Simple Summary
Two rabbit lines are divergently selected for increasing or decreasing the variability of litter size at birth. Decreasing the litter size variability produces more resilient females with less sensitivity to diseases, being an indirect selection way to improve environmental sensitivity. The kits’ survival rate at weaning was higher in the homogeneous line. Moreover, this line led to a greater uniformity of the kits’ weight at weaning, although the weight variability at birth was higher, which could be due to a higher lactation capacity of the homogeneous line.
Abstract
A divergent selection experiment on environmental sensitivity was performed in rabbits. The aim was to estimate the correlated response in kit weight and survival, litter weight, and weight distance from birth to weaning. The weight distance was calculated as the absolute value of the differences between the individual value and the mean value of its litter. The relationship between the probability of survival at 4 d of age, and the weight at birth, was studied. Environmental sensitivity was measured as litter size variability. A total of 2484 kits from 127 does from the low line, and 1916 kits of 114 does from the high line of the 12th generation were weighed. Both of the lines showed similar individual and litter weights at birth and weaning, and a similar survival rate at birth, and at 4 d of age. The survival rate at weaning was higher in the low line (0.67 and 0.62; P = 0.93). The weight distance was higher at birth, but lower at weaning in the low line (47.8 g and 54.1 g; P = 0.98). When the weight at birth was high, the kits had a higher survival rate. In conclusion, selection for environmental sensitivity showed a correlated response in the kits’ survival, and in the homogeneity of litter weight at weaning.
1. Introduction
The aim of genetic selection in maternal rabbit lines has traditionally been to improve the mean of productive traits: Litter size [1], or the length of does’ productive life [2,3]. Overall, this intensive selection for the increase of productivity has been successful, but it has also had negative consequences on animal welfare, increasing culling at early ages [4,5]. Consequently, resistance toward disease and stress are current priorities in rabbit breeding, leading to better doe resilience and welfare.
Selection for environmental sensitivity, measured as litter size variability, is an indirect selection methodology for improving resilience and robustness [6,7]. A divergent selection experiment for this trait has been performed with success [6], leading to lines with high and low litter size variability. Higher litter size variability affects the heterogeneity of littermates, which can produce lower pre-weaning survival rates [8,9]. The aim of this work is to study the correlated response in the pre-weaning survival rates of two rabbit lines, divergently selected for environmental sensitivity.
2. Material and Methods
All experimental procedures involving animals were approved by the Miguel Hernández University of Elche Research Ethics Committee (Reference number 2019/VSC/PEA/0017), in accordance with Council Directives 98/58/EC and 2010/63/EU.
2.1. Animals
A divergent selection experiment for litter size variability was carried out over twelve generations. The selection was based on the phenotypic variance of the litter size of each doe, after correcting the litter size for both year–season and parity–lactation status [6].
All of the animals were reared in the farm of the Miguel Hernández University of Elche (Spain). The rabbits were fed a standard commercial diet (17% crude protein, 16% fiber, 3.5% fat, Nutricun Elite Gra®, De Heus Nutrición Animal, La Coruña, Spain). Food and water were provided ad libitum. The same feeding conditions were provided for both lactating and non-lactating does. Does were housed in individual cages (37.5 × 33 × 90 cm) under a constant photoperiod of 16 h continuous light: 8 h continuous darkness, and with controlled ventilation throughout the experiment. The experiment took place from December to September. The temperature and relative humidity were recorded every 15 min with a Tinytag data logger (Table 1).

Table 1.
Temperature and relative humidity by season.
Does were first mated at 18 weeks of age, and at 10 d after parturition thereafter. Matings took place every week. The nest was made with textile by-products and the doe had free access to the nest, from 2 days before delivery until 21 days after delivery, when the nest was removed. The litters were not standardized, and the kits were weaned at 28 days of age.
Data come from the 12th generation of the selection. The litter size at birth (LS), the number born alive (NBA), the number born dead (NBD), the number of rabbits at 4 days of age (N4), and the number of rabbits at weaning (NW) were recorded. The rabbits were individually weighed and sexed within 24 h after birth. Some of the kits had suckled before being weighed. The milk intake was verified by recording a white mark in the abdominal area. The kits were also weighed at weaning. A total of 2484 kits from 127 does from the low line, and 1916 kits of 114 does from the high line were weighed.
2.2. Traits
The following traits were analyzed: LS; survival at birth (NBA/LS); survival at 4 days of age (N4/NBA); survival at weaning (NW/N4); the individual weight at birth of live and dead kits; the individual weight at weaning; the litter weight at birth of total kits and kits alive; the litter weight at weaning; and the weight distance of live, dead, and weaned rabbits. The weight distance was calculated as the absolute value of the differences between the individual value and the mean value of its litter.
2.3. Statistical Analysis
The model used for analyzing the LS and the litter survival rates was:
where Li is the line effect with two levels (the high and the low lines); Sj is the season effect with three levels (winter, spring, and summer); LPk is the lactation–parity effect with five levels (nulliparous, lactating, and non-lactating primiparous doe, and lactating and non-lactating multiparous doe); pijkl is the dam permanent effect with 241 levels; and eijkl is the residual term.
yijkl = µ + Li +Sj + LPk + pijkl + eijkl
The individual weight at birth for the live and dead kits, and their corresponding distance were analyzed using the following model:
where LKi is the line-survival effect (live kits of the high line, dead kits of the high line, live kits of the low line, and dead kits of the low line); IMl is the intake of milk effect (whether the kit suckled or not before being weighed); SEm is the sex effect (male and female); pijklmn is the dam permanent effect with 241 levels; cijklmno is the common litter effect with 541 levels; b is the regression coefficient of the covariate; LSijklmno is the covariate litter size; and eijklmnop is the residual term.
yijklmnop = LKi + Sj + LPk + IMl + SEm + pijklmn + cijklmno + b × LSijklmno + eijklmnop
Litter weights, individual weights at weaning, and the distance were analyzed with the same model, but the line effect with two levels (high and low lines) was used instead of the line-survival effect.
All of the analyses were performed using Bayesian methodology [10]. Bounded uniform priors were used for all effects. The joint prior distribution for the permanent environmental effect of the doe and the common litter effect was N (0, I⨂Gp), where Gp was the (co)variance matrix between these effects. Residuals priori distribution was N (0, I⨂σ2e). Residuals, permanent environmental effects, and common litter effects are uncorrelated. The priors for the variances were also bounded uniform. Features of the marginal posterior distributions for all of the unknowns were estimated using Gibbs sampling. The Threshold Model program was used [11]. We used a chain of 250,000 samples, with a burn-in period of 50,000. Only one out of every 100 samples was saved for inferences. Convergence was tested using the Z criterion of Geweke [12], and Monte Carlo sampling errors were computed using time-series procedures [13].
The relationship between the probability of survival from birth to 4 d of age, and the individual weight at birth was analyzed by logistic regression. The model included line, season, parity–lactation (with three levels: Nulliparous, lactating, and non-lactating does), milk intake, and sex effects. Table 2 shows the number of kits that survived at 4 d of age, classified by weight at birth, and line. The LOGISTIC procedure of the statistical package SAS was used [14].

Table 2.
Number of kits at birth (number of kits at 4 d of age) by line effect and individual birth weight (g).
3. Results
3.1. Correlated Response to Selection in Litter Survival and Pre-Weaning Weight
Descriptive results of the traits by line are presented in Table 3. The coefficient of variations are moderate and increase from birth to weaning, except for the weight distance, which is high and similar.

Table 3.
General mean, standard deviation (SD), coefficient of variation (CV) for litter size at birth, survival, litter weight, individual weight, and weight distance before weaning.
Table 4 shows the features of the estimated marginal posterior distributions of the differences between the lines for litter survival, individual weight, and weight distances at birth and weaning. The litter size at birth was higher in the low line (H-L = −0.6 kits; P = 1.0). The survival rate at birth and at 4 d of age were similar between the lines, but the survival at weaning was 5% higher in the low line (P = 0.93). Both of the lines showed similar individual weights of kits, and litter weight at birth. There is some evidence that the individual weight at weaning was lower in the low line (H-L = 15 g; P = 0.82), but when the litter weight at weaning was considered, both lines showed similar values (P = 0.78). The weight distance for live kits at birth was higher in the low line (H-L = −0.5 g; P = 0.97); however, the weight distance at weaning was lower in the low line (H-L = 6.3 g; P = 0.98).

Table 4.
Features of the marginal posterior distribution of the differences between the high and the low litter size variability lines for litter size at birth, survival, litter weight, individual weight, and weight distance before weaning.
3.2. Survival at 4 d of Age and Individual Weight at Birth
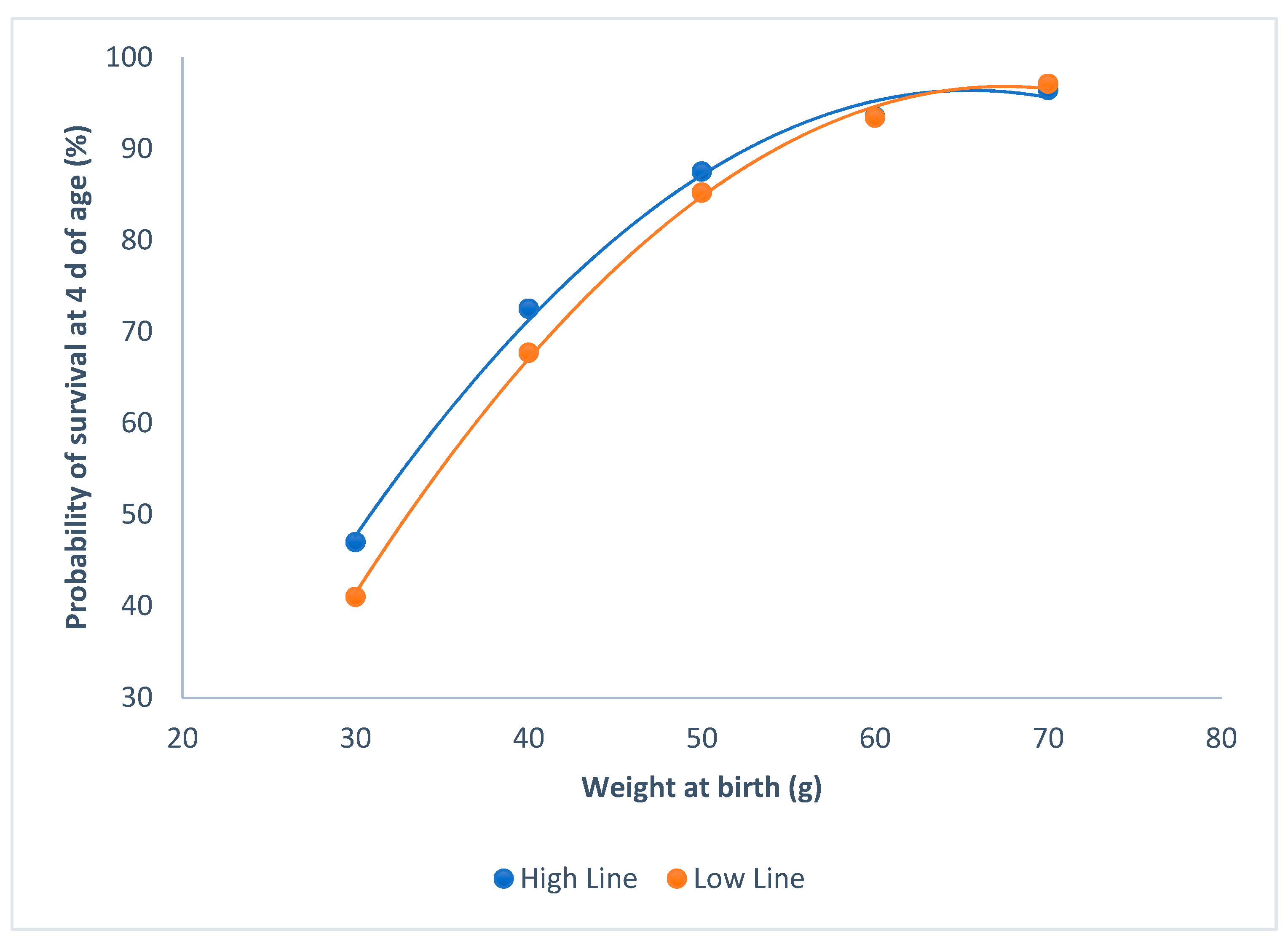
The probability of survival at 4 d of age, and weight at birth were not affected by sex (P = 0.47). Both of the lines showed similar probabilities of survival at 4 d of age, with the same weight at birth (P = 0.12; Figure 1). Probabilities of survival asymptotically increased with the individual birth weights, and raised to more than 90% from 60 g onwards.
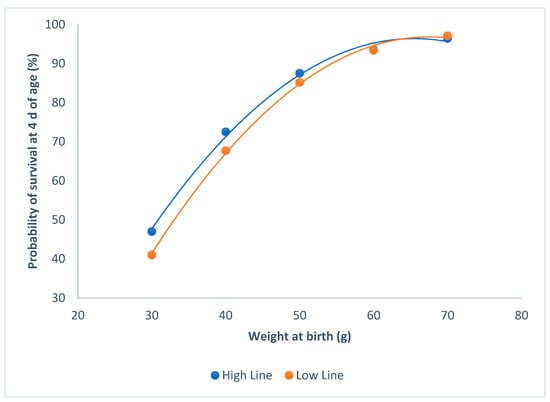
Figure 1.
Relationship between survival at 4 d of age and individual birth weights for the high and the low litter size variability lines.
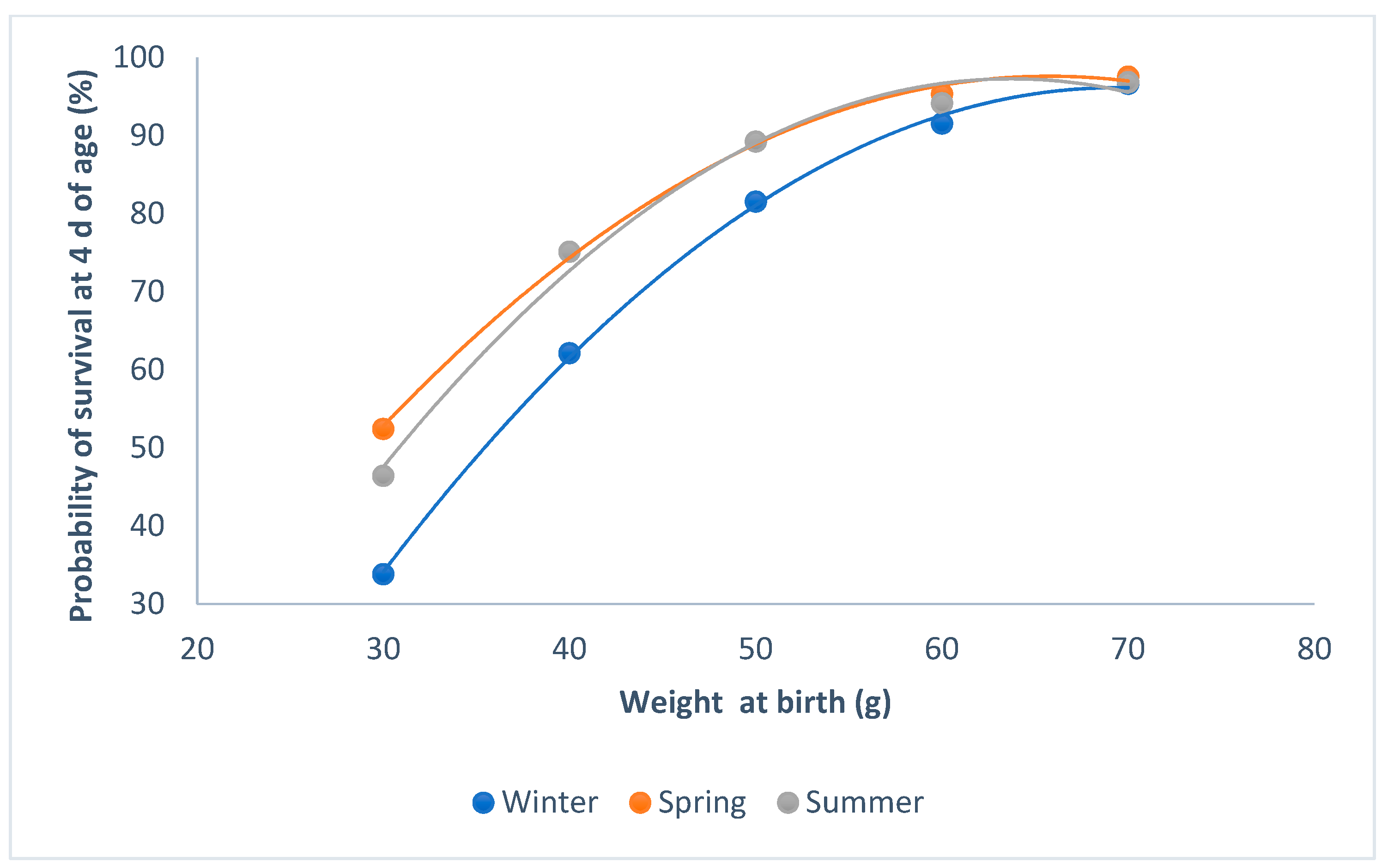
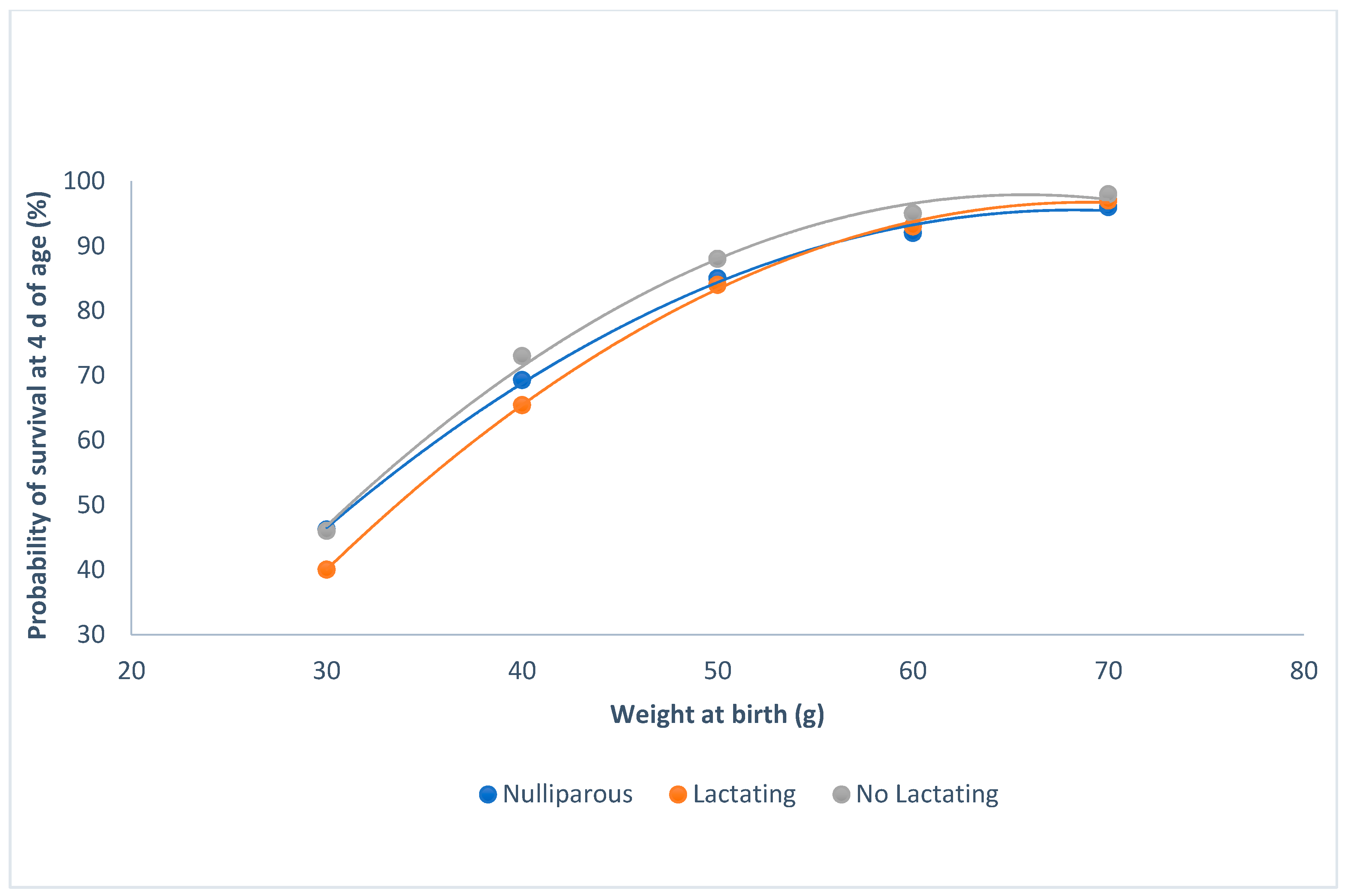
Kits born in winter had less of a probability of survival than those that were born in summer or spring (P < 0.0001; Figure 2). When the weight of the kits was higher than 60 g at birth, the probability of survival was at its maximum, regardless of the parity–lactation status of the doe (P < 0.0001; Figure 3). The minimum probability of survival took place in the lactating does, when the weights ranged from 30 to 60 g; the non-lactating does showed the highest probability of survival.
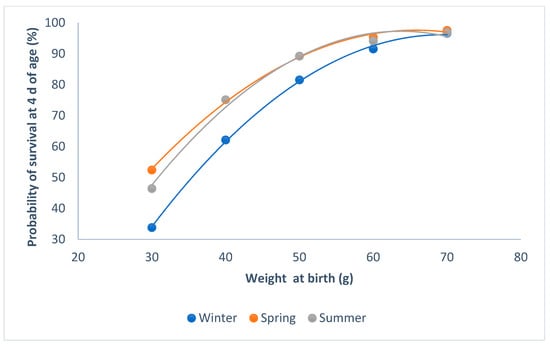
Figure 2.
Relationship between survival at 4 d of age and individual birth weight for the seasons.
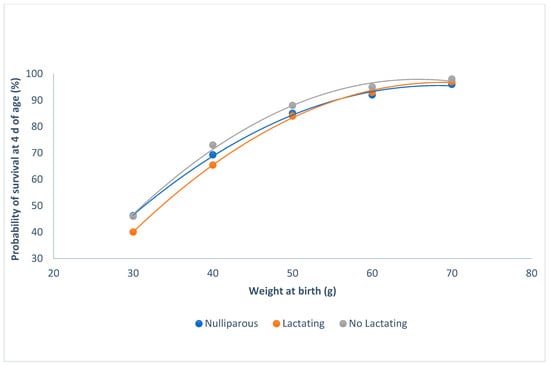
Figure 3.
Relationship between survival at 4 d of age and individual birth weight for the parity-lactation status.
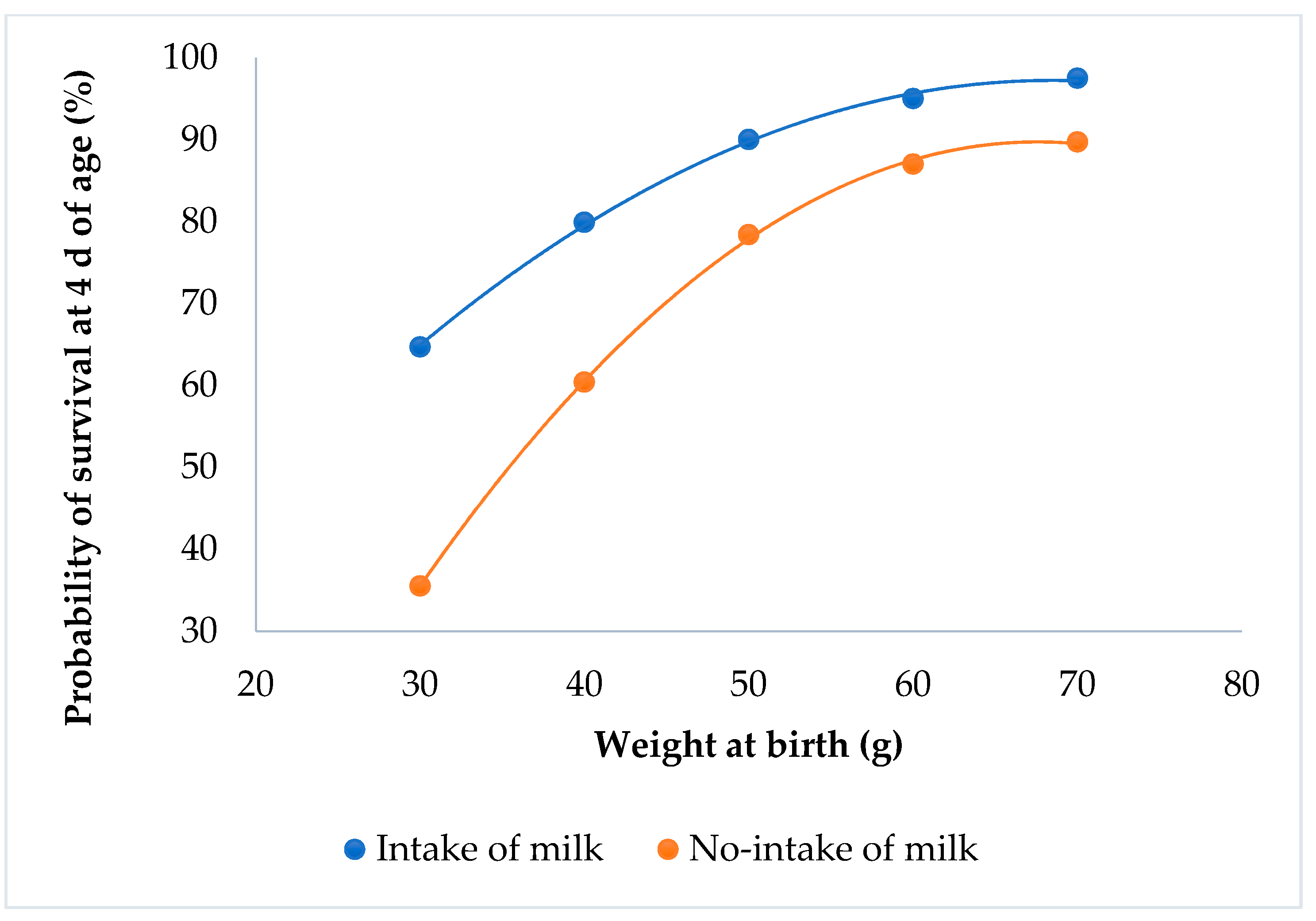
Kits that suckled always had a higher probability of survival than the kits that did not suckle (P < 0.0001; Figure 4). Kits with the minimum weight had a survival probability of 65% when the rabbits suckled, but only 35% if they did not suckle.
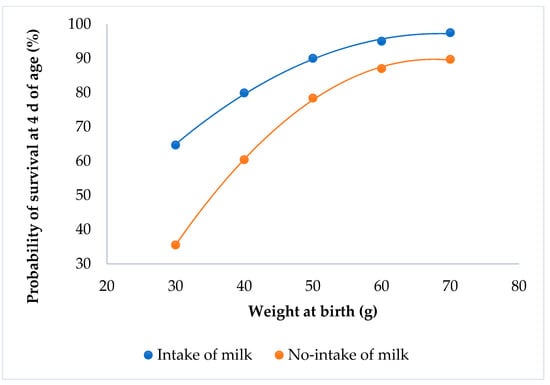
Figure 4.
Relationship between survival at 4 d of age and individual birth weight for the milk intake effect.
4. Discussion
4.1. Correlated Response to Selection in Litter Survival and Pre-Weaning Weight
Our divergent selection experiment for environmental sensitivity has shown that this trait is genetically determined [6]. This has implications for animal welfare, as animals that cope better with their environment have better welfare than the more sensitive animals [7]. After correcting for the litter size, both of the lines had similar individual weights at birth, and the survival rates at birth and the survival rates at four days of age were not modified. Moreover, the relationship between the probability of survival at 4 d of age and the weight at birth was not affected by the line.
Weight distance has been used as the dispersion measure, instead of the standard deviation of the weight of the litter, because it provides one record per individual instead of one per litter. It seems that there is a correlated response on both of the weight distances at birth and at weaning, but with opposite sign; the kits’ weight is more variable at birth in the low line, but then less variable at weaning. To date, there is no information available on the weight distance at birth in rabbits, but Peiró et al. have shown similar values of weight distance at weaning [15].
Maternal care in the first days after parturition is clearly related to the ingestion of energy by the kits, which is directly related to their survival [16]. So, the higher rate of survival at weaning of the low line could indicate higher milk production, and better maternal behavior during lactation. In spite of the greater variability of weight at birth of the low line, this line produces a greater uniformity of weight at weaning than the high line, perhaps due to a higher lactation capacity of the doe. The homogeneity in weight within the litter is an important trait in prolific species such as rabbits [17], because increasing the weight homogeneity within the litter reduces the competition between littermates, and increases the viability of them [18].
4.2. Survival at 4 d of Age and Individual Weight at Birth
The probability of individual survival at 4 d of age is related to birth weight, as the kits with lower birth weight have a lower probability of survival. Neonates require a protective environment, adequate nutrition, and special maternal care in order to survive [19]. So, the season of birth, the intake of milk, and the parity–lactation status of the doe all affect the likelihood of survival. The probability of survival at 4 d of age was lower in winter than in spring and summer, when the weight at birth was less than 50 g. If the birth weight is less than the optimum weight, the energy reserves and the thermoregulatory capacity are reduced, and the perinatal mortality increases [20]. If the temperature in the nest is low during their first five days of life, the instantaneous energy production capacity of the young rabbits is insufficient, being unable to compensate for thermal losses through the skin, and the probability of survival decreases [21].
The kits’ fat tissue is high at birth, and decreases thereafter [22]. The ingestion of milk immediately after birth allows the rabbit to save fat tissue, and thus significantly increase its chances of survival [23,24]. The lack of a milk spot at birth increases the mortality of the kits at 4 d of age, irrespective of their birth weight. Similar results were obtained at the first week of age [24,25].
When lactation and gestation were overlapping, the probability of survival was lower than in nulliparous and non-lactating does. It is well known that does undergo a nutritional deficit when lactation and pregnancy overlap [26,27], and that this deficit affects the probability of the kits’ survival.
5. Conclusions
The low line leads to a greater uniformity of kit weight at weaning than the high line, although the variability of weight at birth is higher, which could be due to a higher lactation capacity of the doe. In conclusion, selection for litter size variability shows a negative correlated response in the uniformity of weights at birth, and a positive correlated response in survival and the uniformity of weights at weaning, without affecting individual and litter weight.
Author Contributions
Conceptualization, M.-L.G., M.-J.A., A.B.; data curation, I.A.; formal analysis: M.-L.G., M.-J.A.; funding acquisition, A.B., M.-J.A.; methodology, I.A., M.-L.G., M.-J.A.; writing and editing, I.A., M.-L.G., A.B.
Funding
This study is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) with the Projects AGL2017- 86083, C2-1-P and C2-2-P.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- García, M.; Baselga, M. Estimation of genetic response to selection in litter size of rabbits using a cryopreserved control population. Livest. Prod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Sánchez, J.P.; Theilgaard, P.; Mínguez, C.; Baselga, M. Constitution and evaluation of a long-lived productive rabbit line. J. Anim. Sci. 2008, 86, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Larzul, C.; Ducrocq, V.; Tudela, F.; Juin, H.; Garreau, H. The length of productive life can be modified through selection: An experimental demonstration in the rabbit. J. Anim. Sci. 2014, 92, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Rosell, J.; De La Fuente, L. Culling and mortality in breeding rabbits. Prev. Vet. Med. 2009, 88, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.; Martínez-Álvaro, M.; García, M.L.; Ibáñez-Escriche, N.; Argente, M.J. Selection for genetic environmental sensitivity of litter size in rabbits. Genet. Sel. Evol. 2017, 49, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Argente, M.J.; García, M.L.; Zbyňovská, K.; Petruška, P.; Capcarová, M.; Blasco, A. Correlated response to selection for litter size environmental variability in rabbits’ resilience. Animal 2019. [Google Scholar] [CrossRef]
- Bolet, G.; Esparbié, J.; Falieres, J. Relations entre le nombre de foetus par corne utérine, la taille de portée à la naissance et la croissance pondérale des lapereaux. Ann. Zootech. 1996, 45, 185–200. [Google Scholar] [CrossRef]
- Poignier, J.; Szendrö, Z.S.; Levai, A.; Radnai, I.; Biro-Nemeth, E. Effect of birth weight and litter size on growth and mortality in rabbit. World Rabbit Sci. 2000, 8, 103–109. [Google Scholar] [CrossRef]
- Blasco, A.; Blasco, P.D.A. Bayesian Data Analysis for Animal Scientists; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Legarra, A.; Varona, L.; López de Maturana, E. TM Threshold Model. Available online: http://snp.toulouse.inra.fr/~alegarra/manualtm.pdf (accessed on 5 July 2019).
- Sorensen, D.; Gianola, D. Likelihood, Bayesian, and MCMC Methods. Quantitative Genetics, 1st ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Geyer, C.M. Practical markow chain Monte Carlo (with discussion). Stat. Sci. 1992, 7, 467–511. [Google Scholar]
- SAS. SAS/STAT User’s Guide 9.4; SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Peiró, R.; Badawy, A.Y.; Blasco, A.; Santacreu, M.A. Correlated responses on growth traits after two-stage selection for ovulation rate and litter size in rabbits. Animal 2019. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.J.; Savietto, D.; Cervera, C.; Baselga, M. Resources allocation in reproductive rabbit does: A review of feeding and genetic strategies for suitable performance. World Rabbit. Sci. 2013, 21, 123–144. [Google Scholar] [CrossRef]
- Bolet, G.; Garreau, H.; Joly, T.; Theau-Clément, M.; Falières, J.; Hurtaud, J.; Bodin, L. Genetic homogenisation of birth weight in rabbits: Indirect selection response for uterine horn characteristics. Livest. Sci. 2007, 111, 28–32. [Google Scholar] [CrossRef]
- Garreau, H.; Bolet, G.; Larzul, C.; Robert-Granié, C.; Saleil, G.; SanCristobal, M.; Bodin, L. Results of four generations of a canalising selection for rabbit birth weight. Livest. Sci. 2008, 119, 55–62. [Google Scholar] [CrossRef]
- Hamilton, H.H.; Lukefahr, S.D.; McNitt, J.I. Maternal nest quality and its influence on litter survival and weaning performance in commercial rabbits. J. Anim. Sci. 1997, 75, 926. [Google Scholar] [CrossRef]
- García-Ximénez, F.; Vicente, J.; Viudes-De-Castro, M. Neonatal performances in 3 lines of rabbit (litter sizes, litter and individual weights). Anim. Res. 1995, 44, 255–261. [Google Scholar]
- Hull, D.; Segall, M.M. The contribution of brown adipose tissue to heat production in the new-born rabbit. J. Physiol. 1965, 181, 449–457. [Google Scholar] [CrossRef]
- Spencer, S.A.; Hull, D. The effect of over-feeding newborn rabbits on somatic and visceral growth, body composition and long-term growth potential. Br. J. Nutr. 1984, 51, 389–402. [Google Scholar] [CrossRef]
- Venge, O. The influence of nursing behaviour and milk production n early growth in rabbits. Anim. Behav. 1963, 11, 500–506. [Google Scholar] [CrossRef]
- Schaal, B.; Coudert, P.; Rideaud, P.; Fortun-Lamothe, L.; Hudson, R.; Orgeur, P. Immediate postnatal sucking in the rabbit: Its influence on pup survival and growth. Reprod. Nutr. Dev. 2000, 40, 19–32. [Google Scholar]
- Argente, M.; Santacreu, M.; Climent, A.; Blasco, A. Phenotypic and genetic parameters of birth weight and weaning weight of rabbits born from unilaterally ovariectomized and intact does. Livest. Prod. Sci. 1999, 57, 159–167. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Sartori, A.; Queaque, P.I. Effect of parity order and litter weaning age on the performance and body energy balance of rabbit dos. Livest. Prod. Sci. 2004, 16, 239–251. [Google Scholar] [CrossRef]
- Rebollar, P.; Pérez-Cabal, M.; Pereda, N.; Lorenzo, P.L.; Arias-Álvarez, M.; García-Rebollar, P. Effects of parity order and reproductive management on the efficiency of rabbit productive systems. Livest. Sci. 2009, 121, 227–233. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).