Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Chemical Composition of Feeds
2.3. Fish Rearing Conditions
2.4. Tissue Sampling and Calculations
2.5. Plasma Metabolic Parameters
2.6. RNA Extraction and cDNA Synthesis
2.7. Real Time PCR
2.8. Histology
2.9. Fourier Transform Infrared Imaging Spectroscopy (FTIRI) Measurements and Data Analysis
2.10. Statistical Analyses
3. Results
3.1. Fish Growth
3.2. Plasma Metabolic Parameters
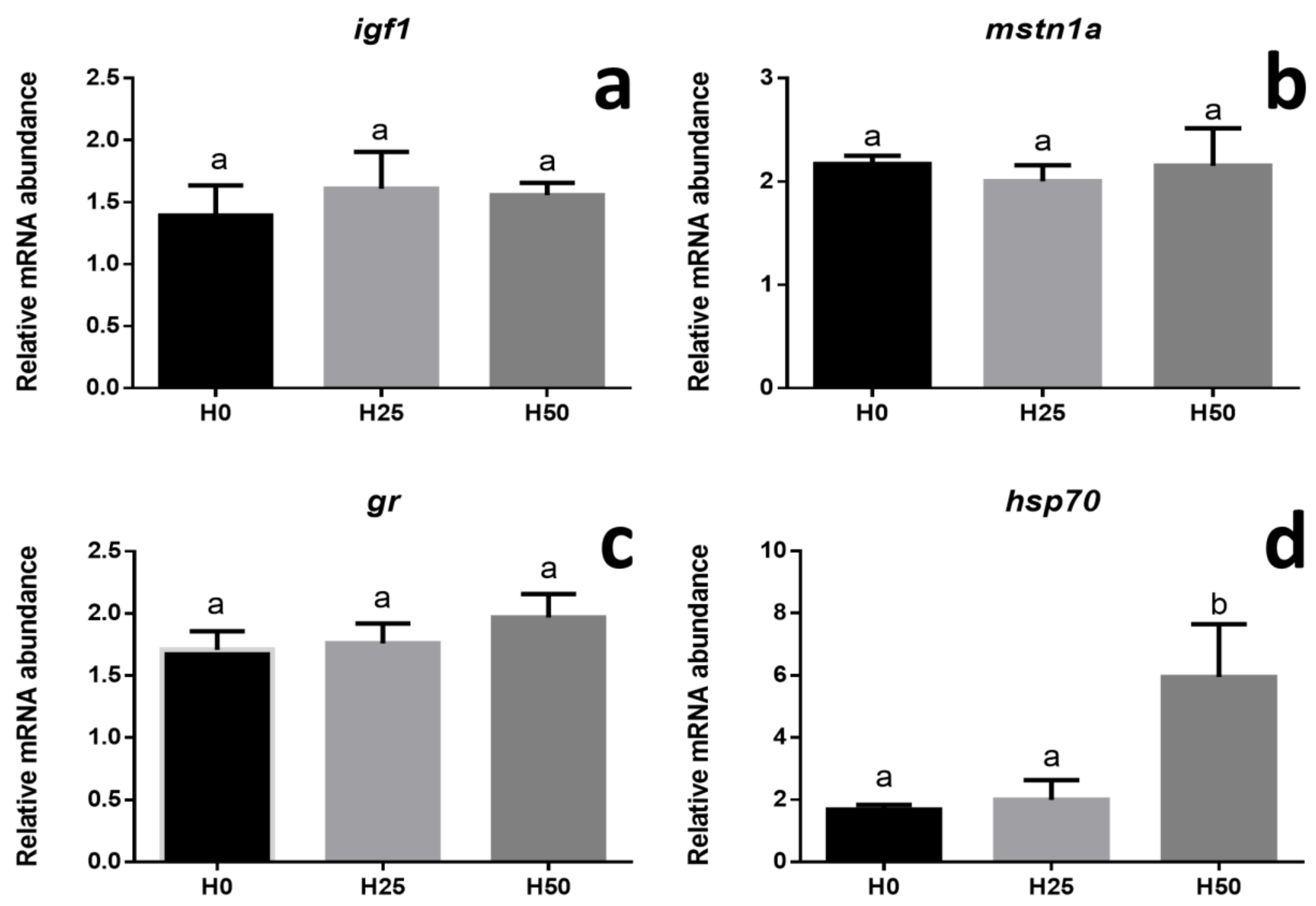
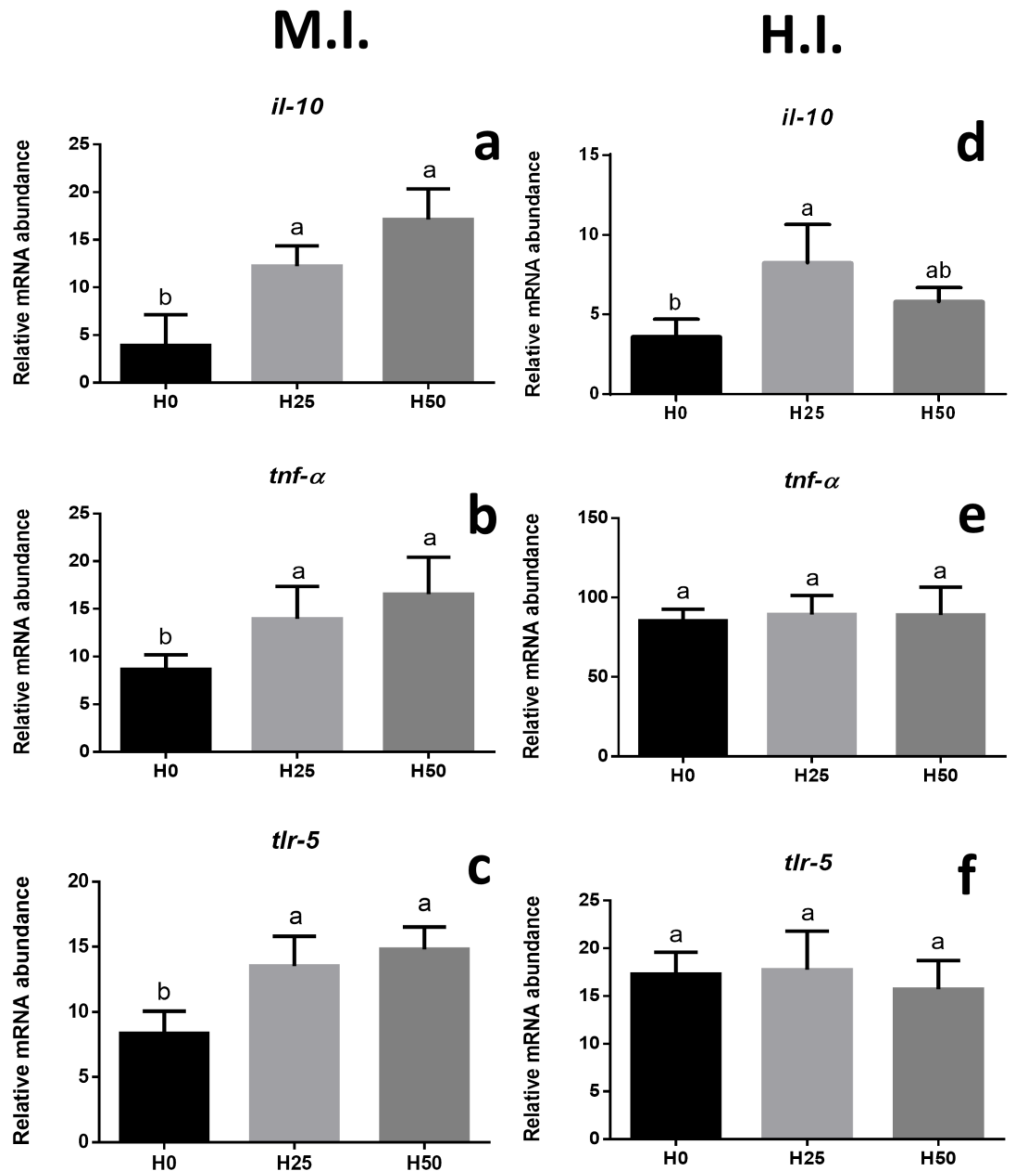
3.3. Gene Expression
3.4. Histology
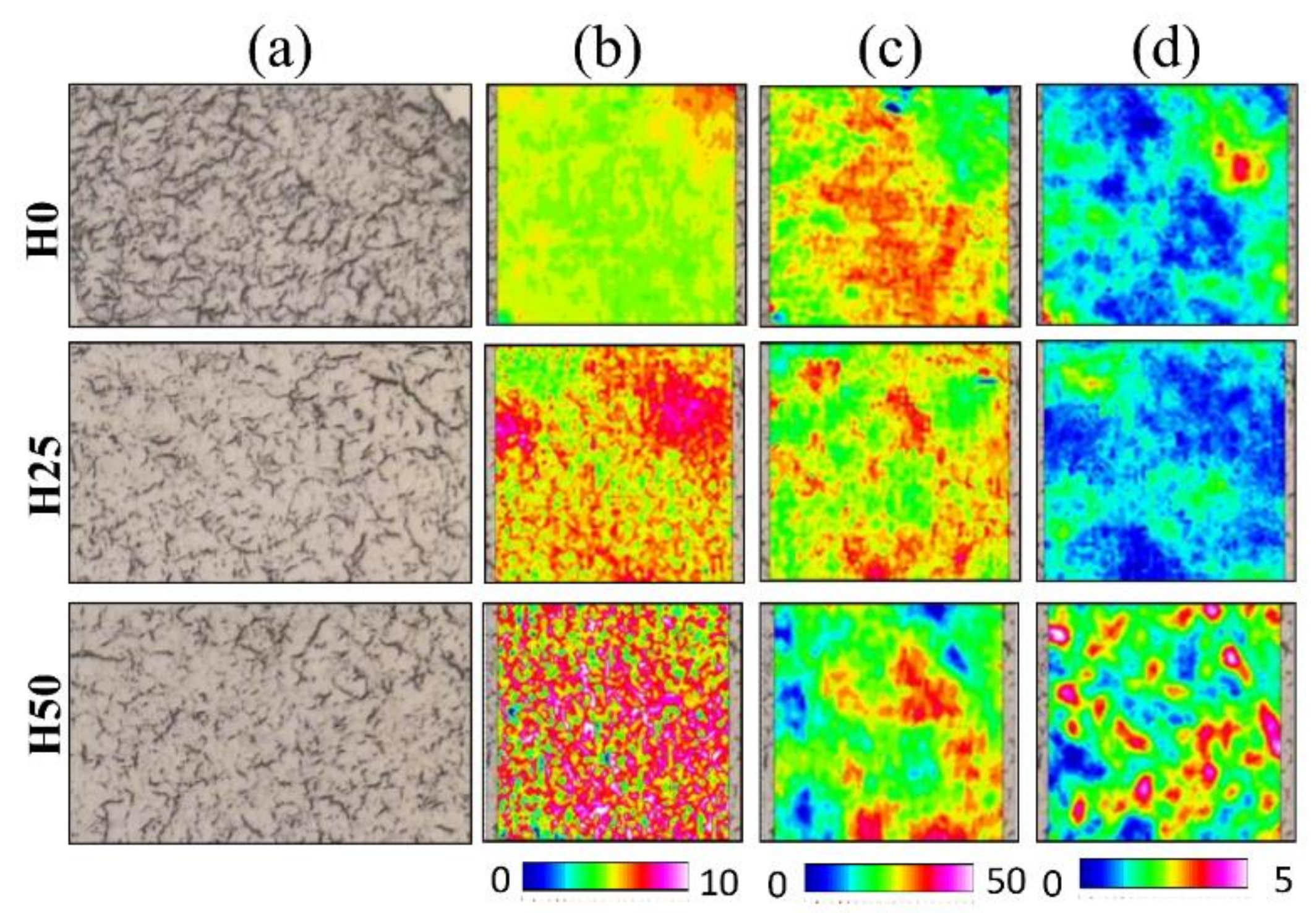
3.5. FTIRI Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tacon, A.; Metian, M. Feed matters: Satisfying the feed demand of aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, Licence: CC BY-NC-SA 3.0 IGO; 2018; p. 227. ISBN 978-92-5-130562-1. [Google Scholar]
- Gatlin, D.; Barrows, F.; Brown, P.; Dabrowski, K.; Gaylord, T.; Hardy, R.; Herman, E.; Hu, G.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquacult. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients—A review of ingredient evaluation strategies for aquaculture feeds. Aquac Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Pahlow, M.; van Oel, P.; Mekonnen, M.; Hoekstra, A. Increasing pressure on freshwater resources due to terrestrial feed ingredients for aquaculture production. Sci. Total Environ. 2015, 536, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeed. In Feed and Feeding Practices in Aquaculture; Davis, A., Ed.; Woodhead Publishing Limited: Waltham, MA, USA, 2015; pp. 203–233. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Mertens, E.; Halloran, A.; Mui, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; pp. 1–201. [Google Scholar]
- Oonincx, D.; de Boer, I. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Makkar, H.; Tran, G.; Henze, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Sanchez-Muros, M.; Barroso, F.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Barroso, F.; de Haro, C.; Sanchez-Muros, M.; Venegas, E.; Martinez-Sanchez, A.; Perez-Banon, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Nogales-Mérida, S.; Gobbi, P.; Jozefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kieronczyk, B.; Jozefiak, A. Insect meal in fish nutrition. Rev. Aquacult. 2018, 1–14. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute—growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.; Mosley, E.; Tomberlin, J.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquacult. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Liland, N.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.; Waagbo, R.; Torstensen, B.; Lock, E. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Newton, L.; Sheppard, C.; Watson, W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Annual Report; Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005; p. 17. [Google Scholar]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.; Irving, S.; Newton, L.; McGuire, M.; Mosley, E.; Hardy, R.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquacult. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Sealey, W.; Gaylord, T.; Barrows, F.; Tomberlin, J.; McGuire, M.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquacult. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56–63. [Google Scholar] [CrossRef]
- Esteban, M.; Cuesta, A.; Ortuno, J.; Meseguer, J. Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol. 2001, 11, 303–315. [Google Scholar] [CrossRef]
- Magalhaes, R.; Sanchez-Lopez, A.; Leal, R.; Martinez-Llorens, S.; Oliva-Telesa, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Li, X.; Rahimnejad, S.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Substituting fish meal with housefly (Musca domestica) maggot meal in diets for bullfrog rana (Lithobates catesbeiana): Effects on growth, digestive enzymes activity, antioxidant capacity and gut health. Aquaculture 2019, 499, 295–305. [Google Scholar] [CrossRef]
- Lock, E.; Arsiwalla, T.; Waagbo, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Elia, A.; Capucchio, M.; Caldaroni, B.; Magara, G.; Dorr, A.; Biasato, I.; Biasibetti, E.; Righetti, M.; Pastorino, P.; Prearo, M.; et al. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 496, 50–57. [Google Scholar] [CrossRef]
- Iwashita, Y.; Yamamoto, T.; Furuita, H.; Sugita, T.; Suzuki, N. Influence of certain soybean antinutritional factors supplemented to a casein-based semipurified diet on intestinal and liver morphology in fingerling rainbow trout Oncorhynchus mykiss. Fish. Sci. 2008, 74, 1075–1082. [Google Scholar] [CrossRef]
- Martin, S.; Dehler, C.; Krol, E. Transcriptomic responses in the fish intestine. Dev. Comp. Immunol. 2016, 64, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Wulff, T.; Petersen, J.; Norrelykke, M.; Jessen, F.; Nielsen, H. Proteome analysis of pyloric ceca: A methodology for fish feed development? J. Agric. Food Chem. 2012, 60, 8457–8464. [Google Scholar] [CrossRef]
- Kortner, T.; Skugor, S.; Penn, M.; Mydland, L.; Djordjevic, B.; Hillestad, M.; Krasnov, A.; Krogdahl, A. Dietary soyasaponin supplementation to pea protein concentrate reveals nutrigenomic interactions underlying enteropathy in Atlantic salmon (Salmo salar). BMC Vet. Res. 2012, 8, 101. [Google Scholar] [CrossRef]
- Giorgini, E.; Randazzo, B.; Gioacchini, G.; Cardinaletti, G.; Vaccari, L.; Tibaldi, E.; Olivotto, I. New insights on the macromolecular building of rainbow trout (O. mykiss) intestine: FTIR imaging and histological correlative study. Aquaculture 2018, 497, 1–9. [Google Scholar] [CrossRef]
- Vargas, A.A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Loreto, N.; Ruschioni, S.; Zarantoniello, M.; Antonucci, M.; et al. Rearing zebrafish on black soldier fly (Hermetia illucens): Biometric, histological, spectroscopic, biochemical, and molecular implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef]
- Carnevali, O.; Notarstefano, V.; Olivotto, I.; Graziano, M.; Gallo, P.; Di Marco Pisciottano, I.; Vaccari, L.; Mandich, A.; Giorgini, E.; Maradonna, F. Dietary administration of edc mixtures: A focus on fish lipid metabolism. Aquat. Toxicol. 2017, 185, 95–104. [Google Scholar] [CrossRef]
- Vargas, A.A.; Randazzo, B.; Foddai, M.; Sanchini, L.; Truzzi, C.; Giorgini, E.; Gasco, L.; Olivotto, I. Insect meal based diets for clownfish: Biometric, histological, spectroscopic, biochemical and molecular implications. Aquaculture 2019, 498, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- ISMEA (2013). Il Settore Ittico in Italia—Check up 2013. Available online: http://www.ismea.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/8845c (accessed on 20 November 2018).
- Collins, S.A.; Desai, A.R.; Mansfield, G.S.; Hill, J.E.; van Kessel, A.G.; Drew, M.D. The effect of increasing inclusion rate of soybean, pea and canola meals and their protein concentrates on the growth of rainbow trout: Concepts in diet formulation and experimental design for ingredient evaluation. Aquaculture 2012, 344–349, 90–99. [Google Scholar] [CrossRef]
- Øverland, M.; SØrensen, M.; Storebakken, T.; Penn, M.; Krogdahl, Å.; Skrede, A. protein concentrate substituting fish meal or soybean meal in diets for Atlantic salmon (Salmo salar)-Effect on growth performance, nutrient digestibility, carcass composition, gut health, and physical feed quality. Aquaculture 2009, 388, 305–311. [Google Scholar] [CrossRef]
- Santigosa, E.; Sànchez, J.; Médale, F.; Kaushil, S.; Pérez-Sànchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 283, 68–74. [Google Scholar] [CrossRef]
- Santigosa, E.; Garcìa-Meilàn, I.; Valentin, J.M.; Pérez-Sànchez, J.; Médale, F.; Kaushil, S.; Gallardo, M.A. Modifications of intestinal nutrient absorption in response to dietary fish meal replacement by plant protein sources in sea bream (Sparus aurata) and rainbow trout (Oncorhynchus mykiss). Aquaculture 2011, 317, 146–154. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 19th ed.; AOAC: Washington, DC, USA, 2006. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, H.S. A simple method for the isolation and purification of the total lipids from animal tissues. J. Biol. Chem. 1997, 226, 497–509. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Tulli, F.; Zittelli, G.; Giorgi, G.; Poli, B.; Tibaldi, E.; Tredici, M. Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization, and fillet composition of European sea bass juveniles fed organic diets. J. Aquat. Food Prod. Technol. 2012, 21, 188–197. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef]
- Randazzo, B.; Rolla, L.; Ofelio, C.; Planas, M.; Gioacchini, G.; Vargas, A.; Giorgini, E.; Olivotto, I. The influence of diet on the early development of two seahorse species (H. guttulatus and H. reidi): Traditional and innovative approaches. Aquaculture 2018, 490, 75–90. [Google Scholar] [CrossRef]
- Giorgini, E.; Sabbatini, S.; Conti, C.; Rubini, C.; Rocchetti, R.; Re, M.; Vaccari, L.; Mitri, E.; Librando, V. Vibrational mapping of sinonasal lesions by Fourier transform infrared imaging spectroscopy. J. Biomed. Opt. 2015, 20, 125003. [Google Scholar] [CrossRef]
- Bondari, K.; Sheppard, D.C. Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Res. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.; Waagbo, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, A.; Lock, E. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Daskalopoulou, E.; Vogiatzis, I.; Rumbos, C.; Mente, E.; Athanassious, C.G. Substitution of Fishmeal by Fly Hermetia illucens Prepupae Meal in the Diet of Gilthead Seabream (Sparus aurata). In Proceedings of the HydroMedit 2014, Volos, Greece, 13–15 November 2014. [Google Scholar]
- Muin, H.; Taufek, N.; Kamarudin, M.; Razak, S. Growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquacult. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Piccinetti, C.; Tulli, F.; Tokle, N.; Cardinaletti, G.; Olivotto, I. The use of preserved copepods in sea bream small-scale culture: Biometric, biochemical and molecular implications. Aquac. Nutr. 2014, 20, 90–100. [Google Scholar] [CrossRef]
- Messina, M.; Bulfon, C.; Beraldo, P.; Tibaldi, E.; Cardinaletti, G. Intestinal morpho-physiology and innate immune status of European sea bass (Dicentrarchus labrax) in response to diets including a blend of two marine microalgae, Tisochrysis lutea and Tetraselmis suecica. Aquaculture 2019, 500, 660–669. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Bruni, L.; Randazzo, B.; Vargas, A.; Gioacchini, G.; Truzzi, C.; Annibaldi, A.; Riolo, P.; Parisi, G.; Cardinaletti, G.; et al. Partial dietary inclusion of Hermetia illucens (black soldier fly) full-fat prepupae in zebrafish feed: Biometric, histological, biochemical, and molecular implications. Zebrafish 2018, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, S.; Xu, J.; Zhuang, L.; Xing, K.; Zhang, M.; Zhu, X.; Wang, L.; Gao, P.; Xi, Q.; et al. Dietary protein-induced hepatic igf-1 secretion mediated by ppar. Activation. PLoS ONE 2017, 12, e0173174. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.; Lawler, A.; Lee, S. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Garikipati, D.; Gahr, S.; Rodgers, B. Identification, characterization, and quantitative expression analysis of rainbow trout myostatin-1a and myostatin-1b genes. J. Endocrinol. 2006, 190, 879–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muddasir, J.; Imtiaz, A. Length weight relationship and condition factor of snow trout, Schizothorax plagiostomus (Heckel, 1838) from Lidder River, Kashmir. Int. J. Fish. Aquat. Stud. 2016, 4, 131–136. [Google Scholar]
- Barton, B. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteiroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, G.; Cardinaletti, G.; Carotti, M.; Palermo, F.A.; Soverchia, L.; Polzonetti-Magni, A.M. Neuroendocrine mechanisms regulating stress response in cultured teleost species. In Fish Endocrinology; Reinecke, M., Zaccone, M.G., Kapoor, B.G., Eds.; CRC Press Taylor & Francis Group: Enfileld, NH, USA, 2006; Volume 2, pp. 693–720. ISBN 978-1-5780-8318-3. [Google Scholar]
- Iwama, G.; Thomas, P.; Forsyth, R.; Vijayan, M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Poltronieri, C.; Negrato, E.; Bertotto, D.; Majolini, D.; Simontacchi, C.; Radaelli, G. Immunohistochemical localization of constitutive and inducible heat shock protein 70 in carp (Cyprinus carpio) and trout (Oncorhynchus mykiss) exposed to transport stress. Eur. J. Histochem. 2008, 52, 191–198. [Google Scholar] [CrossRef]
- Belghit, I.; Waagbø, R.; Lock, E.J.; Liland, N.S. Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquac. Nutr. 2018, 343–357. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.; Hoseinifar, S.; Gheysvandi, N.; Akbarzadeh, A. The effects of stocking density on hemato-immunological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2015, 23, 55–63. [Google Scholar] [CrossRef]
- Gai, F.; Gasco, L.; Daprà, F.; Palmegiano, G.B.; Sicuro, B. Enzymatic and histological evaluations of gut and liver in rainbow trout, Oncorhynchus mykiss, fed with rice protein concentrate based diets. J. World Aquacult. Soc. 2012, 43, 218–229. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Pagliassotti, M. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E275–E281. [Google Scholar] [CrossRef] [Green Version]
- Pfaffenbach, K.; Gentile, C.; Nivala, A.; Wang, D.; Wei, Y.; Pagliassotti, M. Linking endoplasmic reticulum stress to cell death in hepatocytes: Roles of c/ebp homologous protein and chemical chaperones in palmitate-mediated cell death. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1027–E1035. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Finke, M.; van Huis, A. Can diets containing insect promote animal health? J. Insects Food Feed 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Khojasteh, S.M.B.; Sheikhzadeh, F.; Mohammadnejad, D.; Azami, A. Histological, histochemical and ultrastructural study of the intestine of rainbow trout (Oncorhynchus mykiss). World Appl. Sci. J. 2009, 6, 1525–1531. [Google Scholar]
- Leknes, I.L. Goblet cell types in intestine of tiger barb and black tetra (Cyprinidae, Characidae: Teleostei). Anat. Histol. Embryol. 2014, 43, 352–360. [Google Scholar] [CrossRef]
- Dai, X.; Shu, M.; Fang, W. Histological and ultrastructural study of the digestive tract of rice field eel, Monopoterus albus. J. Appl. Ichthyol. 2007, 23, 177–183. [Google Scholar] [CrossRef]
- Swatson, H.; Gous, R.; Iji, P.; Zarrinkalam, R. Effect of dietary protein level, amino acid balance and feeding level on growth, gastrointestinal tract, and mucosal structure of the small intestine in broiler chickens. Anim. Res. 2002, 51, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Trichet, V.; Legrand-Frossi, C.; Frippiat, J. Comparison between intestinal and non-mucosal immune functions of rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2012, 33, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Overland, M.; Sorensen, M.; Penn, M.; Mydland, L.; Shearer, K.; Storebakken, T. Optimal inclusion of lupin and pea protein concentrates in extruded diets for trout (Oncorhynchus mykiss). Aquaculture 2012, 344, 100. [Google Scholar] [CrossRef]




| Diet Formulation and Composition | H | H0 | H25 | H50 |
|---|---|---|---|---|
| Ingredients | ||||
| Chile prime fish meal 1 | 420 | 315 | 210 | |
| Pea protein concentrate 2 | 55 | 78 | 100 | |
| H meal 3 | - | 105 | 210 | |
| Wheat gluten meal 4 | 55 | 78 | 100 | |
| Wheat flour 5 | 290 | 268 | 255 | |
| Fish oil | 70 | 40 | 28 | |
| Palm oil | 70 | 75 | 56 | |
| Mineral supplement $ | 10 | 10 | 10 | |
| Vitamin supplement # | 10 | 10 | 10 | |
| Binder | 20 | 20 | 20 | |
| L-Methionine | - | 1 | 1 | |
| Proximate composition * | ||||
| Moisture | 20.93 ± 0.02 | 4.24 ± 0.03 | 5.49 ± 0.03 | 5.31 ± 0.18 |
| Crude protein, CP | 30.84 ± 0.38 | 40.27 ± 0.45 | 39.98 ± 0.37 | 40.16 ± 0.39 |
| Ether extract, EE | 33.10 ± 0.24 | 18.63 ± 0.27 | 18.56 ± 0.14 | 17.68 ± 0.20 |
| Ash | 10.30 ± 0.18 | 14.30 ± 0.28 | 14.20 ± 0.23 | 14.13 ± 0.31 |
| Gross energy (MJ kg−1) | n.d. 6 | 22.10 ± 0.11 | 22.30 ± 0.03 | 21.28 ± 0.06 |
| Fatty acid composition * | ||||
| SFA | 65.30 ± 2.05 | 33.76 ± 0.14 | 42.13 ± 4.51 | 48.24 ± 1.67 |
| MUFA | 28.37 ± 0.89 | 32.87 ± 1.23 | 34.46 ± 0.61 | 33.04 ± 1.45 |
| PUFA | 6.34 ± 0.41 | 33.00 ± 1.41 | 23.00 ± 1.14 | 18.50 ± 2.12 |
| PUFA n-3 | 0.66 ± 0.04 | 22.01 ± 0.45 | 13.71 ± 0.22 | 10.11 ± 0.33 |
| PUFA n-6 | 5.68 ± 0.21 | 11.33 ± 2.20 | 9.68 ± 1.56 | 8.62 ± 1.50 |
| n-9 | 18.07 ± 1.14 | 26.50 ± 0.71 | 28.00 ± 1.41 | 25.50 ± 2.12 |
| EPA | 0.20 ± 0.02 | 6.85 ± 0.24 | 4.24 ± 0.49 | 3.12 ± 0.74 |
| DHA | - | 13.42 ± 0.89 | 8.24 ± 0.36 | 5.97 ± 0.72 |
| n-3/n-6 | 0.12 ± 0.10 | 1.99 ± 0.43 | 1.44 ± 0.25 | 1.19 ± 0.17 |
| Target Tissue | Gene Name | Primer Sequence | A.T. (°C) | Gene Bank Accession Number | |
|---|---|---|---|---|---|
| Liver | Forward | Reverse | |||
| igf1 | TGGACACGCTGCAGTATGTGTGT | CACTCGTCCACAATACCACGGT | 60 | GQ924783 | |
| mstn1a | CCGCCTTCACATATGCCAA | CAGAACCTGCGTCAGATGCA | 60 | AY839106 | |
| gr | GCCTTTTGGCATGTACTCAAACC | GGACGACTCTCCATACCTGTTC | 60 | AY549305 | |
| hsp70 | CCCTGGGCATCGAAACC | CCCTCGTAGACCTGGATCATG | 60 | AY423555 | |
| Intestine | il-10 | CGACTTTAAATCTCCCATCGA | GCATTGGACGATCTCTTTCTT | 59 | DQ821115 |
| tnf-α | AGCATGGAAGACCGTCAACGAT | AGCATGGAAGACCGTCAACGAT | 60 | DQ070246 | |
| tlr-5 | GGCATCAGCCTGTTGAATTT | ATGAAGAGCGAGAGCCTCAG | 57 | NP001118216 | |
| β-actin | AGACCACCTTCAACTCCATCAT | AGAGGTGATCTCCTTCTGCATC | 60 | AJ537421 | |
| 60S | TTCCTGTCACGACATACAAAGG | GTAAGCAGAAATTGCACCATCA | 60 | DT044641.1 | |
| Growth Metrics | H0 | H25 | H50 |
|---|---|---|---|
| FBW (g) 1 | 301.21 ± 32.21 | 279.59 ± 37.26 | 251.27 ± 22.14 |
| K 2 | 1.13 ± 0.11 | 1.13 ± 0.01 | 1.12 ± 0.07 |
| WG (%) 3 | 119.81 ± 16.83 | 102.91 ± 24.24 | 83.18 ± 14.32 |
| SGR (%) 4 | 0.80 ± 0.08 | 0.71 ± 0.12 | 0.61 ± 0.08 |
| FCR 5 | 1.02 ± 0.17 | 1.22 ± 0.35 | 1.47 ± 0.28 |
| Plasma Parameters | H0 | H25 | H50 |
|---|---|---|---|
| Chol (mg dL−1) | 188.0 ± 20.2 | 178.6 ± 29.9 | 196.7 ± 40.8 |
| Trig (mg dL−1) | 212.4 ± 50.1 | 186.8 ± 55.6 | 202.5 ± 37.8 |
| Glu (mg dL−1) | 112.7 ± 16.8 | 103.6 ± 9.9 | 123.0 ± 31.1 |
| TP (g dL−1) | 3.5 ± 0.5 | 3.2 ± 0.6 | 3.4 ± 0.7 |
| Alb (g dL−1) | 1.3 ± 0.2 | 1.5 ± 0.4 | 1.6 ± 0.3 |
| Wavenumber | Vibrational Mode | Biological Meaning |
|---|---|---|
| ~3019 | Stretching vibration of =CH groups | |
| ~2965, ~2925, ~2858 | Asymmetric and symmetric stretching vibrations of CH3 and CH2 groups | Mainly lipid alkyl chains |
| ~1737 | Stretching vibration of C=O ester moieties | |
| ~1662, ~1544 | Amide I and II bands | Proteins |
| ~1455, ~1395 | Bending vibrations of proteins side chains | |
| ~1237, ~1087 | Asymmetric and symmetric stretching vibrations of PO2- groups | Phosphate groups |
| ~1156 | Stretching vibrations of C-O-H moieties | Carbohydrates and glycogen |
| ~1034 | Stretching vibrations of C-O and C-C moieties and bending of C-O-H groups |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 251. https://doi.org/10.3390/ani9050251
Cardinaletti G, Randazzo B, Messina M, Zarantoniello M, Giorgini E, Zimbelli A, Bruni L, Parisi G, Olivotto I, Tulli F. Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals. 2019; 9(5):251. https://doi.org/10.3390/ani9050251
Chicago/Turabian StyleCardinaletti, Gloriana, Basilio Randazzo, Maria Messina, Matteo Zarantoniello, Elisabetta Giorgini, Andrea Zimbelli, Leonardo Bruni, Giuliana Parisi, Ike Olivotto, and Francesca Tulli. 2019. "Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss)" Animals 9, no. 5: 251. https://doi.org/10.3390/ani9050251
APA StyleCardinaletti, G., Randazzo, B., Messina, M., Zarantoniello, M., Giorgini, E., Zimbelli, A., Bruni, L., Parisi, G., Olivotto, I., & Tulli, F. (2019). Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals, 9(5), 251. https://doi.org/10.3390/ani9050251






