Atmospheric pCO2 Reconstruction of Early Cretaceous Terrestrial Deposits in Texas and Oklahoma Using Pedogenic Carbonate and Occluded Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geologic Setting
2.2. Laboratory Methods
2.3. Estimating Atmospheric CO2
3. Results
3.1. Pedogenic Carbonate
3.2. Organic Matter from Acid-Treated Residues
3.3. Mean Annual Precipitation Estimates
3.4. Soil-Respired CO2 Estimates
3.5. Atmospheric pCO2 Estimates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludvigson, G.A.; Joeckel, R.M.; Gonzalez, L.; Gulbranson, E.L.; Rasbury, T.; Hunt, G.J.; Kirkland, J.I.; Madsen, S. Correlation of Aptian-Albian Carbon Isotope Excursions in Continental Strata of the Cretaceous Foreland Basin, Eastern Utah, U.S.A. J. Sediment. Res. 2010, 80, 955–974. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Faure, M.; Wang, Q. Climatic and environmental indications of carbon and oxygen isotopes from the Lower Cretaceous calcrete and lacustrine carbonates in Southeast and Northwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 171–189. [Google Scholar] [CrossRef]
- Li, X.; Jenkyns, H.C.; Zhang, C.; Wang, Y.; Liu, L.; Cao, K. Carbon isotope signatures of pedogenic carbonates from SE China: Rapid atmospheric pCO2 changes during middle–late Early Cretaceous time. Geol. Mag. 2013, 151, 830–849. [Google Scholar] [CrossRef]
- Ludvigson, G.; Joeckel, R.; Murphy, L.; Stockli, D.; González, L.; Suarez, C.; Kirkland, J.; Al Suwaidi, A. The emerging terrestrial record of Aptian-Albian global change. Cretac. Res. 2015, 56, 1–24. [Google Scholar] [CrossRef]
- Harper, D.T.; Suarez, M.B.; Uglesich, J.; You, H.; Li, D.; Dodson, P. Aptian-Albian clumped isotopes from northwest China: Cool temperatures, variable atmospheric pCO2 and regional shifts in the hydrologic cycle. Clim. Past 2021, 17, 1607–1625. [Google Scholar] [CrossRef]
- Jacobs, L.L.; Winkler, D.A. Mammals, archosaurs, and the Early to Late Cretaceous transition in north-central Texas. In Advances in Vertebrate Paleontology and Geochronology; Tomida, Y., Flynn, L.J., Jacobs, L.L., Eds.; National Science Museum: Tokyo, Japan, 1988; pp. 253–280. [Google Scholar]
- Young, K. Comanche series (Cretaceous), south central Texas. In Comanchean (Lower Cretaceous) Stratigraphy and Paleontology of Texas; Permian Basin Section, Society of Economic Paleontologists and Mineralogists: Midland, TX, USA, 1967; Volume 67, pp. 9–29. [Google Scholar]
- Scott, C. Cephalopods from the Cretaceous Trinity Group of the south-central United States. Univ. Tex. Bull. 1940, 3945, 969–1125. [Google Scholar]
- Young, K. Lower Albian and Aptian (Cretaceous) ammonites of Texas. Geosci. Man. 1974, 8, 175–228. [Google Scholar]
- Young, K. Cretaceous, marine inundations of the San Marcos Platform, Texas. Cretac. Res. 1986, 7, 117–140. [Google Scholar] [CrossRef]
- Harland, B.W.; Cox, V.A.; Llevellyn, G.P.; Pickton, G.C.A.; Smith, G.A.; Walters, R. A Geologic Time Scale; Cambridge University Press: Cambridge, UK, 1982; p. 131. [Google Scholar]
- Winkler, D.A.; Jacobs, L.L.; Branch, J.R.; Murry, P.A. The Proctor Lake dinosaur locality, Lower Cretaceous of Texas. Hunteria 1988, 2, 1–8. [Google Scholar]
- Hall, W.; Geology, B.O.E. Hydrogeologic Significance of Depositional Systems and Facies in Lower Cretaceous Sandstones, North-Central Texas; The University of Texas at Austin: Austin, TX, USA, 1976. [Google Scholar] [CrossRef]
- Andrzejewski, A.K.; Winkler, A.D.; Jacobs, L.L. A new basal ornithopod (Dinosauria: Ornithischia) from the Early Cretaceous of Texas. PLoS ONE 2019, 14, e0207935. [Google Scholar]
- Winkler, D.A.; Murry, P.A. Paleoecology and hypsilophodontid behavior at the Proctor Lake dinosaur locality (Early Cretaceous), Texas. Geol. Soc. Am. Spec. Pap. 1989, 238, 55–62. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Tabor, N.J. Paleoenvironmental and paleoclimatic reconstruction of Cretaceous (Aptian-Cenomanian) terrestrial formations of Texas and Oklahoma using phyllosilicates. Palaeogeogr. Palaeoclim. Palaeoecol. 2020, 543, 109491. [Google Scholar] [CrossRef]
- Winkler, D.A.; Murry, P.A.; Jacobs, L.L. Early Cretaceous (Comanchean) vertebrates of central Texas. J. Vertebr. Paléontol. 1990, 10, 95–116. [Google Scholar] [CrossRef]
- Winkler, D.A.; Rose, P.J. Paleoenvironment at Jones Ranch, an early Cretaceous sauropod quarry in Texas, USA. J. Paleontol. Soc. Korea 2006, 22, 77–89. [Google Scholar]
- Klappa, C.F. Rhizoliths in terrestrial carbonates: Classification, recognition, genesis and significance. Sedimentology 1980, 27, 613–629. [Google Scholar] [CrossRef]
- Hobday, D.K.; Woodruff, C.M.; McBride, M.W.; Ethridge, F.G.; Flores, R.M. Paleotopographic and Structural Controls on Non-Marine Sedimentation of the Lower Cretaceous Antlers Formation and Correlatives, North Texas and Southeastern Oklahoma; University of Texas: Austin, TX, USA, 1981; pp. 71–87. [Google Scholar] [CrossRef]
- Nydam, R.L.; Cifelli, R.L. Lizards from the Lower Cretaceous (Aptian–Albian) Antlers and Cloverly Formations. J. Vertebr. Paléontol. 2002, 22, 286–298. [Google Scholar] [CrossRef]
- Cifelli, R.L.; Gardner, J.D.; Nydam, R.L.; Brinkman, D.L. Additions to the vertebrate fauna of the Antlers Formation (Lower Cretaceous), southeastern Oklahoma. Okla. Geol. Notes 1997, 57, 124–131. [Google Scholar]
- Rennison, C.J. The Stable Carbon Isotope Record Derived from Mid-Cretaceous Terrestrial Plant Fossils form North-Central Texas. Master’s Thesis, Southern Methodist University, Dallas, TX, USA, 1996; 120p. [Google Scholar]
- Cerling, E.T.; Quade, J. Stable carbon and oxygen isotopes in soil carbonates. In Climate Change in Continental Isotopic Records; Swart, P.K., Lohman, K.C., McKenzie, J., Savin, S., Eds.; American Geophyscial Union, Geophyscial Monograph: Washington, DC, USA, 1993; Volume 78, pp. 217–231. [Google Scholar]
- Ekart, D.D.; Cerling, T.E.; Montañez, I.P.; Tabor, N.J. A 400 million year carbon isotope record of pedogenic carbonate: Implications for paleoatmospheric carbon dioxide. Am. J. Sci. 1999, 299, 805–827. [Google Scholar] [CrossRef]
- Boutton, T.W. Stable carbon isotope ratios of natural materials: 1. In Sample Preparation and Mass Spectrometric Analysis; Academic Press, Inc.: San Diego, CA, USA, 1991; pp. 155–171. [Google Scholar]
- Craig, H. Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim. Cosmochim. Acta 1957, 12, 133–149. [Google Scholar] [CrossRef]
- Gofiantini, R. Rep. to the Director General, Proceedings of the Advisory Group Meeting on Stable Isotope Reference Samples for Geochemical and Hydrological Investigations, Viena, Austria, 19–21 September 1983; International Atomic Energy Agency: Vienna, Austria, 1984. [Google Scholar]
- Yapp, C.J.; Poths, H. Carbon isotopes in continental weathering environments and variations in ancient atmospheric CO2 pressure. Earth Planet. Sci. Lett. 1996, 137, 71–82. [Google Scholar] [CrossRef]
- Lee, Y.I. Stable isotopic composition of calcic paleosols of the Early Cretaceous Hasandong Formation, southeastern Korea. Palaeogeogr. Palaeoclim. Palaeoecol. 1999, 150, 123–133. [Google Scholar] [CrossRef]
- Lee, Y.I.; Hisada, K.-I. Stable isotopic composition of pedogenic carbonates of the Early Cretaceous Shimonoseki Subgroup, western Honshu, Japan. Palaeogeogr. Palaeoclim. Palaeoecol. 1999, 153, 127–138. [Google Scholar] [CrossRef]
- Robinson, S.A.; Andrews, J.E.; Hesselbo, S.P.; Radley, J.D.; Dennis, P.F.; Harding, I.C.; Allen, P. Atmospheric pCO2 and depositional environment from stable-isotope geochemistry of calcrete nodules (Barremian, Lower Cretaceous, Wealden Beds, England). J. Geol. Soc. 2002, 159, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Leier, A.; Quade, J.; DeCelles, P.; Kapp, P. Stable isotopic results from paleosol carbonate in South Asia: Paleoenvironmental reconstructions and selective alteration. Earth Planet. Sci. Lett. 2009, 279, 242–254. [Google Scholar] [CrossRef]
- Cerling, T.; Solomon, D.K.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
- Yapp, J.C.; Poths, H. The carbon isotope geochemistry of goethite (α-FeOOH) in ironstone of the Upper Ordovician Neda Formation, Wisconsin, USA: Implications for early Paleozoic continental environments. Geochim. Cosmochim. Acta 1993, 57, 2599–2611. [Google Scholar] [CrossRef]
- Yapp, C.J. Mixing of CO2 in surficial environments as recorded by the concentration and δ13C values of the Fe(CO3)OH component in goethite. Geochim. Cosmochim. Acta 2001, 65, 4115–4130. [Google Scholar] [CrossRef]
- Tabor, J.N.; Montañez, I.P. Morphology and distribution of fossil soils in the Permo-Pennsylvanian Wichita and Bowie Groups, north-central Texas, USA: Implications for western equatorial Pangean palaeoclimate during the icehouse-greenhouse transition. Sedimentology 2004, 51, 851–884. [Google Scholar] [CrossRef]
- Myers, S.T.; Tabor, J.N.; Jacobs, L.L.; Bussert, R. Effects of different organic-matter sources on estimates of atmospheric and soil pCO2 using pedogenic carbonate. J. Sediment. Res. 2016, 86, 800–812. [Google Scholar] [CrossRef]
- Romanek, C.S.; Grossman, E.; Morse, J.W. Carbon isotopic fractionation in synthetic aragonite and calcite: Effects of temperature and precipitation rate. Geochim. Cosmochim. Acta 1992, 56, 419–430. [Google Scholar] [CrossRef]
- Cotton, J.M.; Sheldon, N.D. New constraints on using paleosols to reconstruct atmospheric pCO2. GSA Bull. 2012, 124, 1411–1423. [Google Scholar] [CrossRef] [Green Version]
- Nordt, L.; Driese, S. New weathering index improves paleorainfall estimates from Vertisols. Geology 2010, 38, 407–410. [Google Scholar] [CrossRef]
- Tabor, N.J.; Sidor, C.A.; Smith, R.M.H.; Nesbitt, S.J.; Angielczyk, K.D. Paleosols of the Permian-Triassic: Proxies for rainfall, climate change and major changes in terrestrial tetrapod diversity. J. Vertebr. Paléontol. 2017, 37, 240–253. [Google Scholar] [CrossRef]
- Koch, P.L. Isotopic Reconstruction of Past Continental Environments. Annu. Rev. Earth Planet. Sci. 1998, 26, 573–613. [Google Scholar] [CrossRef] [Green Version]
- Arens, N.C.; Jahren, A.H.; Amundson, R. Can C3 plants faithfully record the carbon isotopic composition of atmospheric carbon dioxide? Paleobiology 2000, 26, 137–164. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Nettleton, W.D.; Staidl, G.J. Soil polygenesis as a function of Quaternary climate change, northern Great Basin, USA. Geoderma 1995, 68, 1–26. [Google Scholar] [CrossRef]
- Haworth, M.; Hesselbo, S.P.; McElwain, J.C.; Robinson, S.A.; Brunt, J.W. Mid-Cretaceous pCO2 based on stomata of the extinct conifer Psedofrenelopsis (Cheirolepidiaceae). Geology 2005, 33, 749–752. [Google Scholar] [CrossRef]
- Hong, S.K.; Lee, Y.I. Evaluation of atmospheric carbon dioxide concentrations during the Cretaceous. Earth Planet. Sci. Lett. 2012, 327, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Sun, B.; Quan, C.; Wu, J.; Lin, Z. Paleo-CO2 variation trends and the Cretaceous greenhouse climate. Earth-Sci. Rev. 2014, 129, 136–147. [Google Scholar] [CrossRef]
- Sun, Y.W.; Li, X.; Zhao, G.W.; Liu, H.; Zhang, Y.L. Aptian and Albian atmospheric CO2 changes during oceanic anoxic events: Evidence from fossil Ginkgo cuticles in Jilin Province, Northeast China. Cretac. Res. 2016, 62, 130–141. [Google Scholar] [CrossRef]
- Jing, D.; Bainian, S. Early Cretaceous atmospheric CO2 estimates based on stomatal index of Pseudofrenelopsis papillosa (Cheirolepidiaceae) from southeast China. Cretac. Res. 2018, 85, 232–242. [Google Scholar] [CrossRef]
- Leckie, R.M.; Bralower, T.J.; Cashman, R. Oceanic anoxic events and plankton evolution: Biotic response to tectonic forcing during the mid-Cretaceous. Paleoceanography 2002, 17, 13-1. [Google Scholar] [CrossRef] [Green Version]
- Coffin, M.F.; Pringle, M.S.; Duncan, R.A.; Gladczenko, G.T.; Storey, M.; Müller, R.D.; Gahagan, L.A. Kerguelen hot spot magma output since 130 Ma. J. Petrol. 2002, 43, 1121–1139. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, E.G.; Caldwell, W.G.E. The Western Interior Basin in Space and Time. Evolution of the Western Interior Basin; Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1933; Volume 39, pp. 1–30. [Google Scholar]
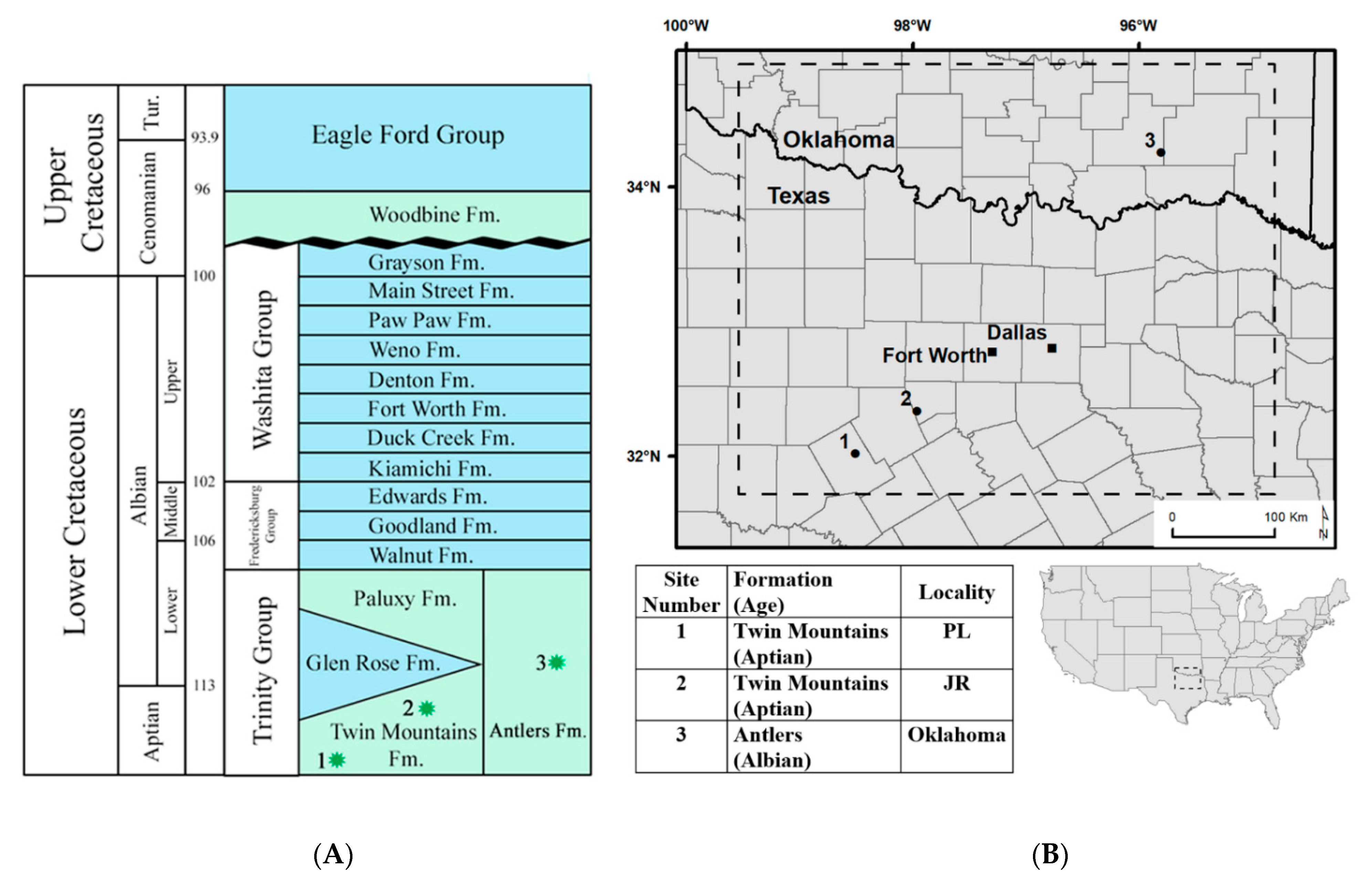

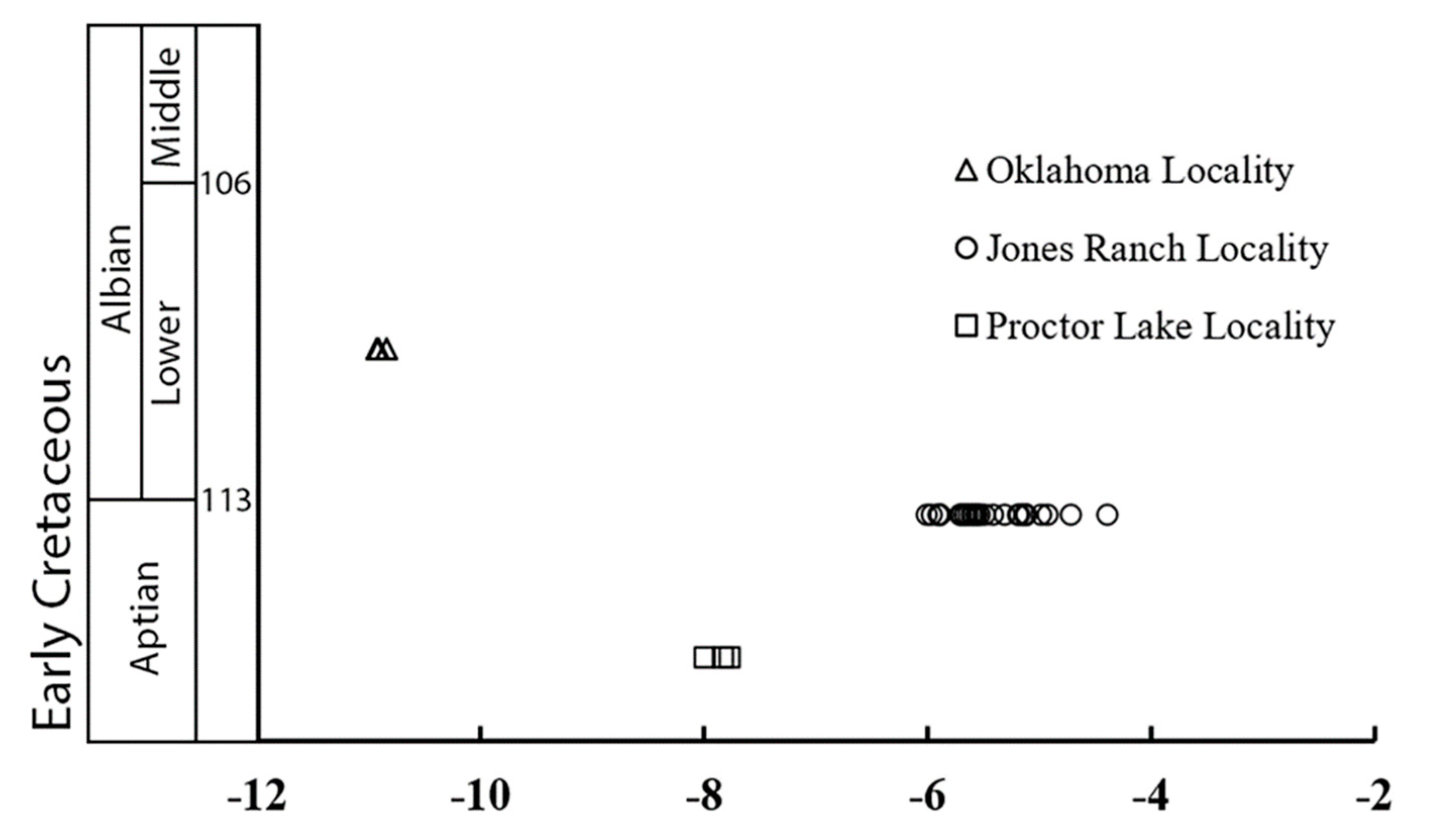

| Location | Sample | Formation (Age) | δ13Ccarb (‰ VPDB) | δ13Coom (‰ VPDB) | Δ13Ccc-om (‰) | δ13CS (‰ VPDB, 30 °C) |
|---|---|---|---|---|---|---|
| Oklahoma | Cross-C-10 | Antlers | −10.84 | −25.22 | 14.38 | −12.55 |
| Cross C-9 | (Albian) | −10.93 | −25.87 | 14.94 | −13.20 | |
| Cross C-1 | −10.92 | −25.00 | 14.08 | −12.33 | ||
| Jones Ranch, TX | ||||||
| CR13A | Twin Mountains | −4.72 | −24.70 | 19.98 | −12.03 | |
| CR13B | (Aptian) | −4.39 | −24.70 | 20.31 | −12.03 | |
| CR12A | −5.12 | −23.75 | 18.63 | −11.07 | ||
| CR12B | −4.99 | −23.75 | 18.76 | −11.07 | ||
| CR11A | −5.18 | −26.05 | 20.87 | −13.38 | ||
| CR11B | −5.19 | −26.05 | 20.86 | −13.38 | ||
| CR10A | −4.92 | −23.85 | 18.93 | −11.17 | ||
| CR10B | −5.13 | −23.85 | 18.72 | −11.17 | ||
| CR9A | −5.96 | −24.87 | 18.91 | −12.20 | ||
| CR9B | −6.01 | −24.87 | 18.86 | −12.20 | ||
| CR8A | −5.56 | −24.59 | 19.03 | −11.92 | ||
| CR8B | −5.32 | −24.59 | 19.27 | −11.92 | ||
| CR7A | −5.12 | −23.77 | 18.65 | −11.09 | ||
| CR7B | −5.42 | −23.77 | 18.35 | −11.09 | ||
| CR6B | −5.91 | −26.02 | 20.11 | −13.35 | ||
| CR5A | −5.61 | −24.16 | 18.55 | −11.49 | ||
| CR5B | −5.59 | −24.16 | 18.57 | −11.49 | ||
| CR4A | −5.49 | −27.30 | 21.81 | −14.64 | ||
| CR4B | −5.64 | −27.30 | 21.66 | −14.64 | ||
| CR3A | −5.54 | −23.53 | 17.99 | −10.85 | ||
| CR3B | −5.70 | −23.53 | 17.83 | −10.85 | ||
| CR2A | −5.71 | −27.03 | 21.32 | −14.37 | ||
| CR2A-R | −5.56 | −27.03 | 21.47 | −14.37 | ||
| CR2B | −5.67 | −27.03 | 21.36 | −14.37 | ||
| CR1A | −5.89 | −23.20 | 17.31 | −10.52 | ||
| CR1A-R | −5.89 | −23.20 | 17.31 | −10.52 | ||
| CR1B | −5.67 | −23.20 | 17.53 | −10.52 | ||
| Proctor Lake, TX | ||||||
| PL-8 | Twin Mountains | −7.84 | −21.08 | 13.24 | −8.39 | |
| PL-6 | (Aptian) | −7.77 | −21.43 | 13.66 | −8.74 | |
| PL-3 | −8.00 | −21.39 | 13.39 | −8.70 | ||
| Locality | Estimate | MAP mm/yr (± 55) Using ΔCcc-om | Cs1 (MAP-Derived) | MAP mm/yr (± 110mm) Using CALMAG | Cs2 (MAP-Derived) |
|---|---|---|---|---|---|
| Oklahoma | Min | 338 | 1649 | 952 | 5128 |
| Max | 366 | 1806 | 1210 | 6591 | |
| Average | 354 | 1736 | 1114 | 6047 | |
| Jones Ranch, TX | Min | 117 | 392 | 288 | 1363 |
| Max | 262 | 1215 | 444 | 2248 | |
| Average | 196 | 839 | 365 | 1800 | |
| Proctor Lake, TX | Min | 380 | 1883 | 268 | 1250 |
| Max | 401 | 2004 | 416 | 1811 | |
| Average | 391 | 1949 | 331 | 1607 |
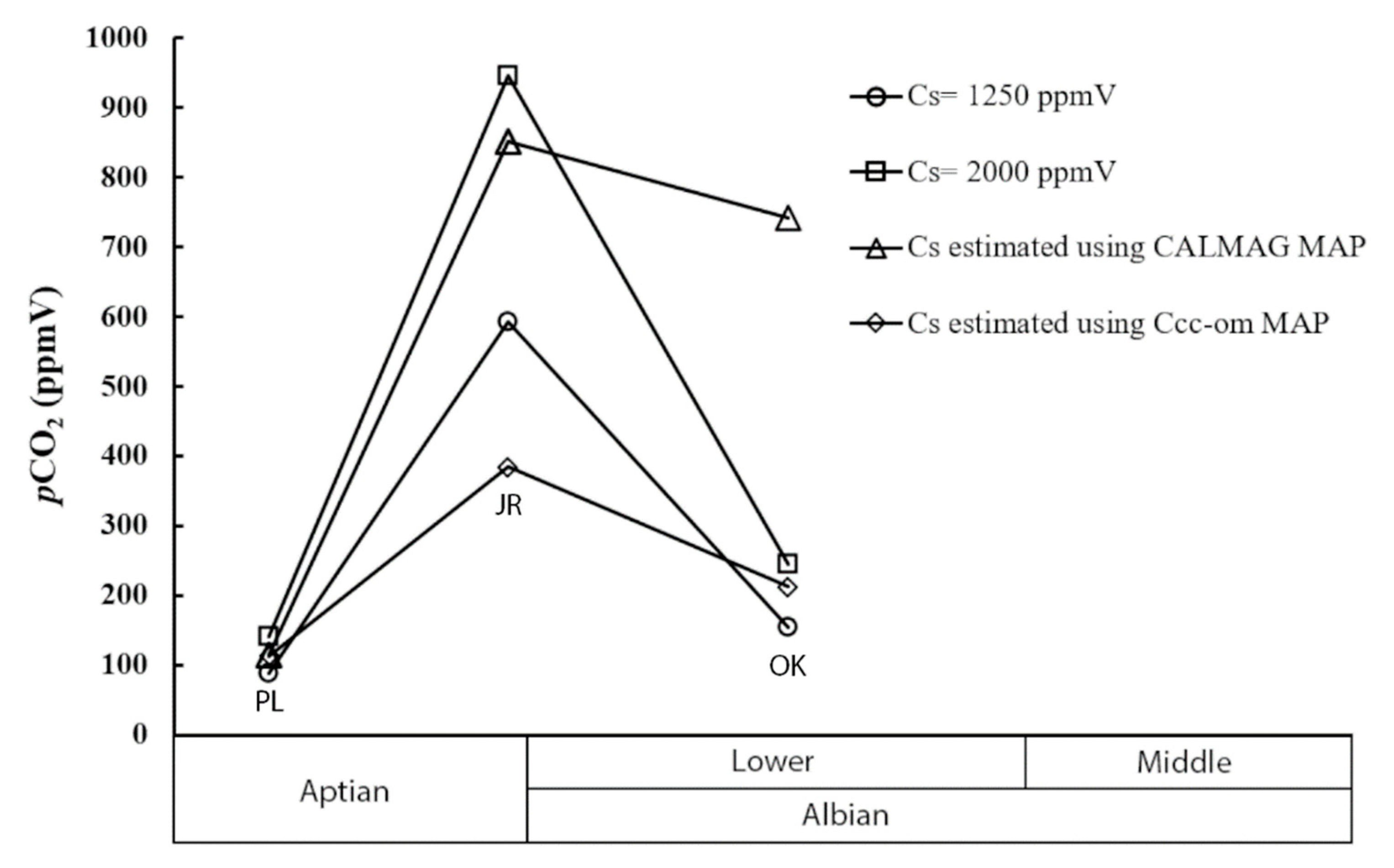
| Locality | Estimate Range | pCO2 (Cs) = 1250 | pCO2 (Cs) = 2000 | pCO2 (Cs) = Δ13Ccc-om MAP avg. | pCO2 (Cs) = CALMAG MAP avg. |
|---|---|---|---|---|---|
| Oklahoma | Min | 124 | 198 | 172 | 600 |
| Max | 188 | 301 | 261 | 910 | |
| Average | 153 | 246 | 213 | 742 | |
| Jones Ranch, TX | Min | 467 | 747 | 313 | 671 |
| Max | 692 | 1108 | 465 | 997 | |
| Average | 592 | 947 | 397 | 852 | |
| Proctor Lake, TX | Min | 67 | 108 | 105 | 86 |
| Max | 115 | 183 | 179 | 147 | |
| Average | 88 | 141 | 138 | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrzejewski, K.; Tabor, N.; Winkler, D.; Myers, T. Atmospheric pCO2 Reconstruction of Early Cretaceous Terrestrial Deposits in Texas and Oklahoma Using Pedogenic Carbonate and Occluded Organic Matter. Geosciences 2022, 12, 148. https://doi.org/10.3390/geosciences12040148
Andrzejewski K, Tabor N, Winkler D, Myers T. Atmospheric pCO2 Reconstruction of Early Cretaceous Terrestrial Deposits in Texas and Oklahoma Using Pedogenic Carbonate and Occluded Organic Matter. Geosciences. 2022; 12(4):148. https://doi.org/10.3390/geosciences12040148
Chicago/Turabian StyleAndrzejewski, Kate, Neil Tabor, Dale Winkler, and Timothy Myers. 2022. "Atmospheric pCO2 Reconstruction of Early Cretaceous Terrestrial Deposits in Texas and Oklahoma Using Pedogenic Carbonate and Occluded Organic Matter" Geosciences 12, no. 4: 148. https://doi.org/10.3390/geosciences12040148






