Morphostructural Characterization of the Black Creole Goat Raised in Central Mexico, a Currently Threatened Zoogenetic Resource
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphometric Variables
2.3. Statistical Analysis
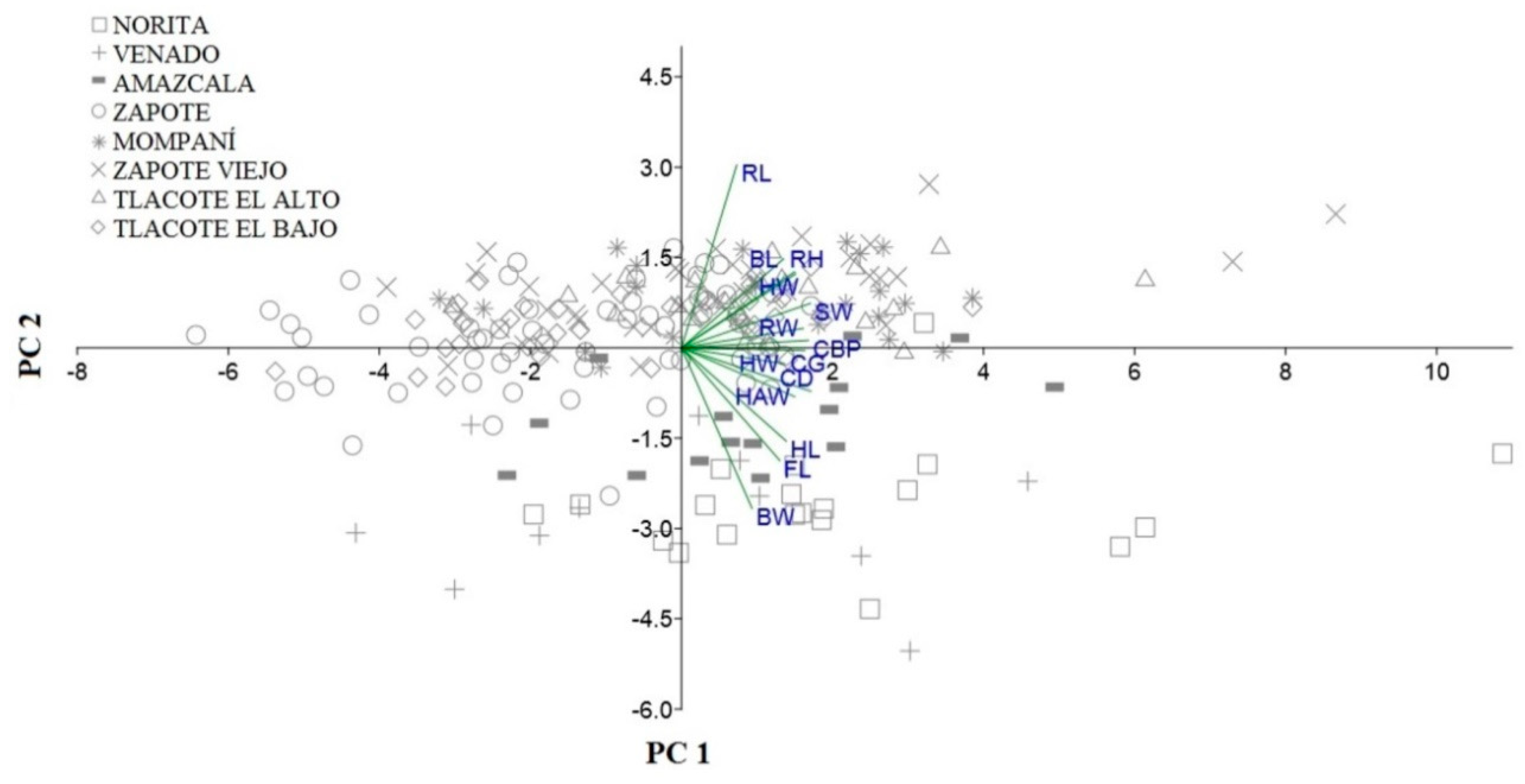
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rege, J.E.O.; Marshall, K.; Notenbaert, A.; Ojango, J.M.K.; Okeyo, A.M. Pro-poor animal improvement and breeding - What can science do? Livest. Sci. 2011, 136, 15–28. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pansu, J.; Pompanon, F. Conservation genetics of cattle, sheep, and goats. Comptes. Rendus. Biol. 2011, 334, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Boyazoglu, J.; Hatziminaoglou, I.; Morand-Fehr, P. The role of the goat in society: Past, present and perspectives for the future. Small Rumin. Res. 2005, 60, 13–23. [Google Scholar] [CrossRef]
- Pereira, F.; Queirós, S.; Gusmão, L.; Nijman, I.J.; Cuppen, E.; Lenstra, J.A.; Consortium, E.; Davis, S.J.M.; Nejmeddine, F.; Amorim, A. Tracing the history of goat pastoralism: New clues from mitochondrial and y chromosome DNA in North Africa. Mol. Biol. Evol. 2009, 26, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- FAO Statistics Database. Available online: http://www.fao.org/faostat/es/#data/QA (accessed on 8 March 2019).
- Boza, J. Papel del ganado caprino en las zonas desfavorecidas. Available online: http://www.ovinos-caprinos.com/MANEJO/47%20-%20Ganado%20Caprino%20en%20zonas%20desfavorecidas.pdf (accessed on 22 March 2019).
- FAO La situación de los recursos zoogenéticos mundiales para la alimentación y la agricultura. Available online: http://www.fao.org/3/a1250s/a1250s00.htm (accessed on 19 March 2019).
- FAO Coping with climate change - the roles of genetic resources for food and agriculture. Available online: http://www.fao.org/news/story/en/item/275041/icode/ (accessed on 30 April 2019).
- Montaldo, H.H.; Torres-Hernández, G.; Valencia-Posadas, M. Goat breeding research in Mexico. Small Rumin. Res. 2010, 89, 155–163. [Google Scholar] [CrossRef]
- Andrade, H.M.; Silva, J.C.; Escalante, I.; Vera, H.R. la cabra criolla negra, un recurso zoogenético en peligro: Bases del programa de recuperación en el bajío mexicano. Rev. Mex. Agroecosistemas 2017, 4, 29–41. [Google Scholar]
- FAO Global plan of action for animal genetic resources and the interlaken declaration. Available online: http://www.fao.org/3/a1404e/a1404e00.htm (accessed on 10 April 2019).
- Mavule, B.S.; Muchenje, V.; Bezuidenhout, C.C.; Kunene, N.W. Morphological structure of Zulu sheep based on principal component analysis of body measurements. Small Rumin. Res. 2013, 111, 23–30. [Google Scholar] [CrossRef]
- Ogah, D.M. Assessing Size and Conformation of the Body of Nigerian Indigenous Turkey. Slovak J. Anim. Sci. 2011, 44, 21–27. [Google Scholar]
- Parés, P.M. Zoometría. In Valoración morfológica de los animales domésticos; Sañudo, C., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, España, 2009; pp. 171–198. [Google Scholar]
- FAO Realización de encuestas y seguimiento de los recursos zoogenéticos. Available online: http://www.fao.org/3/ba0055s/ba0055s00.htm (accessed on 10 April 2019).
- Angulo, Y. Muestreo. In Estadística para las ciencias sociales, del comportamiento y de la salud, 3rd ed.; Pérez-Tejada, H.E., Ed.; Cengage Learning: Distrito Federal, México D.F., 2008; pp. 182–200. [Google Scholar]
- Hernández, J.S.; Franco, F.J.; Herrera, M.; Rodero, E.; Sierra, A.C.; Bañuelos, A.; Delgado, J.V. Estudio de los recursos genéticos de México: Características morfológicas y morfoestructurales de los caprinos nativos de Puebla. Arch. Zootec. 2002, 51, 53–64. [Google Scholar]
- FAO Phenotypic characterization of animal genetic resources. Available online: http://www.fao.org/3/i2686e/i2686e00.htm (accessed on 12 December 2018).
- Herrera, M.; Luque, M. Morfoestructura y sistemas para el futuro en la valoración morfológica. In Valoración morfológica de los animales domésticos; Sañudo, C., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, España, 2009; pp. 83–102. [Google Scholar]
- Kaiser, H.F. The aplication of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 141–151. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing 2017. Available online: https://www.r-project.org/ (accessed on 11 October 2018).
- Gordón-Mendoza, R.; Camargo-Buitrago, I. Selección de estadísticos para la estimación de la precisión experimental en ensayos de maíz. Agron. Mesoam. 2015, 26, 55–63. [Google Scholar] [CrossRef]
- Mohammadi, K.; Beygi Nassiri, M.T.; Fayazi, J.; Roshanfekr, H. Investigation of environmental Factors Influence on Pre-Weaning Grow Traits in Zandi Lambs. J. Anim. Vet. Adv. 2010, 9, 1011–1014. [Google Scholar]
- Dudhe, S.D.; Yadav, S.B.S.; Nagda, R.K.; Pannu, U.; Gahlot, G.C. Genetic and non-genetic factors affecting morphometry of Sirohi goats. Vet. World. 2015, 8, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Hassen, H.; Baum, M.; Rischkowsky, B.; Tibbo, M. Phenotypic characterization of Ethiopian indigenous goat populations. African J. Biotechnol. 2012, 11, 13838–13846. [Google Scholar] [CrossRef]
- Herrera, M.; Rodero, E.; Gutierrez, M.J.; Peña, F.; Rodero, J.M. Application of multifactorial discriminant analysis in the morphostructural differentiation of Andalusian caprine breeds. Small Rumin. Res. 1996, 22, 39–47. [Google Scholar] [CrossRef]
- Vargas, S.; Larbi, A.; Sánchez, M. Analysis of size and conformation of native Creole goat breeds and crossbreds used in smallholder agrosilvopastoral systems in Puebla, Mexico. Trop Anim Heal. Prod. 2007, 279–286. [Google Scholar] [CrossRef]
- Bedotti, D.; Gómez, A.G.; Sánchez, M.; Martos, J. Caracterización morfológica y faneróptica de la cabra colorada pampeana. Arch. Zootec. 2004, 261–271. [Google Scholar]
- Morales-delaNuez, A.; Rodríguez, C.; Santana, M.; Hernandez-Castellano, L.E.; Niño, T.; Reyes-Chacón, R.J.; Moreno-indias, I.; Castro, N.; Capote, J. Morphological measurements and indexes as a tool to support molecular genetic studies: An example in Canary Islands. J. Appl. Anim. Res. 2012, 40, 215–221. [Google Scholar] [CrossRef]
- Fuentes-Mascorro, G.; Martínez, M.S.; Alejandre, M.E.; Chirinos, Z.; Ricardi, L.C. zoometría y distribución de partos de la cabra criolla de los Valles Centrales de Oaxaca. Actas Iberoam. Conserv. Anim. 2013, 3, 150–154. [Google Scholar]
- Dorantes-Coronado, E.J.; Torres-Hernández, G.; Hernández-Mendo, O.; Rojo-Rubio, R. Zoometric measures and their utilization in prediction of live weight of local goats in southern Mexico. Springerplus 2015, 4, 695. [Google Scholar] [CrossRef]
- Gomes, J.K.; Arandas, G.; Vieira, N.M.; de Barros, R.; Pimenta, E.C.; de Albuquerque, L.H.; Ribeiro, M. Multivariate analysis as a tool for phenotypic characterization of an endangered breed. J. Appl. Anim. Res. 2017, 45, 152–158. [Google Scholar]
- Moutchou, N.; González, A.M.; Chentouf, M.; Lairini, K.; Rodero, E. Morphological differentiation of Northern Morocco goat. J. Livest. Sci. Technol. 2017, 5, 33–41. [Google Scholar]
- Khargharia, G.; Kadirvel, G.; Kumar, S.; Doley, S.; Bharti, P.K.; Das, M. Principal component analysis of morphological traits of Assam hill goat in eastern Himalayan India. J. Anim. Plant Sci. 2015, 25, 1251–1258. [Google Scholar]
- Okpeku, M.; Yakubu, A.; Peters, S.; Ozoje, M.; Ikeobi, C.; Adebambo, O.; Imumorin, I. Application of multivariate principal component analysis to morphological characterization of indigenous goats in Southern Nigeria. Acta Agric. Slov. 2011, 98, 101–109. [Google Scholar] [CrossRef]
- Pares, P.M. Body Weight is an Important Trait for Comparisons of Goat Breeds. Iran. J. Appl. Anim. Sci. 2015, 5, 463–466. [Google Scholar]
- Gonzalez-Martinez, A.; Herrera, M.; Luque, M.; Rodero, E. Influence of farming system and production purpose on the morphostructure of Spanish goat breeds. Spanish J. Agric. Res. 2014, 12, 117–124. [Google Scholar] [CrossRef]



| Variable | Mean | SD | CV | Min | Max |
|---|---|---|---|---|---|
| HW | 11.56 | 0.98 | 8.50 | 9.50 | 18.00 |
| HL | 15.66 | 1.40 | 8.96 | 11.00 | 20.50 |
| FL | 12.74 | 1.45 | 11.38 | 9.00 | 19.50 |
| WH | 70.12 | 4.31 | 6.14 | 57.00 | 88.00 |
| CG | 84.07 | 7.36 | 8.76 | 56.00 | 106.00 |
| BL | 71.20 | 6.11 | 8.59 | 52.00 | 87.00 |
| BW | 21.88 | 5.10 | 23.32 | 15.60 | 41.00 |
| CD | 30.31 | 2.63 | 8.69 | 24.00 | 38.00 |
| SW | 16.86 | 1.53 | 9.07 | 13.00 | 24.00 |
| RL | 18.81 | 2.04 | 10.85 | 13.00 | 27.00 |
| RW | 15.61 | 1.40 | 8.95 | 12.50 | 21.00 |
| HAW | 17.17 | 1.79 | 10.43 | 12.20 | 23.00 |
| RH | 70.54 | 3.77 | 5.34 | 61.00 | 86.00 |
| CBP | 8.49 | 0.68 | 8.06 | 6.20 | 11.00 |
| HL | BW | HAW | CD | HW | CG | CBP | RW | SW | RH | WH | BL | RL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FL | 0.87 *** | 0.24 *** | 0.41 *** | 0.49 *** | 0.28 *** | 0.28 *** | 0.36 *** | 0.26 *** | 0.32 *** | 0.35 *** | 0.31 *** | 0.23 *** | 0 |
| HL | 0.25 *** | 0.39 *** | 0.52 *** | 0.27 *** | 0.31 *** | 0.33 *** | 0.38 *** | 0.34 *** | 0.38 *** | 0.34 *** | 0.24 *** | 0.03 | |
| BW | 0.43 *** | 0.51 *** | 0.35 *** | 0.57 *** | 0.38 *** | 0.4 *** | 0.32 ** | 0.15 | 0.17 | 0.2 | −0.11 *** | ||
| HAW | 0.46 *** | 0.39 *** | 0.59 *** | 0.45 *** | 0.58 *** | 0.58 *** | 0.32 *** | 0.34 *** | 0.42 *** | 0.14 | |||
| CD | 0.48 *** | 0.65 *** | 0.59 *** | 0.57 *** | 0.53 *** | 0.49 *** | 0.52 *** | 0.48 *** | 0.22 * | ||||
| HW | 0.43 *** | 0.57 *** | 0.44 *** | 0.42 *** | 0.32 *** | 0.43 *** | 0.35 *** | 0.18 | |||||
| CG | 0.55 *** | 0.58 *** | 0.64 *** | 0.44 *** | 0.44 *** | 0.49 *** | 0.32 *** | ||||||
| CBP | 0.5 *** | 0.56 *** | 0.42 *** | 0.47 *** | 0.49 *** | 0.32 *** | |||||||
| RW | 0.59 *** | 0.49 *** | 0.44 *** | 0.48 *** | 0.4 *** | ||||||||
| SW | 0.48 *** | 0.46 *** | 0.55 *** | 0.4 *** | |||||||||
| RH | 0.78 *** | 0.43 *** | 0.44 *** | ||||||||||
| WH | 0.49 *** | 0.4 *** | |||||||||||
| BL | 0.48 *** |
| INDEX | MEAN | SD | CV | Min | Max |
|---|---|---|---|---|---|
| COI | 85.11 | 8.72 | 10.24 | 62.07 | 151.79 |
| THI | 71.98 | 13.87 | 19.26 | 50.00 | 117.14 |
| CEI | 74.29 | 9.03 | 12.16 | 57.89 | 163.64 |
| PEI | 83.75 | 10.24 | 12.22 | 59.26 | 121.43 |
| PRI | 98.98 | 8.10 | 8.18 | 75.00 | 142.31 |
| MTI | 10.14 | 0.84 | 8.29 | 7.75 | 14.52 |
| RDI | 43.27 | 3.30 | 7.63 | 34.78 | 55.07 |
| TPI | 22.30 | 1.89 | 8.46 | 17.95 | 29.17 |
| LPI | 26.87 | 2.80 | 10.42 | 19.57 | 39.13 |
| Variables | PC 1 | PC 2 | PC 3 | Communality |
|---|---|---|---|---|
| HW | 0.63 | −0.07 | −0.32 | 0.51 |
| HL | 0.65 | −0.39 | 0.55 | 0.87 |
| FL | 0.61 | −0.47 | 0.50 | 0.84 |
| WH | 0.70 | 0.31 | 0.25 | 0.66 |
| CG | 0.76 | −0.01 | −0.29 | 0.66 |
| BL | 0.63 | 0.37 | −0.08 | 0.54 |
| BW | 0.44 | −0.67 | −0.41 | 0.81 |
| CD | 0.80 | −0.18 | 0.00 | 0.67 |
| SW | 0.80 | 0.19 | −0.14 | 0.69 |
| RL | 0.34 | 0.77 | 0.02 | 0.71 |
| RW | 0.75 | 0.08 | −0.12 | 0.59 |
| HAW | 0.70 | −0.20 | −0.10 | 0.54 |
| RH | 0.70 | 0.32 | 0.34 | 0.71 |
| CBP | 0.78 | 0.03 | −0.21 | 0.66 |
| Eigenvalue | 6.39 | 1.88 | 1.18 | |
| % Variance | 45.64 | 13.41 | 8.45 |
| Norita | Venado | Amazcala | Zapote | Mompaní | Z. Viejo | T. Alto | |
|---|---|---|---|---|---|---|---|
| Venado | 5.11 | - | |||||
| Amazcala | 4.81 | 5.23 | - | ||||
| Zapote | 7.77 | 4.91 | 6.07 | - | |||
| Mompaní | 6.04 | 6.60 | 4.71 | 5.70 | - | ||
| Z. Viejo | 5.88 | 5.81 | 4.77 | 4.79 | 3.25 | - | |
| T. Alto | 5.55 | 6.64 | 4.50 | 6.43 | 4.03 | 2.34 | - |
| T. Bajo | 7.03 | 4.92 | 4.82 | 2.60 | 5.17 | 3.68 | 4.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Jarquin, J.C.; Román-Ponce, S.I.; Durán-Aguilar, M.; Vera-Ávila, H.R.; Cambrón-Sandoval, V.H.; Andrade-Montemayor, H.M. Morphostructural Characterization of the Black Creole Goat Raised in Central Mexico, a Currently Threatened Zoogenetic Resource. Animals 2019, 9, 459. https://doi.org/10.3390/ani9070459
Silva-Jarquin JC, Román-Ponce SI, Durán-Aguilar M, Vera-Ávila HR, Cambrón-Sandoval VH, Andrade-Montemayor HM. Morphostructural Characterization of the Black Creole Goat Raised in Central Mexico, a Currently Threatened Zoogenetic Resource. Animals. 2019; 9(7):459. https://doi.org/10.3390/ani9070459
Chicago/Turabian StyleSilva-Jarquin, Juan Carlos, Sergio Iván Román-Ponce, Marina Durán-Aguilar, Héctor Raymundo Vera-Ávila, Víctor Hugo Cambrón-Sandoval, and Héctor Mario Andrade-Montemayor. 2019. "Morphostructural Characterization of the Black Creole Goat Raised in Central Mexico, a Currently Threatened Zoogenetic Resource" Animals 9, no. 7: 459. https://doi.org/10.3390/ani9070459
APA StyleSilva-Jarquin, J. C., Román-Ponce, S. I., Durán-Aguilar, M., Vera-Ávila, H. R., Cambrón-Sandoval, V. H., & Andrade-Montemayor, H. M. (2019). Morphostructural Characterization of the Black Creole Goat Raised in Central Mexico, a Currently Threatened Zoogenetic Resource. Animals, 9(7), 459. https://doi.org/10.3390/ani9070459





