Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Period and Location
2.2. Animals
2.3. Experimental Area
2.4. Feeder and Testing Conditions
2.5. Experimenters
2.6. Experimental Procedure
2.7. Habituation Phase
2.8. Test Phase
2.9. Data Collection
2.9.1. Laterality
2.9.2. Stress
2.9.3. Task Related Behavior
2.10. Statistical Analyses
2.11. Ethical Statement
3. Results
3.1. Horses’ Sex and Innovative Problem-Solving
3.2. Laterality and Innovative Problem-Solving
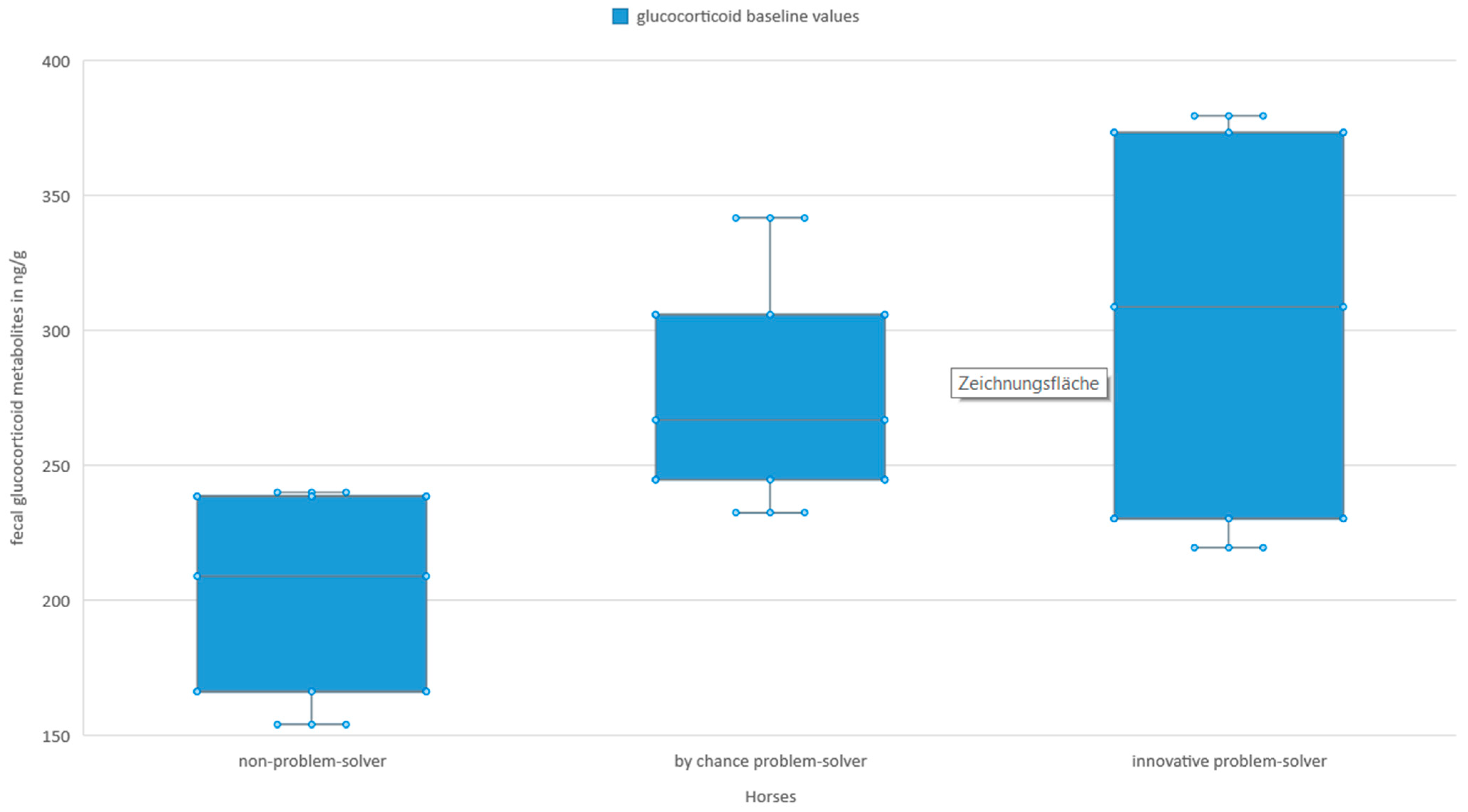
3.3. Stress Hormones and Innovative Problem-Solving
3.4. Task Related Behavior and Innovative Problem-Solving
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGreevy, P.; Winther Christensen, J.; von Borstel, U.K.; McLean, A. Equitation Science, 2nd ed.; John Wiley & Sons Incorporated: Newark, NJ, USA, 2018; ISBN 9781119241416. [Google Scholar]
- Hockenhull, J.; Whay, H.R. A review of approaches to assessing equine welfare. Equine Vet. Educ. 2014, 26, 159–166. [Google Scholar] [CrossRef]
- Brubaker, L.; Udell, M.A.R. Cognition and learning in horses (Equus caballus): What we know and why we should ask more. Behav. Process. 2016, 126, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Reader, S.M.; Laland, K.N. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl. Acad. Sci. USA 2002, 99, 4436–4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, L.; Whittle, P.; Lascaris, E.; Finkelstein, A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997, 53, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Güntürkün, O. The convergent evolution of neural substrates for cognition. Psychol. Res. 2012, 76, 212–219. [Google Scholar] [CrossRef]
- Ramsey, G.; Bastian, M.L.; van Schaik, C. Animal innovation defined and operationalized. Behav. Brain Sci. 2007, 30, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Griffin, A.S.; Guez, D. Innovation and problem solving: A review of common mechanisms. Behav. Process. 2014, 109, 121–134. [Google Scholar] [CrossRef]
- Griffin, A.S. Innovativeness as an emergent property: A new alignment of comparative and experimental research on animal innovation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Meehan, C.L.; Mench, J.A. The challenge of challenge: Can problem solving opportunities enhance animal welfare? Appl. Anim. Behav. Sci. 2007, 102, 246–261. [Google Scholar] [CrossRef]
- Thornton, A.; Samson, J. Innovative problem solving in wild meerkats. Anim. Behav. 2012, 83, 1459–1468. [Google Scholar] [CrossRef]
- Kummer, H.; Goodall, J. Conditions of innovative behaviour in primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 308, 203–214. [Google Scholar] [CrossRef]
- Boogert, N.J.; Reader, S.M.; Hoppitt, W.; Laland, K.N. The origin and spread of innovations in starlings. Anim. Behav. 2008, 75, 1509–1518. [Google Scholar] [CrossRef]
- Cole, E.F.; Quinn, J.L. Personality and problem-solving performance explain competitive ability in the wild. Proc. Biol. Sci. 2012, 279, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.S.; Diquelou, M.; Perea, M. Innovative problem solving in birds: A key role of motor diversity. Anim. Behav. 2014, 92, 221–227. [Google Scholar] [CrossRef]
- Manrique, H.M.; Völter, C.-J.; Call, J. Repeated innovation in great apes. Anim. Behav. 2013, 85, 195–202. [Google Scholar] [CrossRef]
- Morand-Ferron, J.; Quinn, J.L. Larger groups of passerines are more efficient problem solvers in the wild. Proc. Natl. Acad. Sci. USA 2011, 108, 15898–15903. [Google Scholar] [CrossRef] [Green Version]
- Sol, D.; Griffin, A.S.; Bartomeus, I. Consumer and motor innovation in the common myna: The role of motivation and emotional responses. Anim. Behav. 2012, 83, 179–188. [Google Scholar] [CrossRef]
- Webster, S.J.; Lefebvre, L. Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim. Behav. 2001, 62, 23–32. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Ross, S.R.; Linick, S.A.; Milstein, M.S.; Melber, T.N. An experimental, comparative investigation of tool use in chimpanzees and gorillas. Anim. Behav. 2009, 77, 1119–1126. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Holekamp, K.E. Innovative problem solving by wild spotted hyenas. Proc. Biol. Sci. 2012, 279, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R. The role of neophobia and neophilia in the development of innovative behaviour of birds. In Animal Innovation; Reader, S.M., Laland, K.N., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 175–196. ISBN 978-0-19-852622-3. [Google Scholar]
- Reader, S.M.; Laland, K.N. Primate innovation: Sex, age and social rank differences. Int. J. Primatol. 2000, 22, 787–805. [Google Scholar] [CrossRef]
- Cole, E.F.; Cram, D.L.; Quinn, J.L. Individual variation in spontaneous problem-solving performance among wild great tits. Anim. Behav. 2011, 81, 491–498. [Google Scholar] [CrossRef]
- Hunt, G.R.; Corballis, M.C.; Gray, R.D. Laterality in tool manufacture by crows. Nature 2001, 414, 707. [Google Scholar] [CrossRef] [PubMed]
- Magat, M.; Brown, C. Laterality enhances cognition in Australian parrots. Proc. Biol. Sci. 2009, 276, 4155–4162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Brooks, D.E.; Komaromy, A.M.; Kallberg, M.E. Comparative retinal ganglion cell and optic nerve morphology. Vet. Ophthalmol. 1999, 2, 3–11. [Google Scholar] [CrossRef]
- Farmer, K.; Krüger, K.; Byrne, R.W.; Marr, I. Sensory laterality in affiliative interactions in domestic horses and ponies (Equus caballus). Anim. Cogn. 2018. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Lusito, R.; Quaranta, A. Is the left forelimb preference indicative of a stressful situation in horses? Behav. Process. 2014, 107, 61–67. [Google Scholar] [CrossRef]
- Schultheiss, O.C.; Riebel, K.; Jones, N.M. Activity inhibition: A predictor of lateralized brain function during stress? Neuropsychology 2009, 23, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009, 364, 943–954. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Rogers, L.J. Motor and sensory laterality in thoroughbred horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [PubMed]
- Wells, A.E.D.; Blache, D. Horses do not exhibit motor bias when their balance is challenged. Animal 2008, 2, 1645–1650. [Google Scholar] [CrossRef] [Green Version]
- Marr, I.; Farmer, K.; Krueger, K. Evidence for right-sided horses being more optimistic than left-sided horses. Animals (Basel) 2018, 8, 219. [Google Scholar] [CrossRef]
- Rogers, L.J. A Matter of degree: Strength of brain asymmetry and behaviour. Symmetry 2017, 9, 57. [Google Scholar] [CrossRef]
- Marshall-Pescini, S.; Barnard, S.; Branson, N.J.; Valsecchi, P. The effect of preferential paw usage on dogs’ (Canis familiaris) performance in a manipulative problem-solving task. Behav. Process. 2013, 100, 40–43. [Google Scholar] [CrossRef]
- Mendl, M. Performing under pressure: Stress and cognitive function. Appl. Anim. Behav. Sci. 1999, 65, 221–244. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Grace, L.; Hescham, S.; Kellaway, L.A.; Bugarith, K.; Russell, V.A. Effect of exercise on learning and memory in a rat model of developmental stress. Metab. Brain Dis. 2009, 24, 643–657. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.N.; Reader, S.M. Foraging innovation is inversely related to competitive ability in male but not in female guppies. Behav. Ecol. 1999, 10, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Hopper, L.M.; Price, S.A.; Freeman, H.D.; Lambeth, S.P.; Schapiro, S.J.; Kendal, R.L. Influence of personality, age, sex, and estrous state on chimpanzee problem-solving success. Anim. Cogn. 2014, 17, 835–847. [Google Scholar] [CrossRef]
- Tebbich, S.; Sterelny, K.; Teschke, I. The tale of the finch: Adaptive radiation and behavioural flexibility. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 1099–1109. [Google Scholar] [CrossRef]
- Olczak, K.; Winther Christensen, J.; Klocek, C. Food motivation in horses appears stable across different test situations. Appl. Anim. Behav. Sci. 2018, 204, 60–65. [Google Scholar] [CrossRef]
- Krueger, K.; Esch, L.; Byrne, R.W. Animal behaviour in a human world: A crowd sourcing study on horses that open door and gate mechanisms. PLoS ONE 2018. under Review. [Google Scholar]
- Mader, D.R.; Price, E.O. Discrimination learning in horses: Effects of breed, age and social dominance. J. Anim. Sci. 1980, 50, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Heinze, J. Horse sense: Social status of horses (Equus caballus) affects their likelihood of copying other horses’ behavior. Anim. Cogn. 2008, 11, 431–439. [Google Scholar] [CrossRef]
- Krueger, K.; Farmer, K.; Heinze, J. The effects of age, rank and neophobia on social learning in horses. Anim. Cogn. 2014, 17, 645–655. [Google Scholar] [CrossRef]
- Wolff, A.; Hausberger, M. Learning and memorisation of two different tasks in horses: The effects of age, sex and sire. Appl. Anim. Behav. Sci. 1996, 46, 137–143. [Google Scholar] [CrossRef]
- Winther Christensen, J.; Ahrendt, L.P.; Lintrup, R.; Gaillard, C.; Palme, R.; Malmkvist, J. Does learning performance in horses relate to fearfulness, baseline stress hormone, and social rank? Appl. Anim. Behav. Sci. 2012, 140, 44–52. [Google Scholar] [CrossRef]
- Lansade, L.; Simon, F. Horses’ learning performances are under the influence of several temperamental dimensions. Appl. Anim. Behav. Sci. 2010, 125, 30–37. [Google Scholar] [CrossRef]
- Petersen, J.L.; Mickelson, J.R.; Rendahl, A.K.; Valberg, S.J.; Andersson, L.S.; Axelsson, J.; Bailey, E.; Bannasch, D.; Binns, M.M.; Borges, A.S.; et al. Genome-wide analysis reveals selection for important traits in domestic horse breeds. PLoS Genet. 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Pirault, P.; Danvy, S.; Verrier, E.; Leroy, G. Genetic structure and gene flows within horses: A genealogical study at the french population scale. PLoS ONE 2013, 8, e61544. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz. Leitlinien zur Beurteilung von Pferdehaltungen unter Tierschutzgesichtspunkten; Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz: Bonn, Germany, 2009. [Google Scholar]
- Winther Christensen, J.; Zharkikh, T.; Chovaux, E. Object recognition and generalisation during habituation in horses. Appl. Anim. Behav. Sci. 2011, 129, 83–91. [Google Scholar] [CrossRef]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 104–109. [Google Scholar] [CrossRef]
- Roberts, S.M. Equine vision and optics. Vet. Clin. North. Am. Equine Pract. 1992, 8, 451–457. [Google Scholar] [CrossRef]
- Murphy, J.; Hall, C.; Arkins, S. What horses and humans see: A comparative review. Int. J. Zool. 2009, 2009, 1–14. [Google Scholar] [CrossRef]
- McLean, A.N. Short-term spatial memory in the domestic horse. Appl. Anim. Behav. Sci. 2004, 85, 93–105. [Google Scholar] [CrossRef]
- Baragli, P.; Vitale, V.; Paoletti, E.; Mengoli, M.; Sighieri, C. Encoding the object position for assessment of short term spatial memory in horses (Equus caballus). Int. J. Comp. Psychol. 2011, 24, 284–291. [Google Scholar]
- Larose, C.; Richard-Yris, M.-A.; Hausberger, M.; Rogers, L.J. Laterality of horses associated with emotionality in novel situations. Laterality Asymmetries Body Brain Cogn. 2006, 11, 355–367. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Pawluski, J.; Jego, P.; Henry, S.; Bruchet, A.; Palme, R.; Coste, C.; Hausberger, M. Low plasma cortisol and fecal cortisol metabolite measures as indicators of compromised welfare in domestic horses (Equus caballus). PLoS ONE 2017, 12, e0182257. [Google Scholar] [CrossRef]
- Krueger, K.; Marr, I.; Dobler, A.; Palme, R. Preservation of fecal cortisol metabolites (GCM) and immunoglobulin A (IgA) through silica gel drying for field studies in horses: submitted. Conserv. Physiol. 2019. [Google Scholar]
- Flauger, B.; Krueger, K.; Gerhards, H.; Möstl, E. Simplified method to measure glucocorticoid metabolites in faeces of horses. Vet. Res. Commun. 2010, 34, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Van de Waal, E.; Borgeaud, C.; Whiten, A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science 2013, 340, 483–485. [Google Scholar] [CrossRef]
- Sih, A.; Del Giudice, M. Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012, 367, 2762–2772. [Google Scholar] [CrossRef]
- Marchetti, C.; Drent, P.J. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 2000, 60, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Amici, F.; Aureli, F.; Call, J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr. Biol. 2008, 18, 1415–1419. [Google Scholar] [CrossRef]
- Bray, E.E.; MacLean, E.L.; Hare, B.A. Context specificity of inhibitory control in dogs. Anim. Cogn. 2014, 17, 15–31. [Google Scholar] [CrossRef]
- Wimpenny, J.H.; Weir, A.S.A.; Clayton, L.; Rutz, C.; Kacelnik, A. Cognitive processes associated with sequential tool use in New Caledonian crows. PLoS ONE 2009, 4, e6471. [Google Scholar] [CrossRef]
- Griffin, A.S.; Diquelou, M.C. Innovative problem solving in birds: A cross-species comparison of two highly successful passerines. Anim. Behav. 2015, 100, 84–94. [Google Scholar] [CrossRef]
- Greenberg, R.; Mettke-Hofmann, C. Ecological aspects of Neophobia and Neophilia in birds. In Current Ornithology; Springer: Boston, MA, USA, 2001; Volume 319, pp. 119–178. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. Biol. Sci. 2004, 271, S420–S422. [Google Scholar] [CrossRef] [Green Version]
- Hausberger, M.; Fureix, C.; Bourjade, M.; Wessel-Robert, S.; Richard-Yris, M.-A. On the significance of adult play: What does social play tell us about adult horse welfare? Naturwissenschaften 2012, 99, 291–302. [Google Scholar] [CrossRef]
- Duberstein, K.J.; Gilkeson, J.A. Determination of sex differences in personality and trainability of yearling horses utilizing a handler questionnaire. App. Anim. Behav. Sci. 2010, 128, 57–63. [Google Scholar] [CrossRef]
- Miyata, H.; Gajdon, G.K.; Huber, L.; Fujita, K. How do keas (Nestor notabilis) solve artificial-fruit problems with multiple locks? Anim. Cogn. 2011, 14, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Harlow, H.F.; Harlow, M.K.; Meyer, D.R. Learning motivated by a manipulation drive. J. Exp. Psychol. 1950, 40, 228. [Google Scholar] [CrossRef]
- Hagen, K.; Broom, D.M. Emotional reactions to learning in cattle. App. Anim. Behav. Sci. 2004, 85, 203–213. [Google Scholar] [CrossRef]
- Von Bayern, A.M.P.; Heathcote, R.J.P.; Rutz, C.; Kacelnik, A. The role of experience in problem solving and innovative tool use in crows. Curr. Biol. 2009, 19, 1965–1968. [Google Scholar] [CrossRef]
- Deaner, R.O.; Isler, K.; Burkart, J.; van Schaik, C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav. Evol. 2007, 70, 115–124. [Google Scholar] [CrossRef]
- Murphy, J.; Arkins, S. Equine learning behaviour. Behav. Process. 2007, 76, 1–13. [Google Scholar] [CrossRef]
- Reader, S.M.; Hager, Y.; Laland, K.N. The evolution of primate general and cultural intelligence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1017–1027. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esch, L.; Wöhr, C.; Erhard, M.; Krüger, K. Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving. Animals 2019, 9, 265. https://doi.org/10.3390/ani9050265
Esch L, Wöhr C, Erhard M, Krüger K. Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving. Animals. 2019; 9(5):265. https://doi.org/10.3390/ani9050265
Chicago/Turabian StyleEsch, Laureen, Caroline Wöhr, Michael Erhard, and Konstanze Krüger. 2019. "Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving" Animals 9, no. 5: 265. https://doi.org/10.3390/ani9050265
APA StyleEsch, L., Wöhr, C., Erhard, M., & Krüger, K. (2019). Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving. Animals, 9(5), 265. https://doi.org/10.3390/ani9050265




