Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oocyte Collection and In Vitro Maturation (IVM)
2.3. Immunocytochemistry
2.3.1. Nuclear and Cytoskeletal Progression
2.3.2. Subcellular Localization of Phosphorylated p38 (p-p38)
2.4. Western Blotting for p38 Analysis
2.5. Experimental Designs
2.5.1. Experiment 1: Nuclear and Cytoskeletal Progression of Maturing Porcine Oocytes
2.5.2. Experiment 2: Subcellular Localization of p-p38 in Porcine Oocytes during IVM
2.5.3. Experiment 3: Expression of p38 and p-p38 in Porcine Oocytes
2.6. Statistical Analyses
3. Results
3.1. Nuclear and Cytoskeletal Progression of Maturing Porcine Oocytes
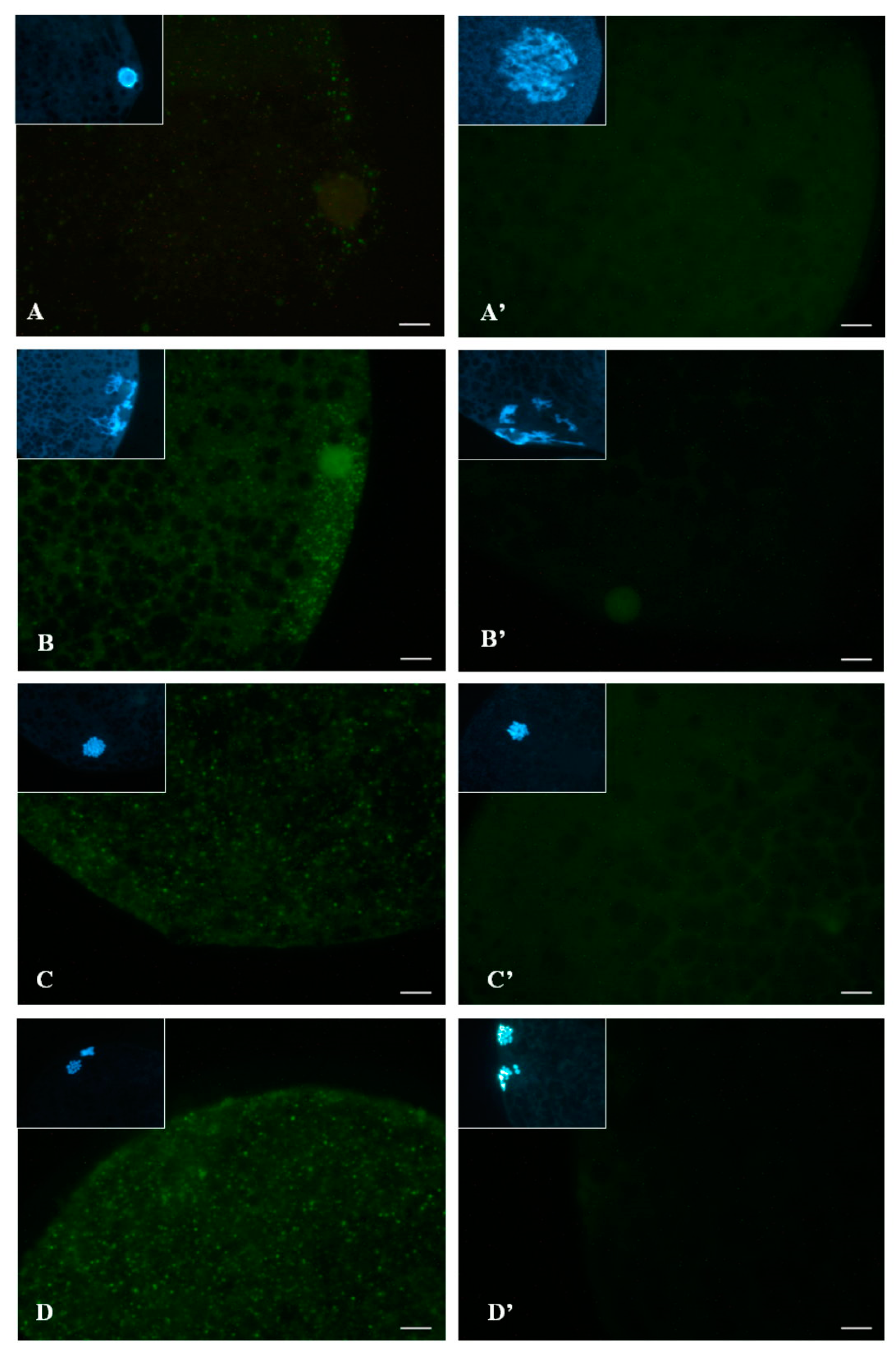
3.2. Subcellular Localization of p-p38 in Porcine Oocytes during IVM
3.3. Expressions of p38 and p-p38 in Porcine Oocytes during IVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pillai, M.S.; Sapna, S.; Shivakumar, K. p38 MAPK regulates G1-S transition in hypoxic cardiac fibroblasts. Int. J. Biochem. Cell Biol. 2011, 43, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Porras, A.; Zuluaga, S.; Black, E.; Valladares, A.; Alvarez, A.M.; Ambrosino, C.; Benito, M.; Nebreda, A.R. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol. Biol. Cell 2004, 15, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.D.; Fulton, D.L.; Richard, S.; Almazan, G. p38 Mitogen-Activated Protein Kinase Pathway Regulates Genes during Proliferation and Differentiation in Oligodendrocytes. PLoS ONE 2015, 10, e0145843. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Xiao, F.; Jiang, Y.; Wu, Z. Involvement of the p38 mitogen-activated protein kinase alpha, beta, and gamma isoforms in myogenic differentiation. Mol. Biol. Cell 2008, 19, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Huth, H.W.; Albarnaz, J.D.; Torres, A.A.; Bonjardim, C.A.; Ropert, C. MEK2 controls the activation of MKK3/MKK6-p38 axis involved in the MDA-MB-231 breast cancer cell survival: Correlation with cyclin D1 expression. Cell Signal. 2016, 28, 1283–1291. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Yen, S.Y.; Tseng, J.K.; Chuang, S.M.; Chen, S.E.; Ju, J.C. Expression and activation of mitogen-activated protein kinases in matured porcine oocytes under thermal stress. J. Reprod. Dev. 2014, 60, 388–394. [Google Scholar] [CrossRef]
- Faerge, I.; Terry, B.; Kalous, J.; Wahl, P.; Lessl, M.; Ottesen, J.L.; Hyttel, P.; Grøndahl, C. Resumption of meiosis induced by meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol. Reprod. 2001, 65, 1751–1758. [Google Scholar] [CrossRef]
- Tunquist, B.J.; Maller, J.L. Under arrest: Cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Gene. Dev. 2016, 17, 683–710. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hoshino, Y.; Tanemura, K.; Sato, E. Distribution of protein disulfide isomerase during maturation of pig oocytes. J. Anim. Sci. 2013, 84, 15–22. [Google Scholar] [CrossRef]
- Sobajima, T.; Aoki, F.; Kohmoto, K. Activation of mitogen-activated protein kinase during meiotic maturation in mouse oocytes. J. Reprod. Fertil. 1993, 97, 389–394. [Google Scholar] [CrossRef]
- Gautier, J.; Norbury, C.; Lohka, M.; Nurse, P.; Maller, J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell 1998, 54, 433–439. [Google Scholar] [CrossRef]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar] [PubMed]
- Suzuki, H.; Fukudome, H.; Takami, A.; Matsuzaki, M. Cytoskeletal and mitochondrial distributions in porcine oocytes at different germinal vesicle stages. J. Mamm. Ova Res. 2008, 25, 177–183. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhang, C.; Xuan, J.; Su, C.; Wang, Y. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene 2015, 577, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Cao, R.; Liu, H.L.; Cui, X.S.; Kim, N.H.; Rui, R.; Sum, S.C. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell Cycle 2014, 13, 3390–3403. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.J.; You, S.Y.; Park, Y.S.; Chang, J.W.; Kim, J.S.; Oh, J.S. TCTP regulates spindle microtubule dynamics by stabilizing polar microtubules during mouse oocyte meiosis. Biochim. Biophys. Acta 2016, 1863, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Pichon, S.; Bryckaert, M.; Berrou, E. Control of actin dynamics by p38 MAP kinase—Hsp27 distribution in the lamellipodium of smooth muscle cells. J. Cell Sci. 2004, 117, 2569–2577. [Google Scholar] [CrossRef]
- Pollard, T.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2013, 124, 453–465. [Google Scholar]
- Brunet, S.; Maro, B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: Integrating time and space. Reproduction 2005, 130, 801–811. [Google Scholar] [CrossRef]
- Bell, C.E.; Watson, A.J. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLoS ONE 2013, 8, e59528. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Tsay, C.; Ruan, C.W. Alterations and reversibility in the chromatin, cytoskeleton and development of pig oocytes treated with roscovitine. Mol. Reprod. Dev. 2003, 64, 482–491. [Google Scholar] [CrossRef]
- Ju, J.C.; Tseng, J.K. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol. Reprod. Dev. 2004, 68, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. Image J. National Institutes of Health: Bethesda, MA, USA, 2006. [Google Scholar]
- Tseng, J.K.; Tang, P.C.; Ju, J.C. In vitro thermal stress induces apoptosis and reduces development of porcine parthenotes. Theriogenology 2006, 66, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS User’s Guide Statistics, Version 6.03; SAS. Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Pan, L.Z.; Zhu, S.; Zhang, M.; Sun, M.J.; Lin, J.; Chen, F.; Tan, J.H. A new classification of the germinal vesicle chromatin configurations in pig oocytes. Biol. Reprod. 2018, 99, 1149–1158. [Google Scholar] [CrossRef]
- Sun, M.J.; Zhu, S.; Li, Y.W.; Lin, J.; Gong, S.; Jiao, G.Z.; Chen, F.; Tan, J.H. An essential role for the intra-oocyte MAPK activity in the NSN-to-SN transition of germinal vesicle chromatin configuration in porcine oocytes. Sci. Rep. 2016, 6, 23555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozen, B.; Ozturk, S.; Yaba, A.; Demir, N. The p38 MAPK signalling pathway is required for glucose metabolism, lineage specification and embryo survival during mouse preimplantation development. Mech. Dev. 2015, 138, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Natale, D.R.; Paliga, A.J.; Beier, F.; D’Souza, S.J.; Watson, A.J. p38 MAPK signaling during murine preimplantation development. Dev. Biol. 2004, 268, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Hishinuma, M.; Shimada, M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J. Ovarian Res. 2009, 2. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK during porcine oocyte maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef]
- Landry, J.; Chretien, P.; Laszlo, A.; Lambert, H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J. Cell. Physiol. 1991, 147, 93–101. [Google Scholar] [CrossRef]
- Huot, J.; Lambert, H.; Lavoie, J.N.; Guimond, A.; Houle, F.; Landry, J. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur. J. Biochem. 1995, 227, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.K.; Jeong, Y.T.; Jo, H.C.; Kang, M.Y.; Chang, I.S.; Baek, J.C.; Park, J.K.; Lee, S.A.; Lee, J.H.; Paik, W.-Y. Increased interaction between heat shock protein 27 and mitogen-activated protein kinase (p38 and extracellular signal-regulated kinase) in pre-eclamptic placentas. J. Obstet. Gynaecol. Res. 2009, 35, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.; Cohen, P.; Trigon, S.; Morange, M.; Alonso-Llamazares, A.; Zamanillo, D.; Hunt, T.; Nebreda, A.R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 1994, 78, 1027–1037. [Google Scholar] [CrossRef]
- Lavoie, J.N.; Lambert, H.; Hickey, E.; Weber, L.A.; Landry, J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 1995, 15, 505–516. [Google Scholar] [CrossRef]
- Guay, J.; Lambert, H.; Gingras-Breton, G.; Lavoie, J.N.; Huot, J.; Landry, J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J. Cell Sci. 1997, 110, 357–368. [Google Scholar]
- Landry, J.; Huot, J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27). Biochem. Soc. Symp. 1999, 64, 79–89. [Google Scholar]
- Schafer, C.; Clapp, P.; Welsh, M.J.; Benndorf, R.; Williams, J.A. HSP27 expression regulates CCK-induced changes of the actin cytoskeleton in CHO-CCK-A cells. Am. J. Physiol. 1999, 277, 1032–1043. [Google Scholar] [CrossRef]
- Murai, H.; Hiragami, F.; Kawamura, K.; Motoda, H.; Koike, Y.; Inoue, S.; Kumagishi, K.; Ohtsuka, A.; Kano, Y. Differential response of heat-shock-induced p38 MAPK and JNK activity in PC12 mutant and PC12 parental cells for differentiation and apoptosis. Acta Med. Okayama 2010, 64, 55–62. [Google Scholar]
- Wang, L.; Qin, W.; Zhang, J.; Bao, C.; Zhang, H.; Che, Y.; Sun, C.; Gu, J.; Feng, X.; Du, C.; et al. Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release. Sci. Rep. 2016, 6, 24058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, K.; Naito, K.; Noguchi, J.; Shimada, A.; Kaneko, H.; Yamashita, M.; Aoki, F.; Tojo, H.; Toyoda, Y. Maturation/M-phase promoting factor: A regulator of aging in porcine oocytes. Biol. Reprod. 2000, 63, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, X.; Wang, W.; Zhu, J.; Li, Y.; Luo, L.; Zhang, W. Activity of MPF and expression of its related genes in mouse MI oocytes exposed to cadmium. Food Chem. Toxicol. 2018, 112, 332–341. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intawicha, P.; Tsai, L.-K.; Yen, S.-Y.; Lo, N.-W.; Ju, J.-C. Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes. Animals 2019, 9, 163. https://doi.org/10.3390/ani9040163
Intawicha P, Tsai L-K, Yen S-Y, Lo N-W, Ju J-C. Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes. Animals. 2019; 9(4):163. https://doi.org/10.3390/ani9040163
Chicago/Turabian StyleIntawicha, Payungsuk, Li-Kuang Tsai, Shih-Ying Yen, Neng-Wen Lo, and Jyh-Cherng Ju. 2019. "Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes" Animals 9, no. 4: 163. https://doi.org/10.3390/ani9040163
APA StyleIntawicha, P., Tsai, L.-K., Yen, S.-Y., Lo, N.-W., & Ju, J.-C. (2019). Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes. Animals, 9(4), 163. https://doi.org/10.3390/ani9040163



