A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sample Collection and Fatty Acids Analysis
2.2. Blood Sampling, DNA Extraction, SNP Discovery, and Genotyping
2.3. Cell Cultures
2.4. In Silico miRNA-Target Interaction Analysis and Luciferase Reporter Assay
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Western Blot
2.7. Statistical Analysis
3. Results
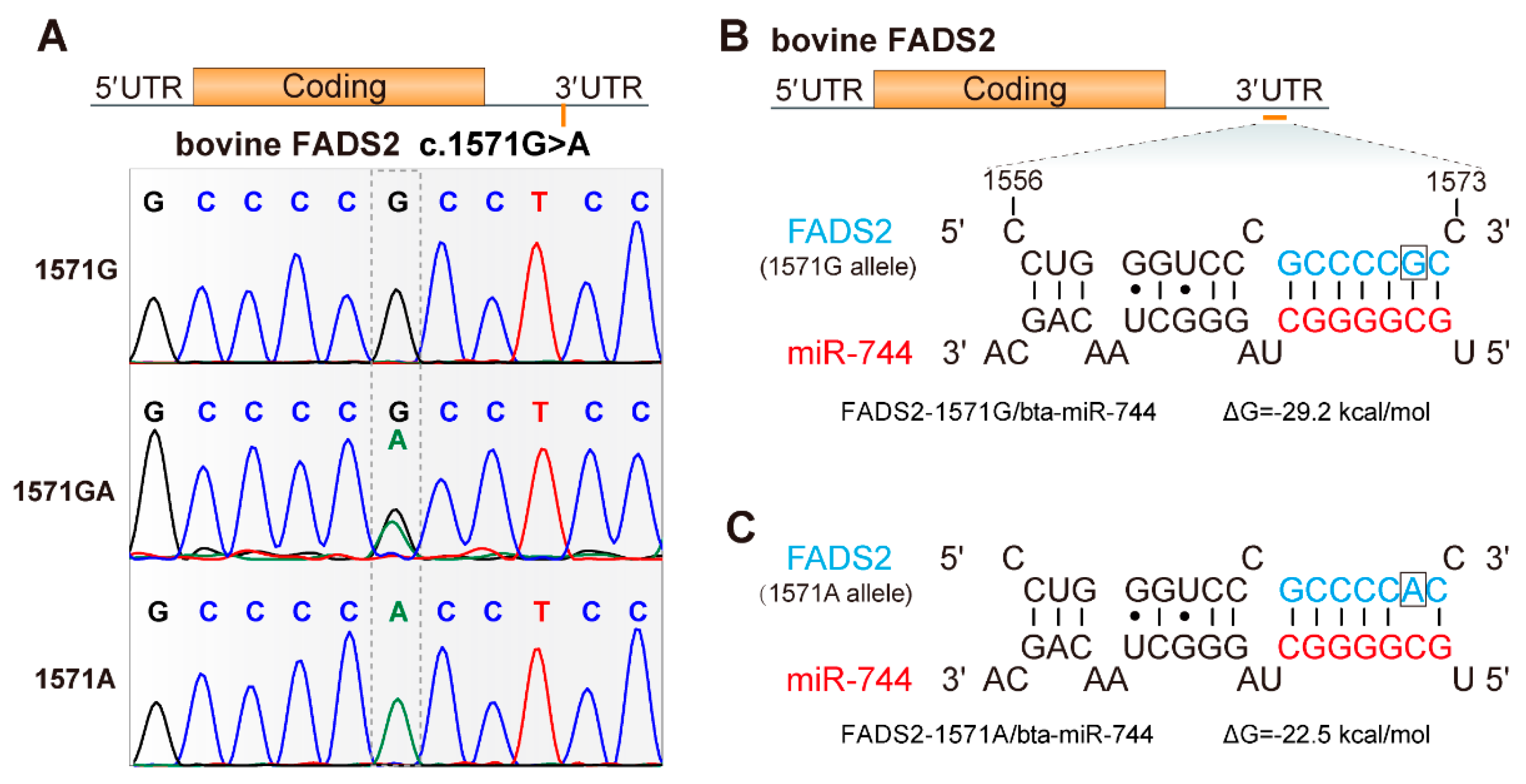
3.1. Identification of SNPs in the 3′ Untranslated Region of FADS2
3.2. Associations between c.1571G>A and Fatty Acids Profiles
3.3. Effects of the SNP c.1571G>A on FADS2 Transcriptional Activity
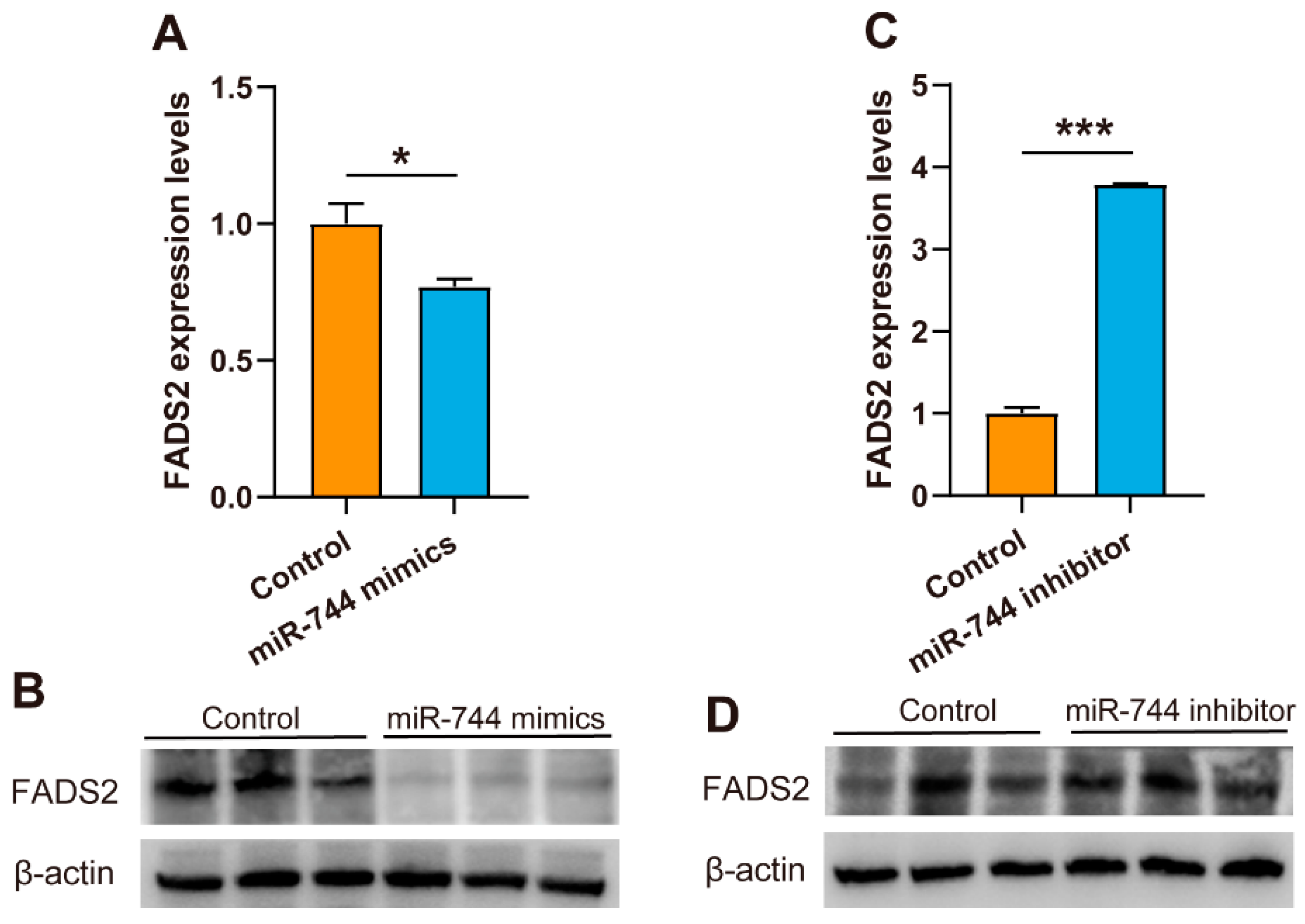
3.4. Mir-744 Regulates FADS2 Expression in MAC-T cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, T.; Tiezzi, F.; Penasa, M.; De Marchi, M.; Cassandro, M. Short communication: Association analysis of diacylglycerol acyltransferase (DGAT1) mutation on chromosome 14 for milk yield and composition traits, somatic cell score, and coagulation properties in Holstein bulls. J. Dairy Sci. 2018, 101, 8087–8091. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Cosenza, G.; Iannaccone, M.; Macciotta, N.P.P.; Guo, Y.; Di Stasio, L.; Pauciullo, A. The single nucleotide polymorphism g.133A>C in the stearoyl CoA desaturase gene (SCD) promoter affects gene expression and quali-quantitative properties of river buffalo milk. J. Dairy Sci. 2019, 102, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viale, E.; Tiezzi, F.; Maretto, F.; De Marchi, M.; Penasa, M.; Cassandro, M. Association of candidate gene polymorphisms with milk technological traits, yield, composition, and somatic cell score in Italian Holstein-Friesian sires. J. Dairy Sci. 2017, 100, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Stoop, W.M.; van Arendonk, J.A.M.; Heck, J.M.L.; van Valenberg, H.J.F.; Bovenhuis, H. Genetic parameters for major milk fatty acids and milk production traits of dutch Holstein-Friesians. J. Dairy Sci. 2008, 91, 385–394. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.Q.; Bu, D.P. Ruminal microbe of biohydrogenation of trans-vaccenic acid to stearic acid in vitro. BMC Res. Notes 2012, 5, 97. [Google Scholar] [CrossRef] [Green Version]
- Bastin, C.; Gengler, N.; Soyeurt, H. Phenotypic and genetic variability of production traits and milk fatty acid contents across days in milk for Walloon Holstein first-parity cows. J. Dairy Sci. 2011, 94, 4152–4163. [Google Scholar] [CrossRef] [Green Version]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Bastin, C.; Gengler, N. Genetic parameters of saturated and monounsaturated fatty acid content and the ratio of saturated to unsaturated fatty acids in bovine milk. J. Dairy Sci. 2008, 91, 3611–3626. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Kothapalli, K.S.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Holz, B.; Jenke, B.; Binczek, E.; Günter, R.H.; Kiss, C.; Karakesisoglou, I.; Thevis, M.; Weber, A.A.; Arnhold, S. Δ6-Desaturase (FADS2) deficiency unveils the role of ω3-and ω6-polyunsaturated fatty acids. EMBO J. 2008, 27, 2281–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroud, C.K.; Nara, T.Y.; Roqueta-Rivera, M.; Radlowski, E.C.; Lawrence, P.; Zhang, Y.; Cho, B.H.; Segre, M.; Hess, R.A.; Brenna, J.T. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J. Lipid Res. 2009, 50, 1870–1880. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef]
- Rzehak, P.; Heinrich, J.; Klopp, N.; Schaeffer, L.; Hoff, S.; Wolfram, G.; Illig, T.; Linseisen, J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 2008, 101, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Malerba, G.; Schaeffer, L.; Xumerle, L.; Klopp, N.; Trabetti, E.; Biscuola, M.; Cavallari, U.; Galavotti, R.; Martinelli, N.; Guarini, P.; et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 2008, 43, 289–299. [Google Scholar] [CrossRef]
- Xie, L.; Innis, S.M. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef] [Green Version]
- Ibeagha-Awemu, E.M.; Akwanji, K.A.; Beaudoin, F.; Zhao, X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet. 2014, 15, 25. [Google Scholar] [CrossRef] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, S.; Ziaugra, L.; Tabbaa, D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009, 60, 2–12. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.-S.; Tam, W.-L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.; Mackay, T. Introduction to Quantitative Genetics, 4th ed.; Longmans: Harlow, UK, 1996. [Google Scholar]
- Hermant, X.; Delay, C.; Flaig, A.; Luque-Bedregal, J.; Briand, G.; Bout, M.A.; Cottel, D.; Wagner, A.; Arveiler, D.; Simon, C.; et al. Identification of a functional FADS1 3′ UTR variant associated with erythrocyte n-6 polyunsaturated fatty acids levels. J. Clin. Lipidol. 2018, 12, 1280–1289. [Google Scholar] [CrossRef]
- Brest, P.; Lapaquette, P.; Souidi, M.; Lebrigand, K.; Cesaro, A.; Vouret-Craviari, V.; Mari, B.; Barbry, P.; Mosnier, J.F.; Hebuterne, X.; et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat. Genet. 2011, 43, 242. [Google Scholar] [CrossRef]
- Yuan, Y.; Weidhaas, J.B. Functional micro RNA binding site variants. Mol. Oncol. 2019, 13, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Lattka, E.; Illig, T.; Koletzko, B.; Heinrich, J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [Google Scholar] [CrossRef]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Kröger, J.; Schulze, M.B. Recent insights into the relation of Δ5 desaturase and Δ6 desaturase activity to the development of type 2 diabetes. Curr. Opin. Lipidol. 2012, 23, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Clop, A.; Marcq, F.; Takeda, H.; Pirottin, D.; Tordoir, X.; Bibé, B.; Bouix, J.; Caiment, F.; Elsen, J.-M.; Eychenne, F. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006, 38, 813. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Deng, D.; Liu, X.; Xiao, Y.; Huang, J.; Wang, F.; Li, X.; Yu, M. A miR-18a binding-site polymorphism in CDC42 3′ UTR affects CDC42 mRNA expression in placentas and is associated with litter size in pigs. Mamm. Genome 2019, 30, 34–41. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Zhang, X.; Ju, Z.; Qi, C.; Zhang, Y.; Li, Q.; Wang, C.; Miao, W.; Zhong, J. One SNP in the 3′-UTR of HMGB1 gene affects the binding of target bta-miR-223 and is involved in mastitis in dairy cattle. Immunogenetics 2012, 64, 817–824. [Google Scholar] [CrossRef]
- Ralston, J.C.; Matravadia, S.; Gaudio, N.; Holloway, G.P.; Mutch, D.M. Polyunsaturated fatty acid regulation of adipocyte FADS1 and FADS2 expression and function. Obesity 2015, 23, 725–728. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Tan, P.; Cai, Z.; Xu, H.; Li, J.; Ren, W.; Xu, H.; Zuo, R.; Zhou, J.; Mai, K. Regulation of FADS2 transcription by SREBP-1 and PPAR-α influences LC-PUFA biosynthesis in fish. Sci. Rep. 2017, 7, 40024. [Google Scholar] [CrossRef]
- Schennink, A.; Heck, J.M.; Bovenhuis, H.; Visker, M.H.; van Valenberg, H.J.; van Arendonk, J.A. Milk fatty acid unsaturation: Genetic parameters and effects of stearoyl-CoA desaturase (SCD1) and acyl CoA: Diacylglycerol acyltransferase 1 (DGAT1). J. Dairy Sci. 2008, 91, 2135–2143. [Google Scholar] [CrossRef] [Green Version]

| SNP | Allele | Allele Frequency | Genotype | Genotype Frequency | Observed Count | Expected Count | χ2 Value of HWE Test | p-Value |
|---|---|---|---|---|---|---|---|---|
| FADS2 c.1571G>A | G | 0.844 | GG | 0.716 | 197 | 195.72 | 0.340 | 0.844 |
| A | 0.156 | GA | 0.255 | 70 | 72.55 | |||
| AA | 0.029 | 8 | 6.72 |
| Fatty Acid-Related Traits 1 | Genotype | Gene Effects 2 | F | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | a | d | α | |||
| C4:0 | 3.311 ab ± 0.050 | 3.216 a ± 0.072 | 3.791 b ± 0.149 | −0.240 | −0.335 | −0.471 | 3.237 | 0.041 |
| C18:0 | 12.045 a ± 0.245 | 14.372 b ± 0.370 | 13.459 ab ± 0.822 | −0.707 | 1.620 | 0.408 | 16.669 | 0.000 |
| C18:2n-6 | 5.583 a ± 0.330 | 3.552 b ± 0.178 | 3.286 b ± 0.181 | 1.149 | −0.883 | 0.541 | 14.068 | 0.000 |
| C18:3n-6 | 0.364 a ± 0.012 | 0.465 b ± 0.013 | 0.480 b ± 0.042 | −0.058 | 0.043 | −0.028 | 21.087 | 0.000 |
| C20:3n-6 | 0.112 a ± 0.007 | 0.147 b ± 0.012 | 0.134 ab ± 0.032 | −0.011 | 0.024 | 0.006 | 5.694 | 0.004 |
| SFA | 65.835 a ± 0.465 | 68.988 b ± 0.618 | 67.35 ab ± 1.314 | −0.758 | 2.395 | 0.890 | 3.384 | 0.000 |
| D6D index | 0.102 a ± 0.004 | 0.140 b ± 0.004 | 0.148 b ± 0.013 | −0.023 | 0.015 | −0.013 | 25.001 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Lu, X.; Gao, Q.; Wang, M.; Arbab, A.A.I.; Sun, Y.; Chen, Z.; Zhang, H.; Karrow, N.A.; Yang, Z.; et al. A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding. Animals 2019, 9, 1090. https://doi.org/10.3390/ani9121090
Li M, Lu X, Gao Q, Wang M, Arbab AAI, Sun Y, Chen Z, Zhang H, Karrow NA, Yang Z, et al. A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding. Animals. 2019; 9(12):1090. https://doi.org/10.3390/ani9121090
Chicago/Turabian StyleLi, Mingxun, Xubin Lu, Qisong Gao, Mengqi Wang, Abdelaziz Adam Idriss Arbab, Yujia Sun, Zhi Chen, Huimin Zhang, Niel A. Karrow, Zhangping Yang, and et al. 2019. "A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding" Animals 9, no. 12: 1090. https://doi.org/10.3390/ani9121090
APA StyleLi, M., Lu, X., Gao, Q., Wang, M., Arbab, A. A. I., Sun, Y., Chen, Z., Zhang, H., Karrow, N. A., Yang, Z., & Mao, Y. (2019). A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding. Animals, 9(12), 1090. https://doi.org/10.3390/ani9121090






