Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Design
2.2. Occurrence and Timing of Estrus Behavior Onset
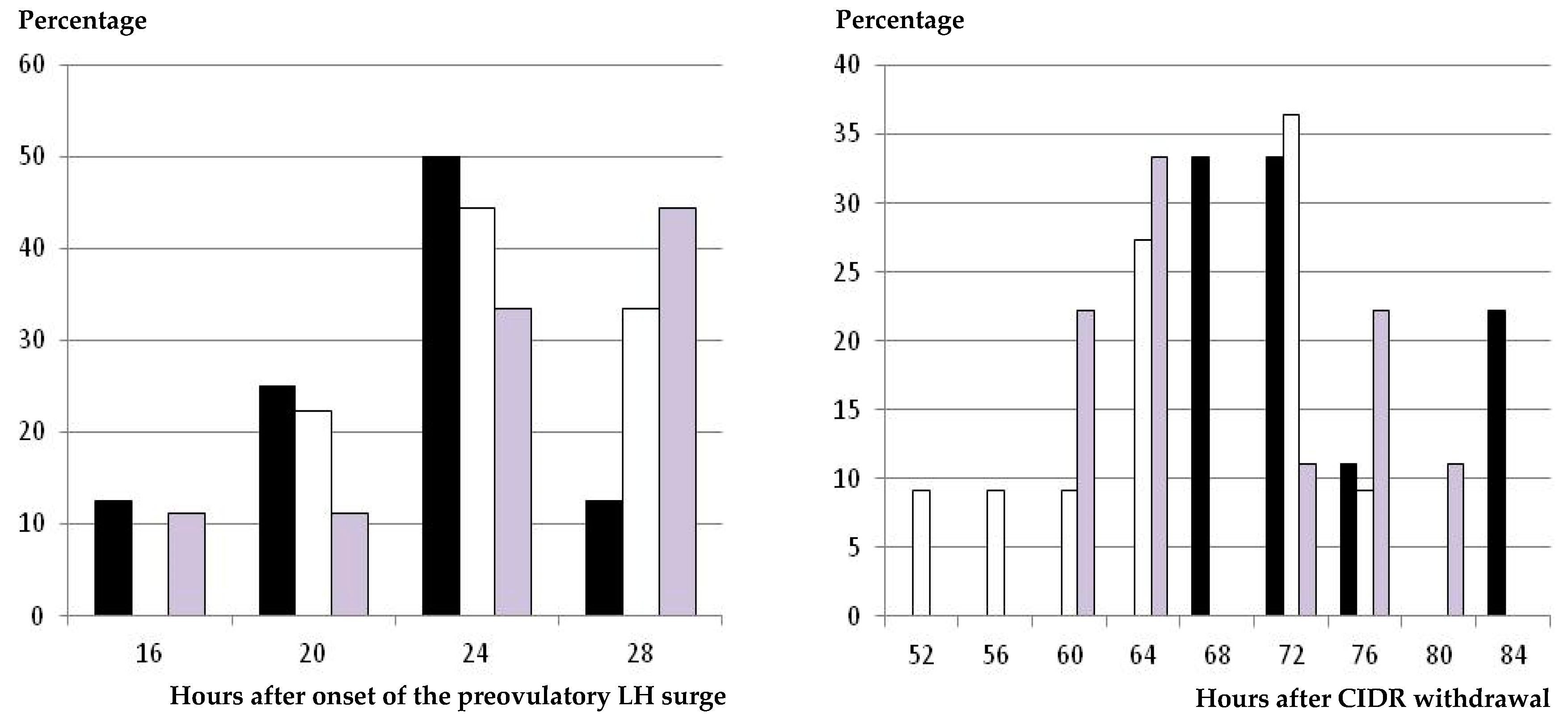
2.3. Occurrence and Timing of Preovulatory LH Surge
2.4. Occurrence and Timing of Ovulation
2.5. Ovulation Rate and Corpora Lutea Functionality
2.6. Fertility Rate
2.7. Statistical Analysis
3. Results
3.1. Occurrence and Timing of Estrus Behavior
3.2. Occurrence and Timing of Preovulatory LH Surge
3.3. Occurrence and Timing of Ovulation
3.4. Ovulation Rate and Corpora Lutea Functionality
3.5. Fertility Rate
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abecia, J.A.; Forcada, F.; Gonzalez-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef]
- Dutt, R.H.; Casida, L.E. Alteration of the estrual cycle in sheep by use of progesterone and its effect upon subsequent ovulation and fertility. Endocrinology 1948, 43, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Dutt, R.H. Induction of oestrus and ovulation in anestrual ewes by the use of progesterone and pregnant mares serum. J. Anim. Sci. 1952, 11, 792. [Google Scholar]
- Martinez-Ros, P.; Rios-Abellan, A.; Gonzalez-Bulnes, A. Influence of progesterone-treatment length and eCG administration on appearance of estrous behavior, ovulatory success and fertility in sheep. Animals 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A.; Rubianes, E. New treatments associated with timed artificial insemination in small ruminants. Reprod. Fertil. Dev. 2004, 16, 403–414. [Google Scholar] [CrossRef]
- Quirke, J.F.; Jennings, J.J.; Hanrahan, J.P.; Gosling, J.P. Oestrus, time of ovulation, ovulation rate and conception rate in progestagen-treated ewes given Gn-RH, Gn-TH analogues and gonadotrophins. J. Reprod. Fertil. 1979, 56, 479–488. [Google Scholar] [CrossRef]
- Cline, M.A.; Ralston, J.N.; Seals, R.C.; Lewis, G.S. Intervals from norgestomet withdrawal and injection of equine chorionic gonadotropin or P.G. 600 to estrus and ovulation in ewes. J. Anim. Sci. 2001, 79, 589–594. [Google Scholar] [CrossRef]
- D’Souza, K.N.; Rastle-Simpson, S.L.; Redhead, A.K.; Baptiste, Q.S.; Smith, B.; Knights, M. Gonadotropin stimulation using P.G. 600® on reproductive success of non-lactating anestrous ewes. Anim. Reprod. Sci. 2014, 148, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; D’Alessandro, A.G. Synchronization of oestrus and ovulation by short time combined FGA, PGF(2α), GnRH, eCG treatments for natural service or AI fixed-time. Anim. Reprod. Sci. 2011, 123, 32–39. [Google Scholar] [CrossRef]
- Titi, H.H.; Kridli, R.T.; Alnimer, M.A. Estrus synchronization in sheep and goats using combinations of GnRH, progestagen and prostaglandin F2alpha. Reprod. Domest. Anim. 2010, 45, 594–599. [Google Scholar]
- Olivera-Muzante, J.; Gil, J.; Viñoles, C.; Fierro, S. Reproductive outcome with GnRH inclusion at 24 or 36 h following a prostaglandin F2α-based protocol for timed AI in ewes. Anim. Reprod. Sci. 2013, 138, 175–179. [Google Scholar] [CrossRef]
- Rekik, M.; Haile, A.; Abebe, A.; Muluneh, D.; Goshme, S.; Ben Salem, I.; Hilali, M.E.; Lassoued, N.; Chanyalew, Y.; Rischkowsky, B. GnRH and prostaglandin-based synchronization protocols as alternatives to progestogen-based treatments in sheep. Reprod. Domest. Anim. 2016, 51, 924–929. [Google Scholar] [CrossRef]
- Wildeus, S. Current concepts y synchronization of estrus: Sheep and goats. J. Anim. Sci. 2000, 77, 1–14. [Google Scholar] [CrossRef]
- Hay, M.F.; Moor, R.M. Functional and structural relationships in the Graafian follicle population of the sheep ovary. J. Reprod. Fertil. 1975, 45, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.W.; Whittier, J.C. Effects of a timed insemination following synchronization of ovulation using the Ovsynch or COSynch protocol in beef cows. Prof. Anim. Sci. 1998, 14, 217–220. [Google Scholar] [CrossRef]
- Bisinotto, R.S.; Pansani, M.B.; Castro, L.O.; Narciso, C.D.; Sinedino, L.D.P.; Martinez, N.; Carneiro, P.E.; Thatcher, W.W.; Santos, J.E.P. Effect of progesterone supplementation on fertility responses of lactating dairy cows with corpus luteum at the initiation of the Ovsynch protocol. Theriogenology 2015, 83, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Lopez, A.; Cocero, M.J.; Dominguez, V.; McNeilly, A.S.; Gonzalez-Bulnes, A. Follicular wave status at the beginning of the FSH treatment modifies reproductive features in superovulated sheep. Reprod. Biol. 2006, 6, 243–264. [Google Scholar]
- Gonzalez-Bulnes, A.; Santiago-Moreno, J.; Garcia Lopez, M.; Gomez-Brunet, A.; Lopez-Sebastian, A. Observacion del ovario en la oveja y eficacia en la deteccion de foliculos y cuerpos luteos mediante ecografía transrectal. Investig. Agric. 1994, 8, 208–218. [Google Scholar]
- Veiga-Lopez, A.; Encinas, T.; McNeilly, A.S.; Gonzalez-Bulnes, A. Timing of preovulatory LH surge andovulation in superovulated sheep are affected by follicular status at start of the FSH treatment. Reprod. Domest. Anim. 2008, 43, 92–98. [Google Scholar]
- Cavalcanti, A.; Brandão, F.Z.; Nogueira, L.A.G.; da Fonseca, J.F. Effects of GnRH administration on ovulation and fertility in ewes subjected to estrous synchronization. R. Bras. Zootec. 2012, 41, 1412–1418. [Google Scholar] [CrossRef]
- Joseph, I.B.J.K.; Currie, W.D.; Rawlings, N.C. Effects of time after ovariectomy, season and oestradiol on luteinizing hormone and follicle-stimulating hormone secretion in ovariectomized ewes. J. Reprod. Fertil. 1992, 94, 511–523. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.S. The control of FSH secretion. Acta Endocrinol. Suppl. 1988, 288, 31–40. [Google Scholar]
- McNeilly, A.S.; Crawford, J.L.; Taragnat, C.; Nicol, L.; McNeilly, J.R. The differential secretion of FSH and LH: Regulation through genes, feedback and packaging. Reprod. Suppl. 2003, 61, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; McNeilly, A.S. Gonadotrophic control of follicular development and function during the oestrous cycle of the ewe. J. Reprod. Fertil. Suppl. 1981, 30, 119–133. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Santiago-Moreno, J.; Cocero, M.J.; Souza, C.J.H.; Groome, N.P.; Garcia-Garcia, R.M.; Lopez-Sebastian, A.; Baird, D.T. Measurement of inhibin A and follicular status predict the response of ewes to superovulatory FSH treatments. Theriogenology 2002, 57, 1263–1272. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Souza, C.J.H.; Campbell, B.K.; Baird, D.T. Systemic and intraovarian effects of dominant follicles on ovine follicular growth. Anim. Reprod. Sci. 2004, 84, 107–119. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Gonzalez-Bulnes, A.; Garcia-Garcia, R.M.; Dominguez, V.; Cocero, M.J. The effects of previous ovarian status on ovulation rate and early embryo development in response to superovulatory FSH treatments in sheep. Theriogenology 2005, 63, 1973–1983. [Google Scholar] [CrossRef]
- Menchaca, A.; Vilariño, M.; Pinczak, A.; Kmaid, S.; Saldaña, J.M. Progesterone treatment, FSH plus eCG, GnRH administration, and Day 0 Protocol for MOET programs in sheep. Theriogenology 2009, 72, 477–483. [Google Scholar] [CrossRef]
- Evans, A.C.O. Ovarian follicle growth and consequences for fertility in sheep. Anim. Reprod. Sci. 2003, 78, 289–306. [Google Scholar] [CrossRef]
- Barrett, D.M.W.; Bartlewski, P.M.; Batista-Arteaga, M.; Symington, A.; Rawlings, N.C. Ultrasound and endocrine evaluation of the ovarian response to a single dose of 500 IU of eCG following a 12-day treatment with progestogen-releasing intravaginal sponges in the breeding and nonbreeding seasons in ewes. Theriogenology 2004, 61, 311–327. [Google Scholar] [CrossRef]



| Event | CIDR-eCG (n = 19) | CIDR-GnRH (n = 19) | GnRH-CIDR-GnRH (n = 19) |
|---|---|---|---|
| Occurrence of estrus behavior (%) | 17/19 (89.5) | 17/19 (89.5) | 16/19 (84.2) |
| Timing of estrus behavior after CIDR removal (range) | 34.1 ± 2.0 a (24–44) | 39.3 ± 2.0 b (28–52) | 39.8 ± 2.2 b (24–52) |
| Occurrence of preovulatory LH surge (%) | 17/17 (100) | 17/17 (100) | 16/16 (100) |
| Timing of preovulatory LH surge after CIDR removal (range) | 42.2 ± 3.0 a (28–56) | 44.4 ± 2.3 a,b (32–52) | 50.7 ± 1.9 b (44–56) |
| Timing of preovulatory LH surge after onset of estrus behavior (range) | 8.0 ± 1.0 (4–12) | 6.7 ± 1.6 (4–16) | 7.5 ± 1.6 (4–16) |
| Occurrence of ovulation (%) | 17/17 (100) | 17/17 (100) | 16/16 (100) |
| Timing of ovulation after CIDR removal (range) | 65.8 ± 2.3 a (52–76) | 68.4 ± 2.5 a,b (60–80) | 73.8 ± 2.1 b (68–84) |
| Timing of ovulation after onset of estrus behavior (range) | 31.6 ± 0.8 (28–36) | 30.7 ± 0.9 (28–36) | 30.2 ± 1.0 (28–36) |
| Timing of ovulation after onset of preovulatory LH surge (range) | 24.0 ± 1.1 (16–28) | 24.0 ± 1.4 (16–28) | 22.5 ± 1.3 (16–28) |
| Parameter | CIDR-eCG | CIDR-GnRH | GnRH-CIDR-GnRH |
|---|---|---|---|
| Number of corpora lutea (range) | 2.1 ± 0.2 a (1–4) | 1.3 ± 0.2 b (1–2) | 1.6 ± 0.2 a (1–2) |
| Plasma progesterone concentrations (range) | 5.9 ± 1.0 (2.3–7.8) | 5.1 ± 0.6 (2.9–7.1) | 4.9 ± 0.7 (1.5–7.0) |
| Fertility rate with regards to ewes ovulating (%) | 13/17 (76.5) | 11/17 (64.7) | 13/16 (81.3) |
| Fertility rate with regards to treated ewes (%) | 13/19 (68.4) | 11/19 (57.9) | 13/19 (68.4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Ros, P.; Gonzalez-Bulnes, A. Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep. Animals 2019, 9, 146. https://doi.org/10.3390/ani9040146
Martinez-Ros P, Gonzalez-Bulnes A. Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep. Animals. 2019; 9(4):146. https://doi.org/10.3390/ani9040146
Chicago/Turabian StyleMartinez-Ros, Paula, and Antonio Gonzalez-Bulnes. 2019. "Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep" Animals 9, no. 4: 146. https://doi.org/10.3390/ani9040146
APA StyleMartinez-Ros, P., & Gonzalez-Bulnes, A. (2019). Efficiency of CIDR-Based Protocols Including GnRH Instead of eCG for Estrus Synchronization in Sheep. Animals, 9(4), 146. https://doi.org/10.3390/ani9040146





