A Primitive Trait in Two Breeds of Equus Caballus Revealed by Comparative Anatomy of the Distal Limb
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal Details
2.3. Dissections
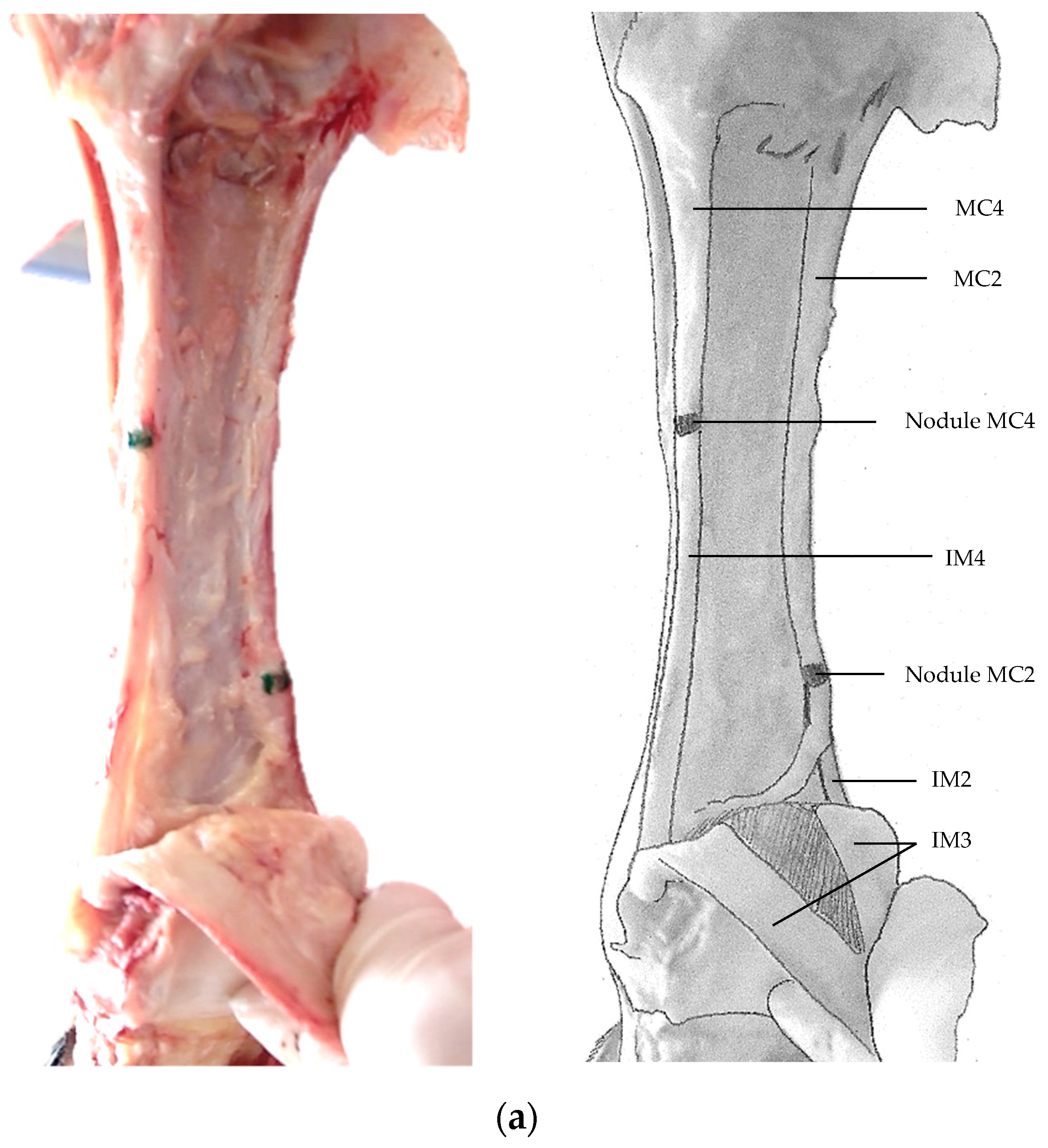
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blits, K. Aristotle: Form, function and comparative anatomy. Anat. Rec. 1999, 257, 58–63. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species; John Murray: London, UK, 1859. [Google Scholar]
- Rogers, G. Brother Surgeons; Random House: London, UK, 1957. [Google Scholar]
- Lucus, J.R. Wilberforce and Huxley: A legendary encounter. Hist. J. 1979, 22, 313–330. [Google Scholar] [CrossRef]
- Thomson, K.S. Huxley, Wilberforce and the Oxford Museum. Am. Sci. 2000, 88, 210–213. [Google Scholar] [CrossRef]
- Zangerl, R. The methods of comparative anatomy and its contribution to the study of evolution. Evolution 1948, 2, 351–374. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, B. Fossil horses—Evidence for evolution. Science 2005, 307, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Matthew, W.D. The evolution of the horse: A record and its interpretation. Q. Rev. Biol. 1926, 1, 139–185. [Google Scholar] [CrossRef]
- MacFadden, B.J. Fossil horses from “Eohippus” (Hyracotherium) to Equus: Scaling Cope’s Law, and the evolution of body size. Paleobiology 1986, 12, 355–369. [Google Scholar] [CrossRef]
- McHorse, B.K.; Biewener, A.A.; Pierce, S.E. Mechanics of evolutionary digit reduction in fossil horses (Equidae). Proc. R. Soc. B 2017, 284, 20171174. [Google Scholar] [CrossRef] [Green Version]
- Thomason, J.J. Estimation of Locomotory forces and stresses in the limb bones of recent and extinct Equids. Paleobiology 1985, 11, 209–220. [Google Scholar] [CrossRef]
- Bradley, O.C. The Topographical Anatomy of the Limbs of the Horse; W. Green & Son: Edinburgh, Scotland, UK, 1946. [Google Scholar]
- Rafati, N.; Andersonn, L.S.; Mikko, S.; Feng, C.; Raudsepp, T.; Pettersson, J.; Janecka, J.; Wattle, O.; Ameur, A.; Thyreen, G.; et al. Large deletions at the SHOX locus in the pseudoautosomal region are associated with skeletal atavism in Shetland ponies. G3-Genes Genom. Genet. 2016, 6, 2213–2223. [Google Scholar] [CrossRef]
- Tyson, R.; Graham, J.P.; Colahan, P.T.; Berry, C.R. Skeletal atavism in a miniature horse. Vet. Radiol. Ultrasound 2004, 45, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Sisson, S. Chapter 16: Equine Syndesmology. In The Anatomy of the Domestic Animals, 5th ed.; Sisson, S., Grossman, J.D., Eds.; Saunders: Philadelphia, PA, USA, 1975; p. 359. [Google Scholar]
- Soffler, C.; Hermanson, J.W. Muscular design in the equine interosseous muscle. J. Morphol. 2006, 267, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, R. Atlas der Anatomie der Pferdes. Part 2; Parey, P., Ed.; Topographische Myologie: Berlin, Germany, 1911. [Google Scholar]
- May-Davis, S.E.R.; Brown, W.Y.; Shorter, K.; Vermeulen, Z.; Butler, R.; Koekkoek, M. A novel non-invasive selection criterion for the preservation of primitive Dutch Konik horses. Animals 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E. Horses of the World; Princeton University Press: Princeton, NJ, USA, 2014; pp. 10–128. [Google Scholar]
- Ashdown, R.R.; Done, S.H. Color Atlas of Veterinary Anatomy Vol. 2; Mosby-Wolfe: London, UK, 2000. [Google Scholar]
- Pasicka, E. Polish Konik horse—Characteristics and historical background of native descendants of Tarpan. Acta Sci. Pol. Med. Vet. 2013, 12, 25–38. [Google Scholar]
- Mesarič, M.; Dolinšek, A.; Dovč, P. Bosnian Mountain Horse: The Oldest Indigenous Breed in the Balkans Facing Extinction; Planibo: Ljubljana, Sovenia, 2015; p. 56. [Google Scholar]
- May-Davis, S.E.R.; Brown, W.Y.; Shorter, K.; Vermeulen, Z. The disappearing lamellae—Implications of new findings in the family Equidae suggest the loss of nuchal ligament lamellae on C6 and C7 occurred after domestication. J. Equine Vet. Sci. 2018, 30, 108–114. [Google Scholar] [CrossRef]
- Nazem, M.N.; Sajjadian, S.M. Anatomical transverse MRI study of ligaments in palmar surface of metacarpus miniature donkey; introduce a new ligament. Folia Morphol. 2017, 76, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Geyer, H.; Fürst, A. Anatomie der griffelbeine und ihrer umgebung unter besonderer berücksichtigung der faszien. Schweiz. Arch. Tierheil 2005, 147, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B. The comparative myology of the forelimb of the hippopotamus, pig and tapir. Am. J. Anat. 1936, 59, 201–247. [Google Scholar] [CrossRef]
- Muir, J. The Malayan Tapir. J. Anat. Physiol. 1871, 6, 131–172. [Google Scholar]
- Evans, H.E.; de Lahunta, A. Guide to the Dissection of the Dog, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 1975; pp. 43–79. [Google Scholar]
- Cunningham, D.J. The intrinsic muscles of the hand of the thylacine (Thylacinus cynocephalus), cuscus (Phaslangista maculate), and phascogale (Phascogale caluraI). J. Anat. Physiol. 1878, 12, 434–444. [Google Scholar]
- Mascarello, F.; Rowlerson, A. Natural involution of muscle in the proximal sesamoidean ligament in sheep. J. Anat. 1995, 186, 75–86. [Google Scholar] [PubMed]
- Constantinescu, G.M.; Reed, S.K.; Constantinescu, I.A. The suspensory apparatus and digital flexor muscles of the llama (Lama glama) 1. The thoracic limb. Int. J. Morphol. 2008, 26, 543–550. [Google Scholar] [CrossRef]
- Nourinezhad, J.; Mazaheri, Y.; Mahabady, M.K. Gross anatomy of the ligaments of fetlock joint in dromedary camel. J. Camel Pract. Res. 2011, 18, 197–202. [Google Scholar]
- El-Shafey, A.; Kassab, A. Computed tomography and cross-sectional anatomy of the metatarsus and digit of one-humped camel (Camelus dromedaries) and buffalo (Bos bubalis). Anat. Histol Embryol. 2012, 42, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.R.; Sardari, K.; Mohammadi, H.R. Normal cross-sectional anatomy of the bovine digit: Comparison of computed tomography and limb anatomy. Anat. Histol. Embryol. 2007, 37, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.B. The evolution of the unguligrade manus in Artiodactyls. J. Vertebr. Paleontol. 2010, 30, 1827–1839. [Google Scholar] [CrossRef]
- Steiner, C.C.; Ryder, O.A. Molecular phylogeny and evolution of Perissodactyla. Zool. J. Linn. Soc. Lond. 2011, 163, 1289–1303. [Google Scholar] [CrossRef]
- Sánchez, I.M.; Cantalapiedra, J.L.; Ríos, M.; Quiralte, V.; Jorge, M. Systematics and evolution of the Miocene three-horned Pataeomerycid ruminates (Mammalia, Cetartiodactyla). PLoS ONE 2015, 10, e0143034. [Google Scholar] [CrossRef]
- Thomason, J.J. The functional morphology of the manus in the Tridactyl equids Merychippus and Mesohippus: Paleontological inferences from neontological models. J. Vertebr. Paleontol. 1986, 6, 143–161. [Google Scholar] [CrossRef]
- Solounis, N.; Danowitz, M.; Stachtiaras, E.; Khurana, A.; Araim, M.; Sayegh, M.; Natale, J. The evolution and anatomy of the horse manus with an emphasis on digit reduction. R. Soc. Open Sci. 2018, 5, 171782. [Google Scholar] [CrossRef] [Green Version]
- Bough, J. From value to vermin: A history of the donkey in Australia. Aust. Zool. 2006, 33, 388–397. [Google Scholar] [CrossRef]
- Burnham, S. Anatomical differences of the donkey and mule. Proc. Annu. Conv. Am. Assoc. Equine Pract. 2002, 48, 102–109. [Google Scholar]
- Bőkőnyi, S. The Przevalsky Horse; Souvenir Press: London, UK, 1974; p. 38. [Google Scholar]



| Species | Column Title | Distal Limbs (n) 1 | IM2 (n) | IM4 (n) | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| Equus caballus: Domestic horse | Fore | 121 | 121 | 1 * | 1 * | 1 * | 1 * |
| (17 breeds n = 121; DL n = 484) | Hind | 121 | 121 | 1 * | 1 * | 1 * | 1 * |
| Equus caballus: Primitive horse | Fore | 15 | 14 | 13 | 12 | 13 | 12 |
| (Dutch Konik n = 17; DL = 61) | Hind | 15 | 17 | 15 | 15 | 15 | 16 |
| Equus przewalskii | Fore | 9 | 2 | 9 | 2 | 9 | 2 |
| (Przewalski’s horse n = 9; DL = 15) | Hind | 2 | 2 | 2 | 2 | 2 | 2 |
| Equus asinus | Fore | 3 | 3 | 3 | 3 | 3 | 3 |
| (Donkey n = 3; DL = 11) | Hind | 3 | 2 | 3 | 2 | 2 | 2 |
| Equus quagga boehmi | Fore | 1 | 1 | 0 | 0 | 1 | 1 |
| (Grant’s zebra n = 1; DL = 4) | Hind | 1 | 1 | 0 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May-Davis, S.; Vermeulen, Z.; Brown, W.Y. A Primitive Trait in Two Breeds of Equus Caballus Revealed by Comparative Anatomy of the Distal Limb. Animals 2019, 9, 355. https://doi.org/10.3390/ani9060355
May-Davis S, Vermeulen Z, Brown WY. A Primitive Trait in Two Breeds of Equus Caballus Revealed by Comparative Anatomy of the Distal Limb. Animals. 2019; 9(6):355. https://doi.org/10.3390/ani9060355
Chicago/Turabian StyleMay-Davis, Sharon, Zefanja Vermeulen, and Wendy Y. Brown. 2019. "A Primitive Trait in Two Breeds of Equus Caballus Revealed by Comparative Anatomy of the Distal Limb" Animals 9, no. 6: 355. https://doi.org/10.3390/ani9060355
APA StyleMay-Davis, S., Vermeulen, Z., & Brown, W. Y. (2019). A Primitive Trait in Two Breeds of Equus Caballus Revealed by Comparative Anatomy of the Distal Limb. Animals, 9(6), 355. https://doi.org/10.3390/ani9060355







