Stable Isotope Tracers of Cretaceous Arctic Paleoprecipitation
Abstract
:1. Introduction
2. Materials and Methods
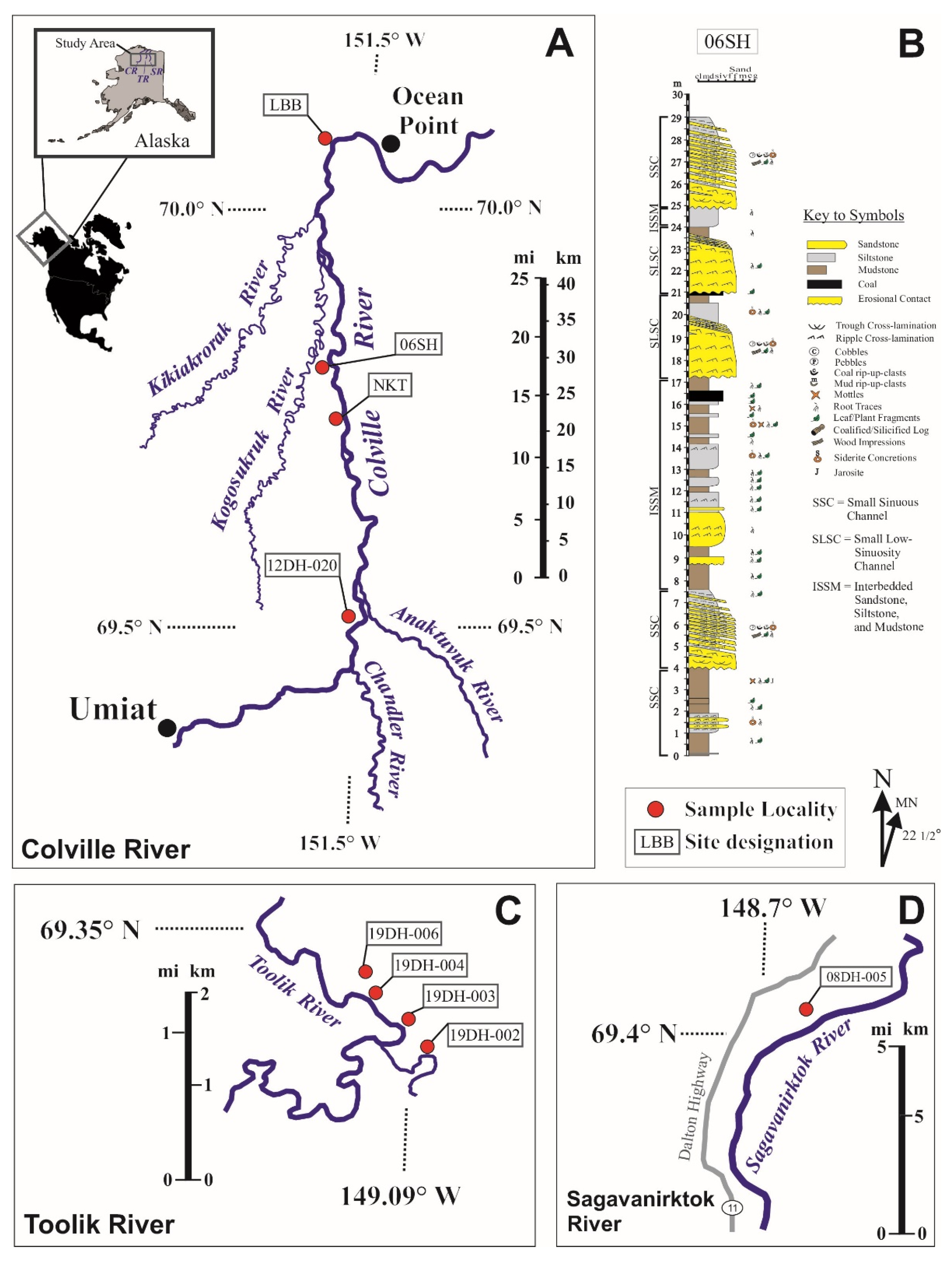
2.1. Sample Materials from the Prince Creek Formation, Colville Basin, Alaska
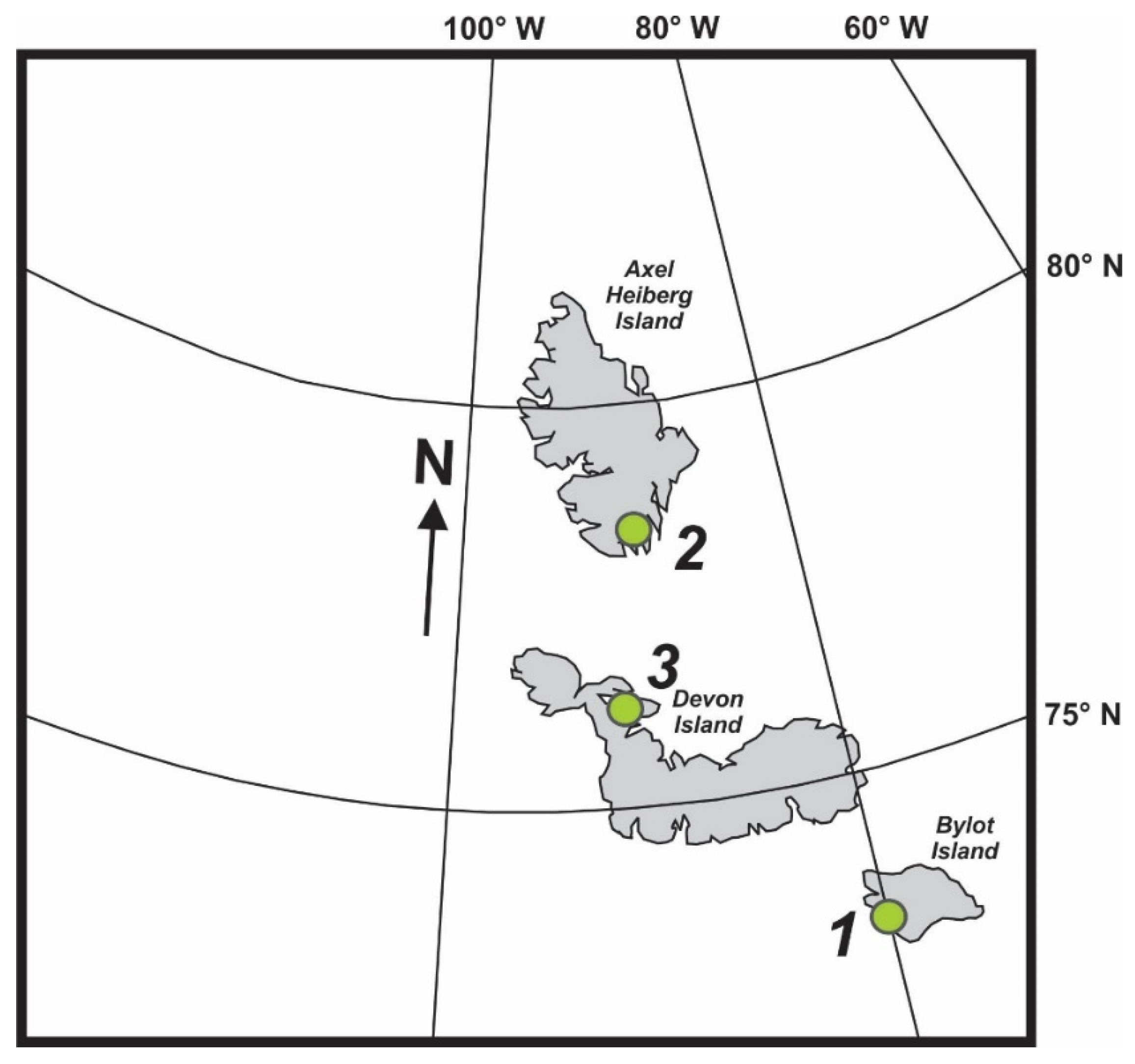
2.2. Sample Materials from the Canadian High Arctic
2.3. Organic Geochemistry Sample Preparation and Extraction
2.4. Compound Identification and Quantification
2.5. Compound-Specific Isotope Analysis
2.6. Bulk Sample Organic Carbon Analysis
3. Results
3.1. Organic Matter Characterization of Carbonaceous Mudstones of the Prince Creek Formation
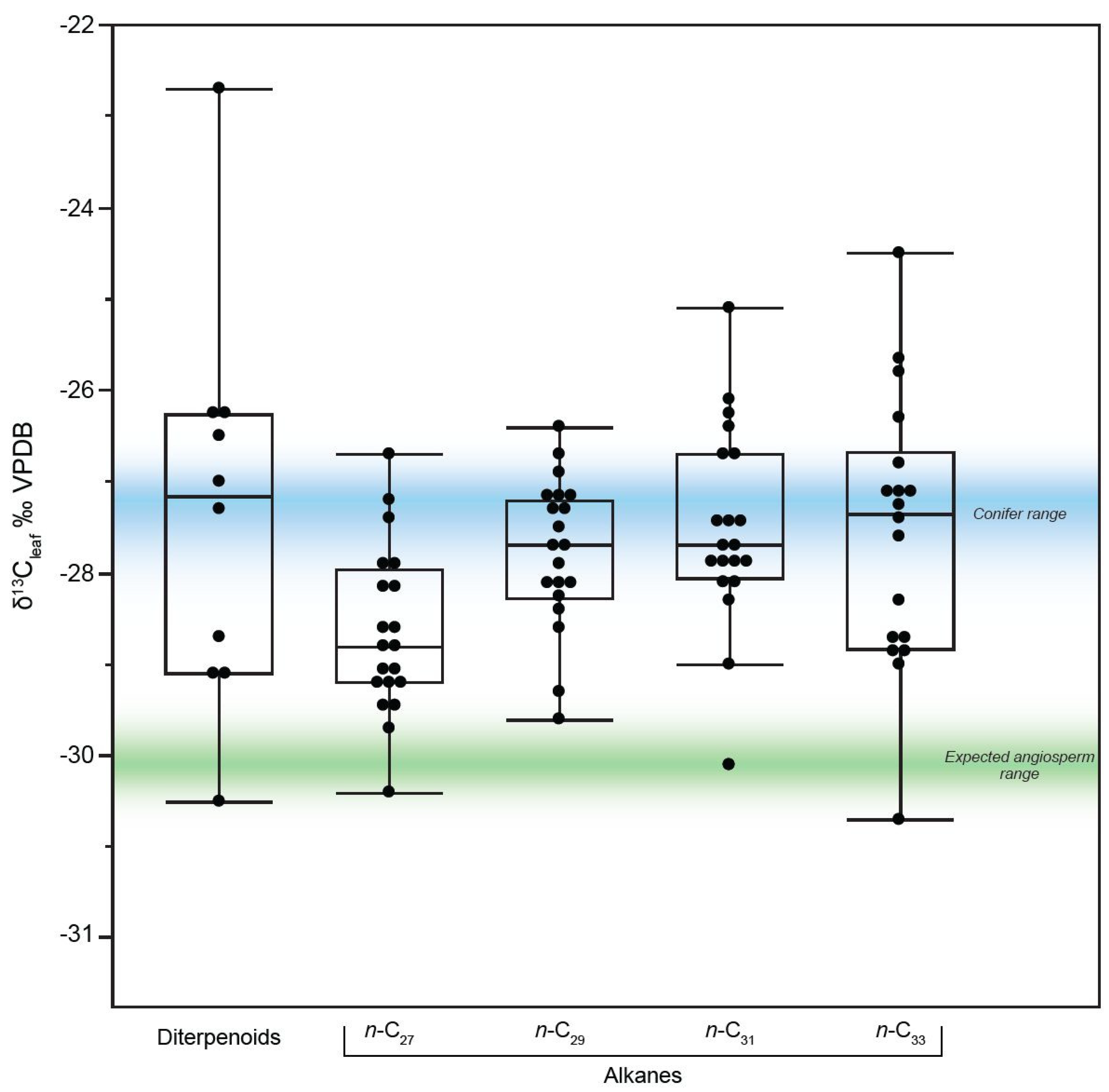
3.2. Carbon Isotopes of n-Alkanes, Diterpenoids, and Hopanes from the Prince Creek Formation
3.3. Hydrogen Isotopes of n-Alkanes
3.4. Organic Matter from the Hassel Formation of the Canadian High Arctic
3.5. Quantitative Re-Evaluation of Paleosol Siderite Oxygen Isotopes
3.6. Integration of Water Hydrogen and Oxygen Isotopic Values from Cretaceous Units
4. Discussion
4.1. The Utility of Isotopic Results from Cretaceous Terrestrial Arctic Organic Matter
4.2. Interpretation of Apparent Deuterium Excess in Paleoprecipitation of the Cretaceous Arctic
4.3. The Possible Influence of Continuous Transpiration by Cretaceous Arctic Paleofloras
4.4. The Possible Influence of Hydrogenotrophic Methanogenesis in Cretaceous Arctic Soils
4.5. The Path Forward
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barron, E.J. Warm, equable Cretaceous: The nature of the problem. Earth Sci. Rev. 1983, 19, 305–338. [Google Scholar] [CrossRef]
- Ruddiman, W.F. Earth’s Climate: Past and Future, 2nd ed.; W. H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Hay, W.W. Can humans force a return to a ‘Cretaceous’ climate? Sediment. Geol. 2011, 235, 5–26. [Google Scholar] [CrossRef]
- Foster, G.L.; Royer, D.L.; Lunte, D.J. Future climate forcing potentially without precedent in the last 420 million years. Nat. Commun. 2017, 8, 14845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, C.J. Palaeoclimate: A balmy Arctic. Nature 2004, 432, 814. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, G.R., Jr.; Kiehl, J.; Shields, C.; Scherer, J.; Scotese, C. Latitudinal temperature gradients and high-latitude temperatures during the latest Cretaceous: Congruence of geologic data and climate models. Geology 2015, 43, 683–686. [Google Scholar] [CrossRef]
- Poulsen, C.J.; Tabor, C.; White, J.D. Long-term climate forcing by atmospheric oxygen concentrations. Science 2015, 348, 1238–1241. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Pagani, M.; Pedentchouk, N.; Huber, M.; Sluijs, A.; Schouten, S.; Brinkhuis, H.; Damsté, J.S.S.; Dickens, G.R. Arctic hydrology during global warming at the Palaeocene/Eocene thermal maximum. Nature 2006, 442, 671–675. [Google Scholar] [CrossRef]
- Speelman, E.N.; Sewall, J.O.; Noone, D.; Huber, M.; Von der Heydt, A.S.; Damste, J.S.; Reichart, G.-J. Modeling the influence of a reduced equator-to-pole sea surface temperature gradient on the distribution of water isotopes in the Early/Middle Eocene. Earth Planet. Sci. Lett. 2010, 298, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Winnick, M.J.; Caves, J.K.; Chamberlin, C.P. A mechanistic analysis of early Eocene latitudinal gradients of isotopes in precipitation. Geophys. Res. Lett. 2015, 42, 8216–8224. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.; Fernandez, A.; Bernasconi, S.M.; Rugenstein, J.K.C.; Passey, S.R.; White, T. Spatial pattern of super-greenhouse warmth controlled by elevated specific humidity. Nat. Geosci. 2020, 13, 739–744. [Google Scholar] [CrossRef]
- Ufnar, D.F.; Gonzalez, L.; Ludvigson, G.A.; Brenner, R.L.; Witzke, B.J. The mid-Cretaceous water bearer: Isotope mass balance quantification of the Albian hydrologic cycle. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 188, 51–71. [Google Scholar] [CrossRef]
- Ufnar, D.F.; Ludvigson, G.A.; Gonzalez, L.A.; Brenner, R.L.; Witzke, B.J. High latitude meteoric δ18O compositions: Paleosol siderite in the Middle Cretaceous Nanushuk Formation, North Slope, Alaska. Geol. Soc. Am. Bull. 2004, 116, 463–473. [Google Scholar] [CrossRef]
- Suarez, M.B.; González, L.A.; Ludvigson, G.A. Quantification of a Greenhouse Hydrologic Cycle from Equatorial to Polar Latitudes: The Mid-Cretaceous Water Bearer Revisited. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 307, 301–312. [Google Scholar] [CrossRef]
- Lollar, C.J. Probing a Paleoclimate Model-Data Misfit in Arctic Alaska from the Cretaceous Greenhouse World. Unpublished Master’s Thesis, University of Kansas, Lawrence, KS, USA, 2011. Available online: https://kuscholarworks.ku.edu/handle/1808/8373 (accessed on 31 January 2022).
- Suarez, C.A.; Ludvigson, G.A.; Gonzalez, L.; Fiorillo, A.; Flaig, P.; McCarthy, P.J. Use of Multiple Oxygen Isotope Proxies for Elucidating Arctic Cretaceous Palaeo-Hydrology. In Isotopic Studies in Cretaceous Research; Bojar, A.-V., Melinte-Dobrinescu, M.C., Smit, J., Eds.; Geological Society London, Special Publications: London, UK, 2013; Volume 382, pp. 185–202. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Jaramillo, S.; McCarthy, P.J.; Ochoa, A.; Fowell, S.J.; Longstaffe, F.J. Paleoclimate reconstruction of the Prince Creek Formation, Arctic Alaska, during Maastrichtian global warming. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 532, 109265. [Google Scholar] [CrossRef]
- Ross, J.B.; Ludvigson, G.A.; Schröder-Adams, C.J.; Suarez, M.B. High Latitude Meteoric δ18O Compositions from the Cenomanian Bastion Ridge Formation, Axel Heiberg Island, Canadian Arctic Archipelago: A Palaeoclimate Proxy from the Sverdrup Basin. In Cretaceous Climate Events and Short-Term Sea-Level Changes; Wagreich, M., Hart, M.B., Sames, B., Yilmaz, I.O., Eds.; Geological Society, London, Special Publications: London, UK, 2020; Volume 498, pp. 57–74. [Google Scholar]
- Ludvigson, G.A.; González, L.A.; Metzger, R.A.; Witzke, B.J.; Brenner, R.L.; Murillo, A.; White, T.S. Meteoric sphaerosiderite lines and their use for paleohydrology and paleoclimatology. Geology 1998, 26, 1039–1042. [Google Scholar] [CrossRef]
- Richard, Z.D.; Pollard, D.; Kump, L.R.; White, T.S. Anomalously low δ18O values of high-latitude Permo-Triassic paleosol siderite. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 492, 26–40. [Google Scholar] [CrossRef]
- Ufnar, D.; González, L.; Ludvigson, G.; Brenner, R.; Witzke, B. Evidence for increased latent heat transport during the Cretaceous (Albian) greenhouse warming. Geology 2004, 32, 1049–1052. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zwiers, F.W.; Hegerl, G.C.; Lambert, F.H.; Gillett, N.P.; Solomon, S.; Stott, P.A.; Nozawa, T. Detection of human influence on twentieth-century precipitation trends. Nature 2007, 448, 461–466. [Google Scholar] [CrossRef]
- Curry, R.; Dickson, B.; Yashayaev, I. A change in the freshwater balance of the Atlantic Ocean over the past four decades. Nature 2003, 426, 826–829. [Google Scholar] [CrossRef]
- Poulsen, C.J.; Pollard, D.; White, T.S.; Poulsen, C. General circulation model simulation of the δ18O content of continental precipitation in the middle Cretaceous: A model–proxy comparison. Geology 2007, 35, 199–202. [Google Scholar] [CrossRef]
- Super, J.R.; Chin, K.; Pagani, M.; Li, H.; Tabor, C.; Harwood, D.M.; Hull, P.M. Late Cretaceous climate in the Canadian Arctic: Multi-proxy constraints from Devon Island. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 504, 1–22. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.A.; Gonzalez, L.A.; Ludvigson, G.A.; Kirkland, J.I.; Cifelli, R.L.; Kohn, M.J. Multi-taxa isotopic investigation of paleohydrology in the Lower Cretaceous Cedar Mountain Formation, eastern Utah, U.S.A.: Deciphering effects of the Nevadaplano plateau on regional climate. J. Sediment. Res. 2014, 84, 975–987. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.; Fernandez, A.; Storck, J.C.; White, T.S.; Lever, M.; Müller, I.A.; Bishop, S.; Seifert, R.F.; Driese, S.G.; Krylov, A.; et al. Experimental calibration of clumped isotopes in siderite between 8.5 and 62 °C and its application as paleo-thermometer in paleosols. Geochim. Cosmochim. Acta 2019, 254, 1–20. [Google Scholar] [CrossRef]
- Flaig, P.P.; McCarthy, P.J.; Fiorillo, A.R. A Tidally Influenced, High-Latitude Coastal-Plain: The Upper Cretaceous (Maastrichtian) Prince Creek Formation, North Slope, Alaska. In From River to Rock Record: The Preservation of Fluvial Sediments and Their Subsequent Interpretation; Davidson, S., Leleu, S., North, C., Eds.; SEPM Special Publication: Broken Arrow, OK, USA, 2011; Volume 97, pp. 233–264. [Google Scholar]
- Flaig, P.P.; McCarthy, P.J.; Fiorillo, A.E. Anatomy, Evolution, and Paleoenvironmental Interpretation of an Ancient Arctic Coastal Plain: Integrated Paleopedology and Palynology from the upper Cretaceous (Maastrichtian) Prince Creek Formation, North Slope, Alaska, USA. In New Frontiers in Paleopedology and Terrestrial Paleoclimatology, Paleosols and Soil Surface Analogue Systems; Driese, S.G., Nordt, L.C., Eds.; SEPM Special Publication: Broken Arrow, OK, USA, 2013; Volume 114, pp. 179–230. [Google Scholar]
- Bonelli, J.R.; Flaig, P.P. Quantitative biofacies analysis to identify relationships and refine controls on paleosol development, Prince Creek Formation, North slope Alaska, USA. Geosciences 2021, 11, 460. [Google Scholar] [CrossRef]
- Jaramillo, S.S.; McCarthy, P.J.; Trainor, T.P.; Fowell, S.J.; Fiorillo, A.R. Origin of clay minerals in alluvial paleosols, Prince Creek Formation, North Slope, Alaska, U.S.A.: Influence of volcanic ash on pedogenesis in the Late Cretaceous Arctic. J. Sediment. Res. 2015, 85, 192–208. [Google Scholar] [CrossRef]
- Flaig, P.; Fiorillo, A.; McCarthy, P.J. Dinosaur-bearing hyperconcentrated flows of Cretaceous Arctic Alaska: Recurring catastrophic event beds on a distal paleopolar coastal plain. PALAIOS 2014, 29, 594–611. [Google Scholar] [CrossRef]
- Van der Kolk, D.; Flaig, P.; Hasiotis, S.T. Paleoenvironmental reconstruction of a Late Cretaceous, muddy, river-dominated polar deltaic system: Schrader Bluff—Prince Creek Formation transition, Shivugak Bluffs, North Slope of Alaska, U.S.A. J. Sediment. Res. 2015, 85, 903–936. [Google Scholar] [CrossRef]
- Flaig, P.P.; Van der Kolk, D.A. Depositional Environments of the Prince Creek Formation along the East Side of the Toolik River, Sagavanirktok Quadrangle, North Slope, Alaska. Alaska Division of Geological & Geophysical Surveys Preliminary Interpretive Report; DGGS PIR 2015-4; Alaska Division of Geological & Geophysical Surveys: Fairbanks, AK, USA, 2015. [Google Scholar] [CrossRef] [Green Version]
- Miall, A.D.; Balkwill, H.R.; Hopkins, W.S., Jr. Cretaceous and Tertiary Sediments of Eclipse trough, Bylot Island Area, Arctic Canada, and Their Regional Settings: Geological Survey of Canada; Paper 79–23; Energy, Mines and Resources Canada: Nunavut, NC, Canada, 1980; 20p. [Google Scholar]
- Ludvigson, G.A.; González, L.A.; Fowle, D.A.; Roberts, J.A.; Driese, S.G.; Villarreal, M.A.; Smith, J.J.; Suarez, M.B. Paleoclimatic applications and modern process studies of pedogenic siderite. In New Frontiers in Paleopedology and Terrestrial Paleoclimatology; Driese, S.G., Nordt, L.C., McCarthy, P.J., Eds.; SEPM Special Publication: Broken Arrow, OK, USA, 2013; Volume 104, pp. 79–87. [Google Scholar] [CrossRef] [Green Version]
- Schröder-Adams, C.; Herrle, J.O.; Embry, A.F.; Haggart, J.W.; Galloway, J.; Pugh, A.T.; Harwood, D.M. Aptian to Santonian foraminiferal biostratigraphy and paleoenvironmental change in the Sverdrup Basin as revealed at Glacier Fiord, Axel Heiberg Island, Canadian Arctic Archipelago. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 413, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.M.; Sweet, A.R.; Pugh, A.; Schröder-Adams, C.J.; Swindles, G.T.; Haggart, J.W.; Embry, A.F. Correlating middle Cretaceous palynological records from the Canadian High Arctic based on a section from the Sverdrup Basin and samples from the Eclipse Trough. Palynology 2012, 36, 277–302. [Google Scholar] [CrossRef]
- SSchlanser, K.M.; Diefendorf, A.F.; West, C.K.; Greenwood, D.R.; Basinger, J.F.; Meyer, H.W.; Lowe, A.J.; Naake, H.H. Conifers are a major source of sedimentary leaf wax n-alkanes when dominant in the landscape: Case studies from the Paleogene. Org. Geochem. 2020, 147, 104069. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide. In Biomarkers and Isotopes in Petroleum Exploration and Earth History, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005; Volume 2. [Google Scholar]
- Larson, T.E.; Heikoop, J.M.; Perkins, G.; Chipera, S.J.; Hess, M.A. Pretreatment technique for siderite removal for organic carbon isotope and C:N ratio analysis in geological samples. Rapid Commun. Mass Spectrom. 2008, 22, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; Toman, B.; Verkouteren, R.M. New guidelines for δ13C measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ficken, K.J.; Li, B.; Swain, D.L.; Eglinton, G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000, 31, 745–749. [Google Scholar] [CrossRef]
- Marzi, R.; Torkelson, B.E.; Olson, R.K. A revised carbon preference index. Org. Geochem. 1993, 20, 1303–1306. [Google Scholar] [CrossRef]
- Bray, E.E.; Evans, E.D. Distribution of n-paraffins as a clue to recognition of source beds. Geochim. Cosmochim. Acta 1961, 22, 2–15. [Google Scholar] [CrossRef]
- Eglinton, G.; Hamilton, R.J. Leaf epicuticular waxes. Science 1967, 156, 1322–1335. [Google Scholar] [CrossRef]
- Freeman, K.H.; Pancost, R.D. 12.15—Biomarkers for terrestrial plants and climate. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 395–416. [Google Scholar]
- Diefendorf, A.F.; Freimuth, E.J. Extracting the most from terrestrial plant-derived n-alkyl lipids and their carbon isotopes from the sedimentary record: A review. Org. Geochem. 2017, 103, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Diefendorf, A.F.; Leslie, A.B.; Wing, S.L. A phylogenetic analysis of conifer diterpenoids and their carbon isotopes for chemotaxonomic applications. Org. Geochem. 2019, 127, 50–58. [Google Scholar] [CrossRef]
- Schlanser, K.; Diefendorf, A.F.; Greenwood, D.; Mueller, K.E.; West, C.K.; Lowe, A.J.; Basinger, J.F.; Currano, E.D.; Flynn, A.G.; Fricke, H.C.; et al. On geologic timescales, plant carbon isotope fractionation responds to precipitation similarly to modern plants and has a small negative correlation with pCO2. Geochim. Cosmochim. Acta 2020, 270, 264–281. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Mueller, K.E.; Wing, S.L.; Koch, P.L.; Freeman, K.H. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. USA 2010, 107, 5738–5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, A.; Gomez, B.; Legendre, S.; Lécuyer, C. Evolution of the carbon isotope composition of atmospheric CO2 throughout the Cretaceous. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 471, 40–47. [Google Scholar] [CrossRef]
- Inglis, G.N.; Naafs, B.D.A.; Zheng, Y.; Schellekens, J.; Pancost, R.D. δ13C values of bacterial hopanoids and leaf waxes as tracers for methanotrophy in peatlands. Geochim. Cosmochim. Acta 2019, 260, 244–256. [Google Scholar] [CrossRef] [Green Version]
- McFarlin, J.M.; Axford, Y.; Masterson, A.L.; Osburn, M.R. Calibration of modern sedimentary δ2H plant wax-water relationships in Greenland lakes. Quat. Sci. Rev. 2019, 225, 105978. [Google Scholar] [CrossRef]
- Sachse, D.; Billault, I.; Bowen, G.J.; Chikaraishi, Y.; Dawson, T.E.; Feakins, S.J.; Freeman, K.H.; Magill, C.R.; McInerney, F.A.; Van der Meer, M.T.; et al. Molecular paleohydrology: Interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu. Rev. Earth Planet. Sci. 2012, 40, 221–249. [Google Scholar] [CrossRef] [Green Version]
- Bowen, G.J. The Online Isotopes in Precipitation Calculator, Version 2.2. 2009. Available online: https://wateriso.utah.edu/waterisotopes/pages/data_access/oipc_citation.html (accessed on 6 June 2021).
- Bowen, G.J.; Cai, Z.; Fiorella, R.P.; Putman, A.L. Isotopes in the Water Cycle: Regional- to Global-Scale Patterns and Applications. Annu. Rev. Earth Planet. Sci. 2019, 47, 453–479. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.; Van Dijk, J.; Müller, I.A.; Bernasconi, S.M. Siderite acid fractionation factors for sealed and open vessel digestions at 70 and 100 °C. Chem. Geol. 2016, 444, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.; Fernandez, A.; Müller, I.A.; Lever, M.A.; Bernasconi, S.M. Oxygen isotope fractionation in the siderite-water system between 8.5 and 62 °C. Geochim. Cosmochim. Acta 2018, 220, 535–551. [Google Scholar] [CrossRef]
- Spicer, R.A.; Herman, A.B. The Late Cretaceous environment of the Arctic: A quantitative reassessment based on plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 295, 423–442. [Google Scholar] [CrossRef] [Green Version]
- Forster, A.; Schouten, S.; Baas, M.; Damste, J.S. Mid-Cretaceous (Albian–Santonian) sea surface temperature record of the tropical Atlantic Ocean. Geology 2007, 35, 919–922. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Sberna, D.T.; Taylor, D.W. Effect of thermal maturation on plant-derived terpenoids and leaf wax n-alkyl components. Org. Geochem. 2015, 89–90, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Schimmelmann, A.; Sessions, A.L.; Mastalerz, M. Hydrogen Isotopic (D/H) Composition of Organic Matter During Diagenesis and Thermal Maturation. Annu. Rev. Earth Planet. Sci. 2006, 34, 501–533. [Google Scholar] [CrossRef] [Green Version]
- Diefendorf, A.F.; Freeman, K.H.; Wing, S.L. A comparison of terpenoid and leaf fossil vegetation proxies in Paleocene and Eocene Bighorn Basin sediments. Org. Geochem. 2014, 71, 30–42. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Leslie, A.B.; Wing, S. Leaf wax composition and carbon isotopes vary among major conifer groups. Geochim. Cosmochim. Acta 2015, 170, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Craig, H.; Gordon, L.I. Deuterium and Oxygen-18 Variations in the Ocean and the Marine Atmosphere; Tongiorgi, E. Ed. In Proceedings of the Conference on Stable Isotopes in Oceanographic Studies and Paleotemperatures, Pisa, Italy, 26–30 July 1965; pp. 9–130. [Google Scholar]
- Gat, J.R.; Bowser, C.J.; Kendall, C. The contribution of evaporation from the Great Lakes to the continental atmosphere: Estimate based on stable isotope data. Geophys. Res. Lett. 1994, 21, 557–560. [Google Scholar] [CrossRef]
- Good, S.P.; Mallia, D.V.; Lin, J.C.; Bowen, G.J. Stable isotope analysis of precipitation samples obtained via crowdsourcing reveals the spatiotemporal evolution of Superstorm Sandy. PLoS ONE 2014, 9, e91117. [Google Scholar] [CrossRef] [Green Version]
- Cropper, S.; Solander, K.; Newman, B.D.; Tuinenburg, O.A.; Staal, A.; Theeuwen, J.J.E.; Xu, C. Comparing deuterium excess to large-scale precipitation recycling models in the tropics npj. Clim. Atmos. Sci. 2021, 4, 60. [Google Scholar] [CrossRef]
- Mellat, M.; Bailey, H.; Mustonen, K.-R.; Marttila, H.; Klein, E.S.; Gribanov, K.; Bret-Harte, M.S.; Chupakov, A.V.; Divine, D.V.; Else, B.; et al. Hydroclimate controls on the isotopic (d18O, d2H, d-excess) traits of Pan Arctic summer rainfall events. Front. Earth Sci. 2021, 9, 651731. [Google Scholar] [CrossRef]
- Bailey, A.; Posmentier, E.; Feng, X. Patterns of Evaporation and Precipitation Drive Global Isotopic Changes in Atmospheric Moisture. Geophys. Res. Lett. 2018, 45, 7093–7101. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Pagani, M.; Briggs, D.E.G.; Equiza, M.A.; Jagels, R.; Leng, Q.; Lepage, B.A. Carbon and hydrogen isotope fractionation under continuous light: Implications for paleoenvironmental interpretations of the High Arctic during Paleogene warming. Oecologia 2009, 160, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, W.; Leng, Q.; Hren, M.T.; Pagani, M. Variation in n-alkane δD values from terrestrial plants at high latitude: Implications for paleoclimate reconstruction. Org. Geochem. 2011, 42, 283–288. [Google Scholar] [CrossRef]
- Talbot, M.R.; Kelts, K. Primary and diagenetic carbonates in the anoxic sediments of Lake Bosumtwi, Ghana. Geology 1986, 14, 912–916. [Google Scholar] [CrossRef]
- Mozley, P.S.; Wersin, P. Isotopic composition of siderite as an indicator of depositional environment. Geology 1992, 20, 817–820. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Schoell, M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 1980, 44, 649–661. [Google Scholar] [CrossRef]
- North, J.C.; Frew, R.D.; Van Hale, R. Can stable isotopes be used to monitor landfill leachate impact on surface waters? J. Geophys. Explor. 2006, 88, 49–53. [Google Scholar] [CrossRef]
- Andrei, F.; Barbieri, M.; Sappa, G. Application of 2H and 18O isotopes for tracing municipal solid waste landfill contamination of groundwater: Two Italian case histories. Water 2021, 13, 1065. [Google Scholar] [CrossRef]
- Castañeda, S.; Sucgang, R.; Almoneda, R.; Mendoza, N.; David, C. Environmental isotopes and major ions for tracing leachate contamination from a municipal landfill in Metro Manila, Philippines. J. Environ. Radioact. 2012, 110, 20–37. [Google Scholar] [CrossRef]
- Kulongoski, J.T.; McMahon, P.B.; Land, M.T.; Wright, M.T.; Johnson, T.A.; Landon, M.K. Origin of methane and sources of high concentrations in Los Angeles groundwater. J. Geophys. Res. Biogeosci. 2018, 123, 818–831. [Google Scholar] [CrossRef]
- Siegel, D.I.; Chanton, J.R.; Glaser, P.H.; Chasar, L.S.; Rosenberry, D. Estimating methane production rates in bogs and landfills by deuterium enrichment of pore water: Global Biogeochem. Cycles 2001, 15, 967–975. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Tabor, N.J. Paleoenvironmental and paleoclimatic reconstruction of Cretaceous (Aptian-Cenomanian) terrestrial formations of Texas and Oklahoma using phyllosilicates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 543, 109491. [Google Scholar] [CrossRef]
- Rozanski, K.; Araguás-Araguás, L.; Gonfiantini, R. Isotopic patterns in modern global precipitation. Climate Change in Continental Isotopic Records. Am. Geophys. Union Monogr. 1993, 78, 1–36. [Google Scholar]

| Organic Compound | Organic δD vs. VSMOW | Water δD vs. VSMOW |
|---|---|---|
| n-C25 | −227.1 ± 3.1‰ | −119.1 ± 25‰ |
| n-C27 | −221.4 ± 3.1‰ | −113.4 ± 25‰ |
| n-C29 | −202.7 ± 3.1‰ | −94.7 ± 25‰ |
| n-C31 | −195.0 ± 3.1‰ | −87 ± 25‰ |
| n-C33 | −226.4 ± 3.1‰ | −118.4 ± 25‰ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludvigson, G.A.; Diefendorf, A.F.; Suarez, M.B.; González, L.A.; Corcoran, M.C.; Schlanser, K.; Flaig, P.P.; McCarthy, P.J.; van der Kolk, D.; Houseknecht, D.; et al. Stable Isotope Tracers of Cretaceous Arctic Paleoprecipitation. Geosciences 2022, 12, 143. https://doi.org/10.3390/geosciences12040143
Ludvigson GA, Diefendorf AF, Suarez MB, González LA, Corcoran MC, Schlanser K, Flaig PP, McCarthy PJ, van der Kolk D, Houseknecht D, et al. Stable Isotope Tracers of Cretaceous Arctic Paleoprecipitation. Geosciences. 2022; 12(4):143. https://doi.org/10.3390/geosciences12040143
Chicago/Turabian StyleLudvigson, Greg A., Aaron F. Diefendorf, Marina B. Suarez, Luis A. González, Megan C. Corcoran, Kristen Schlanser, Peter P. Flaig, Paul J. McCarthy, Dolores van der Kolk, David Houseknecht, and et al. 2022. "Stable Isotope Tracers of Cretaceous Arctic Paleoprecipitation" Geosciences 12, no. 4: 143. https://doi.org/10.3390/geosciences12040143






