The Alpha Hypothesis: Did Lateralized Cattle–Human Interactions Change the Script for Western Culture?
Abstract
:Simple Summary
Abstract
1. Introduction
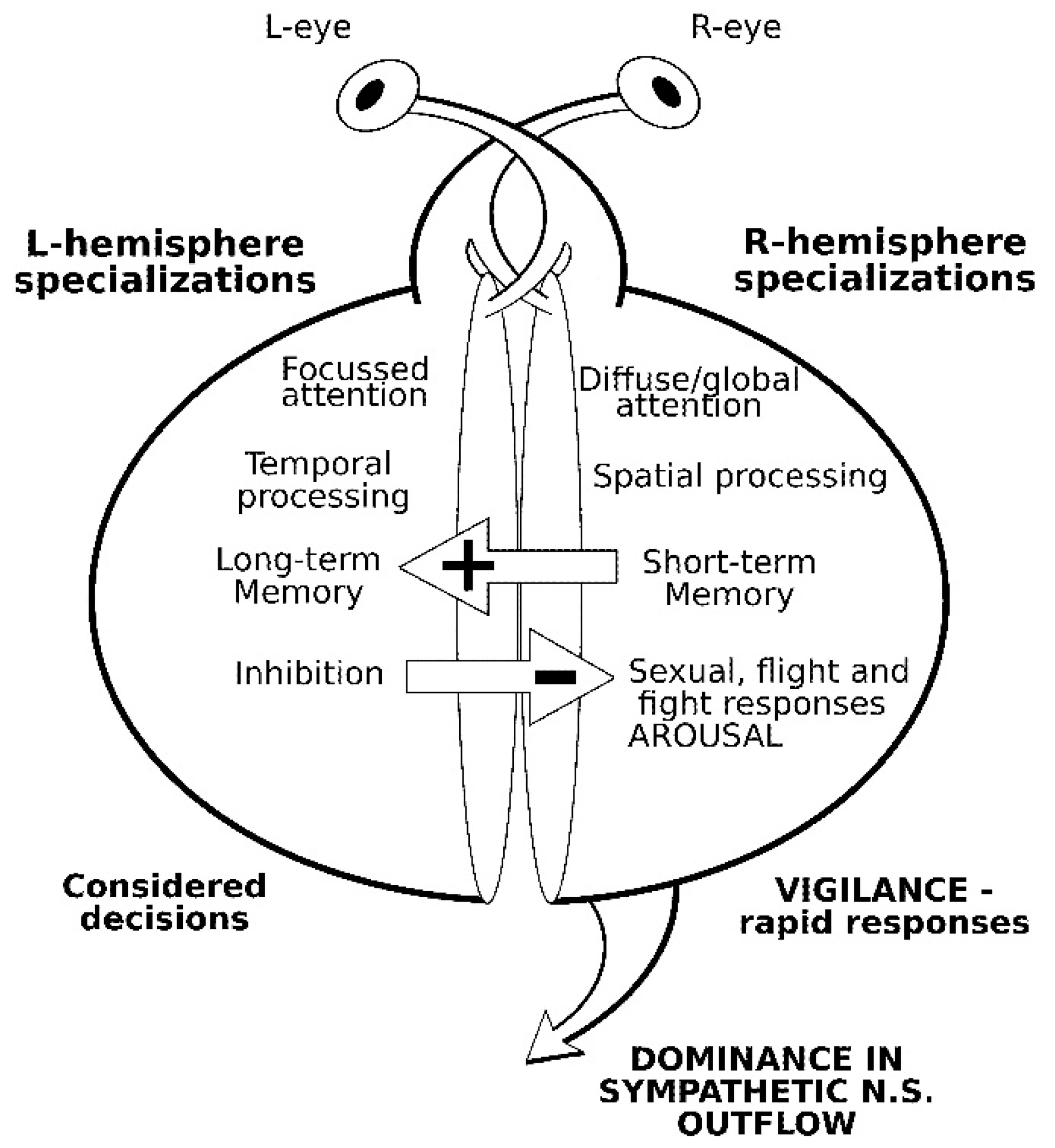
2. Comparative Vertebrate Lateralization
2.1. Social Facilitation Hypothesis and Domestication
2.2. Lateralized Visual Processing in Domestic Cattle
3. Cattle Domestication and Early Religious Symbolism
3.1. Cattle in Early Religion
4. Text and Context—Starting the Alphabetic Code
4.1. Egyptian Hieroglyphics (3500 BCE)
4.2. Semitic Inscriptions at Wadi el-Hol (1900–1850 BCE)
4.3. Inscriptions at Serabit el-Khadem (1850–1700 BCE)
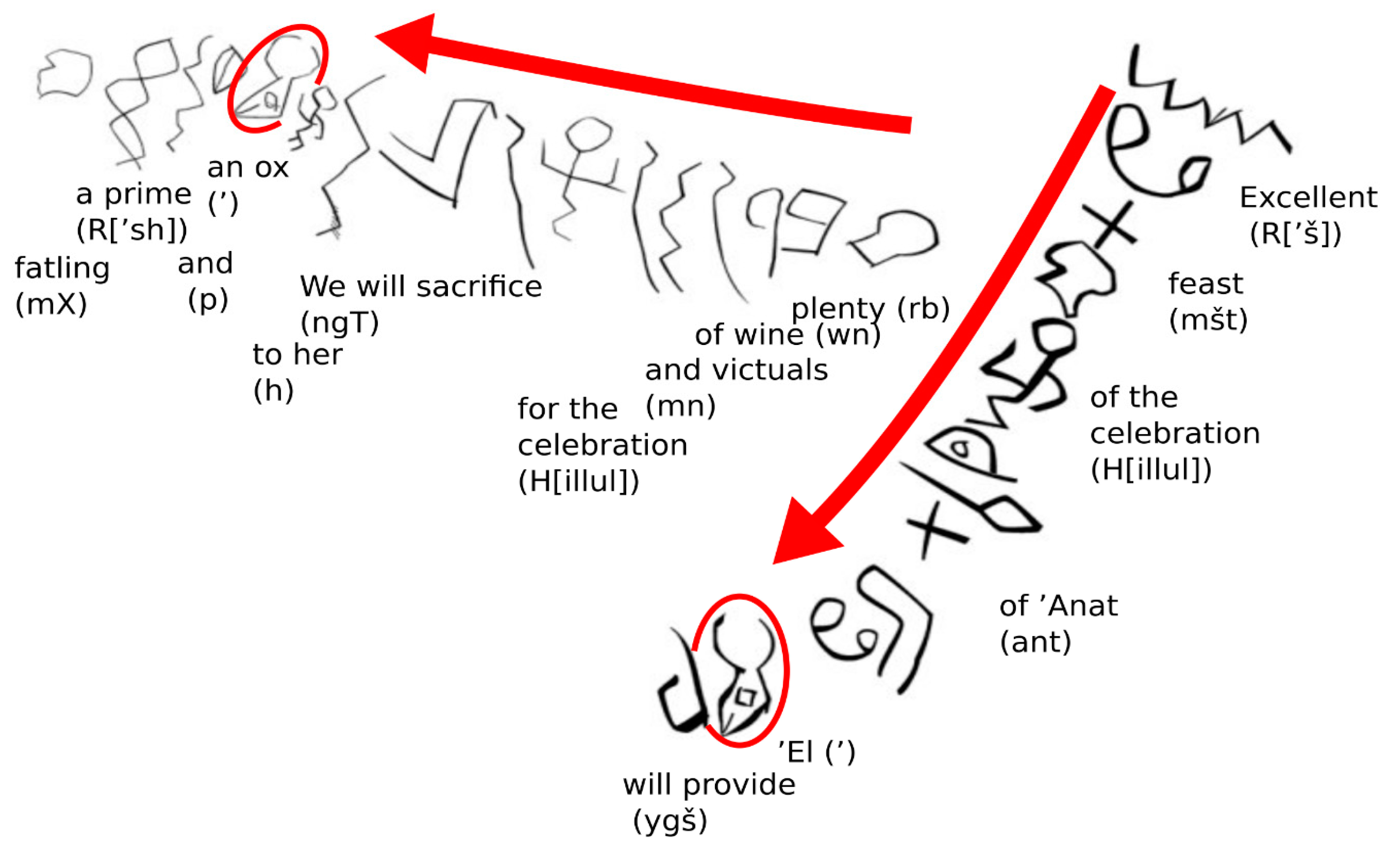
4.4. Proto-Canaanite Script ( >1150 BCE)
4.5. Phoenician Abjad (1150 BCE–150 CE)
4.6. Aramaic (800 BCE to 600 CE), and Child Scripts of Hebrew and Arabic
4.7. Greek Alphabet (>750 y BCE)
4.8. Etruscan Alphabet (700 BCE)
4.9. Roman/Latin Script (600 BCE)
4.10. Roman/Latin Minuscule Development
5. Discussion
5.1. Conservation of the ‘Alpha’ Position
5.2. Early Religious Significance
5.3. The Lateralization of Domestication
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogers, L.J. Lateralization in vertebrates: Its early evolution, general pattern, and development. In Advances in the Study of Behavior; Slater, P.J.B., Rosenblatt, J.S., Snowdon, C.T., Roper, T.J., Eds.; Academic Press: San Diego, CA, USA, 2002; Volume 31, pp. 107–161. [Google Scholar]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Ghirlanda, S.; Vallortigara, G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proc. R. Soc. B Biol. Sci. 2004, 271, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left–right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Ocklenburg, S. Ontogenesis of Lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, MA, USA, 2013; ISBN 978-1-107-00535-8. [Google Scholar]
- Karenina, K.; Giljov, A.; Ingram, J.; Rowntree, V.J.; Malashichev, Y. Lateralization of mother–infant interactions in a diverse range of mammal species. Nat. Ecol. Evol. 2017, 1, 0030. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.; Phillips, C.J. Visual preferences in cow-calf dyads: Lateralised vigilance and maternal bonding in domestic cattle. In Proceedings of the 37th Annual Conference of the Australasian Society for the Study of Animal Behaviour (ASSAB), Narrabri, NSW, Australia, 6–10 April 2010. [Google Scholar]
- Freund, N.; Valencia-Alfonso, C.E.; Kirsch, J.; Brodmann, K.; Manns, M.; Güntürkün, O. Asymmetric top-down modulation of ascending visual pathways in pigeons. Neuropsychologia 2016, 83, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Duboué, E.R.; Hong, E.; Eldred, K.C.; Halpern, M.E. Left Habenular Activity Attenuates Fear Responses in Larval Zebrafish. Curr. Biol. 2017, 27, 2154–2162.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggs, D.J.; Miller, P.E.; Ofri, R. Slatter’s Fundamentals of Veterinary Ophthalmology, 4th ed.; Saunders Elsevier: St. Louis, MO, USA, 2008; p. 9. ISBN 978-0-7216-0561-6. [Google Scholar]
- Gibbs, M.E.; Andrew, R.J.; Ng, K.T. Hemispheric lateralization of memory stages for discriminated avoidance learning in the chick. Behav. Brain Res. 2003, 139, 157–165. [Google Scholar] [CrossRef]
- Robins, A.; Chen, P.; Beazley, L.D.; Dunlop, S.A. Lateralized predatory responses in the ornate dragon lizard (Ctenophorus ornatus). Neuroreport 2005, 16, 849–852. [Google Scholar] [CrossRef]
- Robins, A.; Rogers, L.J. Complementary and lateralized forms of processing in Bufo marinus for novel and familiar prey. Neurobiol. Learn. Mem. 2006, 86, 214–227. [Google Scholar] [CrossRef]
- Robins, A.; Phillips, C. Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Later. Asymmetries Body Brain Cogn. 2010, 15, 514–534. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.J.; Friedman, B.H. Autonomic responses to lateralized cold pressor and facial cooling tasks. Psychophysiology 2015, 52, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Wittling, W. Brain asymmetry and autonomic control of the heart. Eur. Psychol. 1997, 2, 313–327. [Google Scholar] [CrossRef]
- Andrew, R.J. Neural and Behavioural Plasticity: The Use of the Domestic Chick as a Model; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Andrew, R.J. Left and right hemisphere memory traces: Their formation and fate. Evidence from events during memory formation in the chick. Later. Asymmetries Body Brain Cogn. 1997, 2, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Cantalupo, C.; Capocchiano, M.; Vallortigara, G. Population lateralisation and social behaviour: A study with 16 species of fish. Later. Asymmetries Body Brain Cogn. 2000, 5, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Evolution of hemispheric specialization: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. The evolutionary psychology of left and right: Costs and benefits of lateralization. Dev. Psychobiol. 2006, 48, 418–427. [Google Scholar] [CrossRef]
- Zeder, M.A. Pathways to animal domestication. In Biodiversity in Agriculture: Domestication, Evolution, and Sustainability; Gepts, P., Famula, T.R., Bettinger, R.L., Brush, S.B., Damania, A.B., McGuire, P.E., Qualset, C.O., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 227–259. [Google Scholar]
- Ghirlanda, S.; Frasnelli, E.; Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 861–866. [Google Scholar] [CrossRef]
- Frasnelli, E.; Vallortigara, G. Individual-level and population-level lateralization: Two sides of the same coin. Symmetry 2018, 10, 739. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species; Routledge: London, UK, 1859; ISBN 978-1-134-43992-8. [Google Scholar]
- Darwin, C. The Variations of Plants and Animals under Domestication; John Murray: London, UK, 1868; Volume 2. [Google Scholar]
- Daisley, J.N.; Vallortigara, G.; Regolin, L. Logic in an asymmetrical (social) brain: Transitive inference in the young domestic chick. Soc. Neurosci. 2010, 5, 309–319. [Google Scholar] [CrossRef]
- Robins, A.; Goma, A.A.; Ouine, L.; Phillips, C.J.C. The eyes have it: Lateralized coping strategies in cattle herds responding to human approach. Anim. Cogn. 2018, 21, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Andrew, R. Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-1-139-43747-9. [Google Scholar]
- Farmer, K.; Krueger, K.; Byrne, R.W. Visual laterality in the domestic horse (Equus caballus) interacting with humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.J.C.; Oevermans, H.; Syrett, K.L.; Jespersen, A.Y.; Pearce, G.P. Lateralization of behavior in dairy cows in response to conspecifics and novel persons. J. Dairy Sci. 2015, 98, 2389–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goma, A.A.; Pearce, P.G.; Uddin, J.; Rimon, E.; Davies, H.; Phillips, C.J.C. A forced lateralisation test for dairy cows and its relation to their behaviour. Appl. Anim. Behav. Sci. 2018, 207, 8–19. [Google Scholar]
- Rizhova, L.Y.; Kokorina, E.P. Behavioural asymmetry is involved in regulation of autonomic processes: Left side presentation of food improves reproduction and lactation in cows. Behav. Brain Res. 2005, 161, 75–81. [Google Scholar] [CrossRef]
- Kappel, S.; Mendl, M.T.; Barrett, D.C.; Murrell, J.C.; Whay, H.R. Lateralized behaviour as indicator of affective state in dairy cows. PLoS ONE 2017, 12, e0184933. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Haselton, M.G.; Nettle, D. The paranoid optimist: An integrative evolutionary model of cognitive biases. Personal. Soc. Psychol. Rev. 2006, 10, 47–66. [Google Scholar] [CrossRef]
- Van Vuure, T. History, morphology and ecology of the Aurochs (Bos primigenius). Lutra 2002, 45, 1–16. [Google Scholar]
- Zhang, H.; Paijmans, J.L.A.; Chang, F.; Wu, X.; Chen, G.; Lei, C.; Yang, X.; Wei, Z.; Bradley, D.G.; Orlando, L.; et al. Morphological and genetic evidence for early Holocene cattle management in northeastern China. Nat. Commun. 2013, 4, 2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramod, R.K.; Velayutham, D.; Sajesh, P.K.; Beena, P.S.; Zachariah, A.; Zachariah, A.; Chandramohan, B.; Sujith, S.S.; Ganapathi, P.; Kumar, B.D.; et al. Complete mitogenome reveals genetic divergence and phylogenetic relationships among Indian cattle (Bos indicus) breeds. Anim. Biotechnol. 2019, 30, 219–232. [Google Scholar] [CrossRef]
- Ajmone-Marsan, P.; Garcia, J.F.; Lenstra, J.A. On the origin of cattle: How aurochs became cattle and colonized the world. Evol. Anthropol. Issues News Rev. 2010, 19, 148–157. [Google Scholar] [CrossRef]
- Beja-Pereira, A.; Caramelli, D.; Lalueza-Fox, C.; Vernesi, C.; Ferrand, N.; Casoli, A.; Goyache, F.; Royo, L.J.; Conti, S.; Lari, M.; et al. The origin of European cattle: Evidence from modern and ancient DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 8113–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götherström, A.; Anderung, C.; Hellborg, L.; Elburg, R.; Smith, C.; Bradley, D.G.; Ellegren, H. Cattle domestication in the Near East was followed by hybridization with aurochs bulls in Europe. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 2345–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, L. The first aurochs genome reveals the breeding history of British and European cattle. Genome Biol. 2015, 16, 225–228. [Google Scholar] [CrossRef]
- Upadhyay, M.R.; Chen, W.; Lenstra, J.A.; Goderie, C.R.J.; MacHugh, D.E.; Park, S.D.E.; Magee, D.A.; Matassino, D.; Ciani, F.; Megens, H.-J.; et al. Genetic origin, admixture and population history of aurochs (Bosprimigenius) and primitive European cattle. Heredity 2017, 118, 169–176. [Google Scholar] [CrossRef]
- Wright, E.; Viner-Daniels, S. Geographical variation in the size and shape of the European aurochs (Bos primigenius). J. Archaeol. Sci. 2015, 54, 8–22. [Google Scholar] [CrossRef]
- Hristov, P.; Spassov, N.; Iliev, N.; Radoslavov, G. An independent event of Neolithic cattle domestication on the South-eastern Balkans: Evidence from prehistoric aurochs and cattle populations. Mitochondrial DNA Part A 2017, 28, 383–391. [Google Scholar] [CrossRef]
- Meier, J.S.; Goring-Morris, A.N.; Munro, N.D. Aurochs bone deposits at Kfar HaHoresh and the southern Levant across the agricultural transition. Antiquity 2017, 91, 1469–1483. [Google Scholar] [CrossRef]
- Goring-Morris, A.N. Life, death and the emergence of differential status in the Near Eastern Neolithic: Evidence from Kfar HaHoresh, Lower Galilee, Israel. In Archaeological Perspectives on the Transmission and Transformation of Culture in the Eastern Mediterranean; Levant Supplementary Series; Council for British Research in the Levant and Oxbow Books: London, UK, 2005; Volume 2, pp. 89–105. [Google Scholar]
- Twiss, K.C.; Russell, N. Taking the bull by the horns: Ideology, masculinity, and cattle horns at Çatalhöyük (Turkey). Paléorient 2009, 35, 19–32. [Google Scholar] [CrossRef]
- Isaac, E. On the domestication of cattle: Zoology and cultural history both illuminate the view that the original motive was religious, not economic. Science 1962, 137, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D. The Oxford Companion to World Mythology; Oxford University Press: New York, NY, USA, 2005; ISBN 978-0-19-515669-0. [Google Scholar]
- Smith, M.S. The Origins of Biblical Monotheism: Israel’s Polytheistic Background and the Ugaritic Texts; Oxford University Press: New York, NY, USA, 2001; ISBN 978-0-19-513480-3. [Google Scholar]
- Hart, G. Routledge Dictionary of Egyptian Gods and Goddesses.; Taylor & Francis: Hoboken, NI, USA, 2005; ISBN 978-0-203-02362-4. [Google Scholar]
- Monaghan, P. Goddesses in World Culture; Praeger: Santa Barbara, CA, USA, 2010; ISBN 978-0-313-35465-6. [Google Scholar]
- Ellis, N. Sekhmet, Bast, and Hathor: Power, passion, and transformation through the Egyptian goddess Trinity. In Goddesses in World Culture; Praeger: Santa Barbara, CA, USA, 2010; ISBN 978-0-313-35465-6. [Google Scholar]
- Daniels, P.T. The first civilizations. In The World’s Writing Systems; Oxford University Press: New York, NY, USA, 1996; pp. 21–32. [Google Scholar]
- Daniels, P.T. Scripts of semitic languages. In The Semitic Languages; Routledge: New York, NY, USA, 2013; pp. 16–45. [Google Scholar]
- Daniels, P.T. The World’s Writing Systems; Oxford University Press: New York, NY, USA, 1996; ISBN 978-0-19-507993-7. [Google Scholar]
- Ritner, R.K. Egyptian writing. In The World’s Writing Systems; Oxford University Press: New York, NY, USA, 1996; pp. 73–87. [Google Scholar]
- Robinson, A. Writing systems. In The Book: A Global History; Oxford University Press: Oxford, UK, 2013; pp. 3–18. [Google Scholar]
- Darnell, J.C.; Dobbs-Allsopp, F.W.; Lundberg, M.J.; McCarter, P.K.; Zuckerman, B.; Manassa, C. Two early alphabetic inscriptions from the Wadi el-Ḥôl: New evidence for the origin of the alphabet from the Western Desert of Egypt. Annu. Am. Sch. Orient. Res. 2005, 5, 64–124. [Google Scholar]
- Darnell, J.C. Wadi el-Hol. UCLA Encycl. Egyptol. 2013, 1, 1–19. [Google Scholar]
- Colles, B.E. Proto–Alphabetic inscriptions from the Wadi Arabah. Antig. Oriente 2010, 8, 75–96. [Google Scholar]
- Simons, F. Proto-Sinaitic–progenitor of the alphabet. Rosetta 2011, 9, 16–40. [Google Scholar]
- Kaufman, S.A. The pitfalls of typology: On the early history of the alphabet. Hebr. Union Coll. Annu. 1986, 57, 1–14. [Google Scholar]
- Millard, A.R. The infancy of the alphabet. World Archaeol. 1986, 17, 390–398. [Google Scholar] [CrossRef]
- Coogan, M.D. Alphabets and elements. Bull. Am. Sch. Orient. Res. 1974, 216, 61–63. [Google Scholar] [CrossRef]
- Stieglitz, R.R. The Ugaritic cuneiform and Canaanite linear alphabets. J. East. Stud. 1971, 30, 135–139. [Google Scholar] [CrossRef]
- O’Connor, M. Epigraphic Semitic scripts. In The World’s Writing Systems; Oxford University Press: New York, NY, USA, 1996; pp. 88–107. [Google Scholar]
- Naveh, J. Some semitic epigraphical considerations on the antiquity of the Greek alphabet. Am. J. Archaeol. 1973, 77, 1–8. [Google Scholar] [CrossRef]
- Threatte, L. The Greek alphabet. In The World’s Writing Systems; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Luraghi, N. The local scripts from nature to culture. Class. Antiq. 2010, 29, 68–91. [Google Scholar] [CrossRef]
- Chrisomalis, S. The Egyptian origin of the Greek alphabetic numerals. Antiq. Camb. 2003, 77, 485–496. [Google Scholar] [CrossRef]
- Pande, N.A. Numeral systems of great ancient human civilizations. J. Sci. Arts 2010, 2, 209. [Google Scholar]
- Cauvin, J. The symbolic foundations of the Neolithic Revolution in the Near East. In Life in Neolithic Farming Communities; Springer: New York, NY, USA, 2002; pp. 235–252. [Google Scholar]
- Ibáñez, J.J.; González-Urquijo, J.; Teira-Mayolini, L.C.; Lazuén, T. The emergence of the Neolithic in the Near East: A protracted and multi-regional model. Quat. Int. 2018, 470, 226–252. [Google Scholar]
- Zeder, M.A. The neolithic macro-(R)evolution: Macroevolutionary theory and the study of culture change. J. Archaeol. Res. 2009, 17, 1–63. [Google Scholar]
- Zeder, M.A. Evolutionary biology and the emergence of agriculture: The value of co-opted models of evolution in the study of culture change. In Macroevolution in Human Prehistory: Evolutionary Theory and Processual Archaeology; Prentiss, A., Kuijt, I., Chatters, J.C., Eds.; Springer: New York, NY, USA, 2009; pp. 157–210. ISBN 978-1-4419-0682-3. [Google Scholar]
- Cross, F.M. Canaanite Myth and Hebrew Epic: Essays in the History of the Religion of Israel; Harvard University Press: Cambridge, MA, USA, 2009; ISBN 978-0-674-03008-4. [Google Scholar]
- Benner, J.A. The Ancient Hebrew lexicon of the Bible; Virtualbookworm.com Publishing Inc.: College Station, TX, USA, 2005; ISBN 1-58939-776-2. [Google Scholar]
- Siniscalchi, M.; D’Ingeo, S.; Quaranta, A. Lateralized functions in the dog brain. Symmetry 2017, 9, 71. [Google Scholar] [CrossRef]
- Fabre-Thorpe, M.; Fagot, J.; Lorincz, E.; Levesque, F.; Vauclair, J. Laterality in cats: Paw preference and performance in a visuomotor activity. Cortex 1993, 29, 15–24. [Google Scholar] [CrossRef]
- Wells, D.L.; Millsopp, S. Lateralized behaviour in the domestic cat, Felis silvestris catus. Anim. Behav. 2009, 78, 537–541. [Google Scholar] [CrossRef]
- McDowell, L.J.; Wells, D.L.; Hepper, P.G. Lateralization of spontaneous behaviours in the domestic cat, Felis silvestris. Anim. Behav. 2018, 135, 37–43. [Google Scholar] [CrossRef]
- Camerlink, I.; Menneson, S.; Turner, S.P.; Farish, M.; Arnott, G. Lateralization influences contest behaviour in domestic pigs. Sci. Rep. 2018, 8, 12116. [Google Scholar] [CrossRef] [PubMed]
- Espmark, Y.; Kinderås, K. Behavioural lateralisation in reindeer. Rangifer 2002, 22, 51–59. [Google Scholar] [CrossRef]
- Anderson, D.M.; Murray, L.W. Sheep laterality. Laterality Asymmetries Body Brain Cogn. 2013, 18, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, S.; Matthews, L.; Messori, S.; Podaliri-Vulpiani, M.; Ferri, N. Laterality as an indicator of emotional stress in ewes and lambs during a separation test. Anim. Cogn. 2016, 19, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Versace, E.; Morgante, M.; Pulina, G.; Vallortigara, G. Behavioural lateralization in sheep (Ovis aries). Behav. Brain Res. 2007, 184, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Phillips, C. A note on behavioural laterality in neonatal lambs. Appl. Anim. Behav. Sci. 2004, 86, 161–167. [Google Scholar] [CrossRef]
- Nawroth, C.; Albuquerque, N.; Savalli, C.; Single, M.-S.; McElligott, A.G. Goats prefer positive human emotional facial expressions. R. Soc. Open Sci. 2018, 5, 180491. [Google Scholar] [CrossRef] [Green Version]
- Austin, N.P.; Rogers, P.L.J. Asymmetry of flight and escape turning responses in horses. Lateral. Asymmetries Body Brain Cogn. 2007, 12, 464–474. [Google Scholar] [CrossRef]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; Scott, S.K.; McComb, K. Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalisations. Sci. Rep. 2018, 8, 13052. [Google Scholar] [CrossRef] [PubMed]
- Hamp, E. A rule of the road. Gen. Linguist. Univ. Park Pa 2001, 41, 147–158. [Google Scholar]
- Phillips, C. Cattle Behaviour and Welfare; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-4743-9. [Google Scholar]
- Sullivan, R.M.; Gratton, A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999, 19, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C. The concept of allostasis: Coping with a capricious environment. J. Mammal. 2005, 86, 248–254. [Google Scholar] [CrossRef]
- Evans, C.S.; Evans, L.; Marler, P. On the meaning of alarm calls: Functional reference in an avian vocal system. Anim. Behav. 1993, 46, 23–38. [Google Scholar] [CrossRef]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 2005, 162, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Revell, D.; Maynard, B.; Erkelenz, P.; Thomas, D. ‘Rangeland Self Herding’-positively influencing grazing distribution to benefit livestock, landscapes and people. In Proceedings of the 18th Australian Rangeland Society Biennial Conference, Australian Rangeland Society, Parkside, South Australia, 12–16 April 2015; p. 3. [Google Scholar]
- Cerqueira, J.J.; Almeida, O.F.X.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain. Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Meador, K.J.; Loring, D.W.; Ray, P.G.; Helman, S.W.; Vazquez, B.R.; Neveu, P.J. Role of cerebral lateralization in control of immune processes in humans. Ann. Neurol. 2004, 55, 840–844. [Google Scholar] [CrossRef]
- Neveu, P.J. Cerebral lateralization and the immune system. Int. Rev. Neurobiol. 2002, 52, 303–323. [Google Scholar]
- Stoyanov, Z.; Decheva, L.; Pashalieva, I.; Nikolova, P. Brain asymmetry, immunity, handedness. Open Med. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Sumner, R.C.; Parton, A.; Nowicky, A.V.; Kishore, U.; Gidron, Y. Hemispheric lateralisation and immune function: A systematic review of human research. J. Neuroimmunol. 2011, 240–241, 1–12. [Google Scholar] [CrossRef]

 | Right Eye/Left hemisphere “Off Side” |  | Left Eye/Right hemisphere “Near Side” |
| Rapid familiarization (habituation/learning) [15,32] |  | Novelty detection [15,32] | |
| Cohort * of cattle initiate flight in response to novelty [29] Variable responses [29] | Suppresses right hemisphere reactions including flight Permits close approach by humans Predictable responses [29,31] | ||
| Most cows transition from left to right eye preference when passing a novel human repeatedly over multiple trials, indicating familiarization [32,33] | Cohort * persistently choosing to pass a novel human within their left visual field are comparatively more anxious on a range of other measures, although produce significantly higher milk yield than their counterparts choosing the right visual field [32,33] | ||
Increased production associated with left-side feeding of high-quality ration
| |||
| Most cattle tested without interrupting their approach from distance subsequently investigate static, novel stimuli (checkerboard, balloon) on the right and not left side when presented simultaneously on both left and right visual field [35] | Cohort * of cattle hesitant in approach subsequently choose to investigate static, novel yellow balloon on the left and not right side when presented simultaneously [35] | ||
| More likely to dominate agonistic social encounters [32] | More likely to submit in agonistic social encounters [32] | ||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robins, A. The Alpha Hypothesis: Did Lateralized Cattle–Human Interactions Change the Script for Western Culture? Animals 2019, 9, 638. https://doi.org/10.3390/ani9090638
Robins A. The Alpha Hypothesis: Did Lateralized Cattle–Human Interactions Change the Script for Western Culture? Animals. 2019; 9(9):638. https://doi.org/10.3390/ani9090638
Chicago/Turabian StyleRobins, Andrew. 2019. "The Alpha Hypothesis: Did Lateralized Cattle–Human Interactions Change the Script for Western Culture?" Animals 9, no. 9: 638. https://doi.org/10.3390/ani9090638
APA StyleRobins, A. (2019). The Alpha Hypothesis: Did Lateralized Cattle–Human Interactions Change the Script for Western Culture? Animals, 9(9), 638. https://doi.org/10.3390/ani9090638





