin) with or without Bentonite Supplementation. Animals. 2019; 9(12):1081. https://doi.org/10.3390/ani9121081
Chicago/Turabian StyleTaszkun, Iwona, Ewa Tomaszewska, Piotr Dobrowolski, Andrzej Żmuda, Wiesław Sitkowski, and Siemowit Muszyński. 2019. "Evaluation of Collagen and Elastin Content in Skin of Multiparous Minks Receiving Feed Contaminated with Deoxynivalenol (DON, vomitoxin) with or without Bentonite Supplementation" Animals 9, no. 12: 1081. https://doi.org/10.3390/ani9121081






