Effect of Culture pH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Synechococcus PCC7942 Strain
2.2. EPS Extraction and Purification
2.3. Chemical Composition of EPS
2.3.1. FT-IR Spectroscopy
2.3.2. Protein, Sugar and Glycosaminoglycan Assays
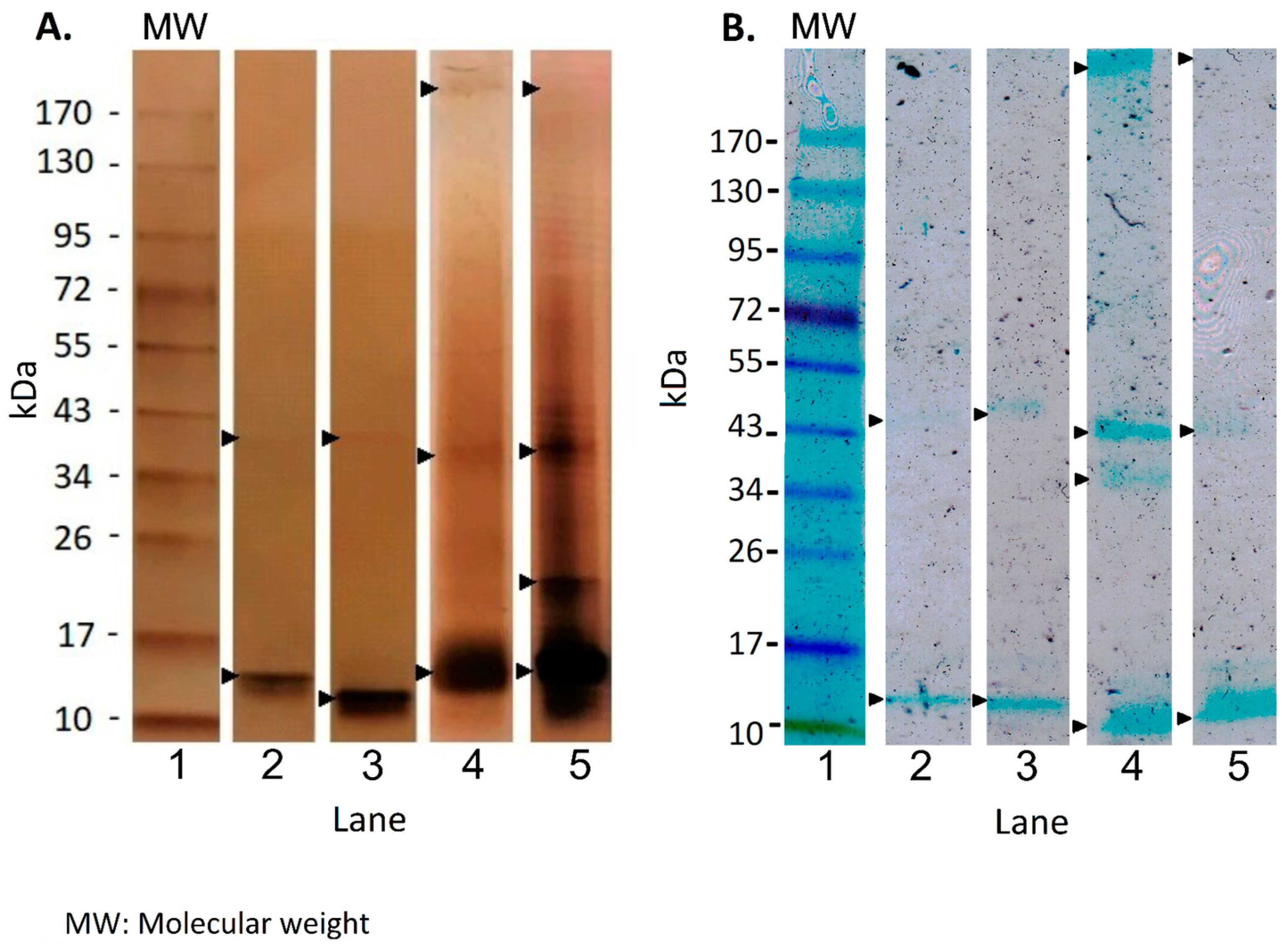
2.3.3. Molecular Size Distribution Using SDS-PAGE
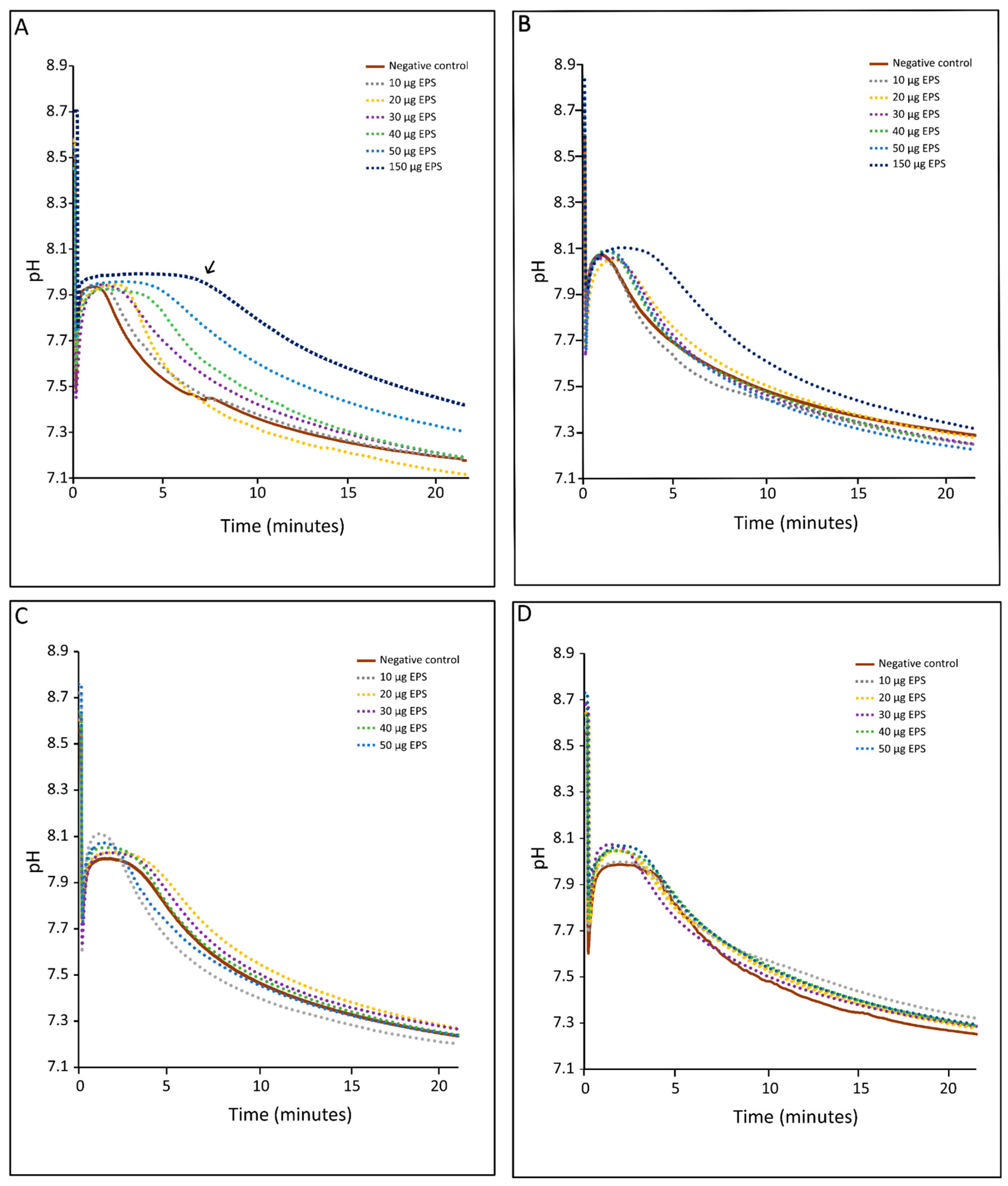
2.3.4. Inhibition of CaCO3 Precipitation Using pH-Drift Assay
2.3.5. Forced Precipitation of Calcium Carbonate
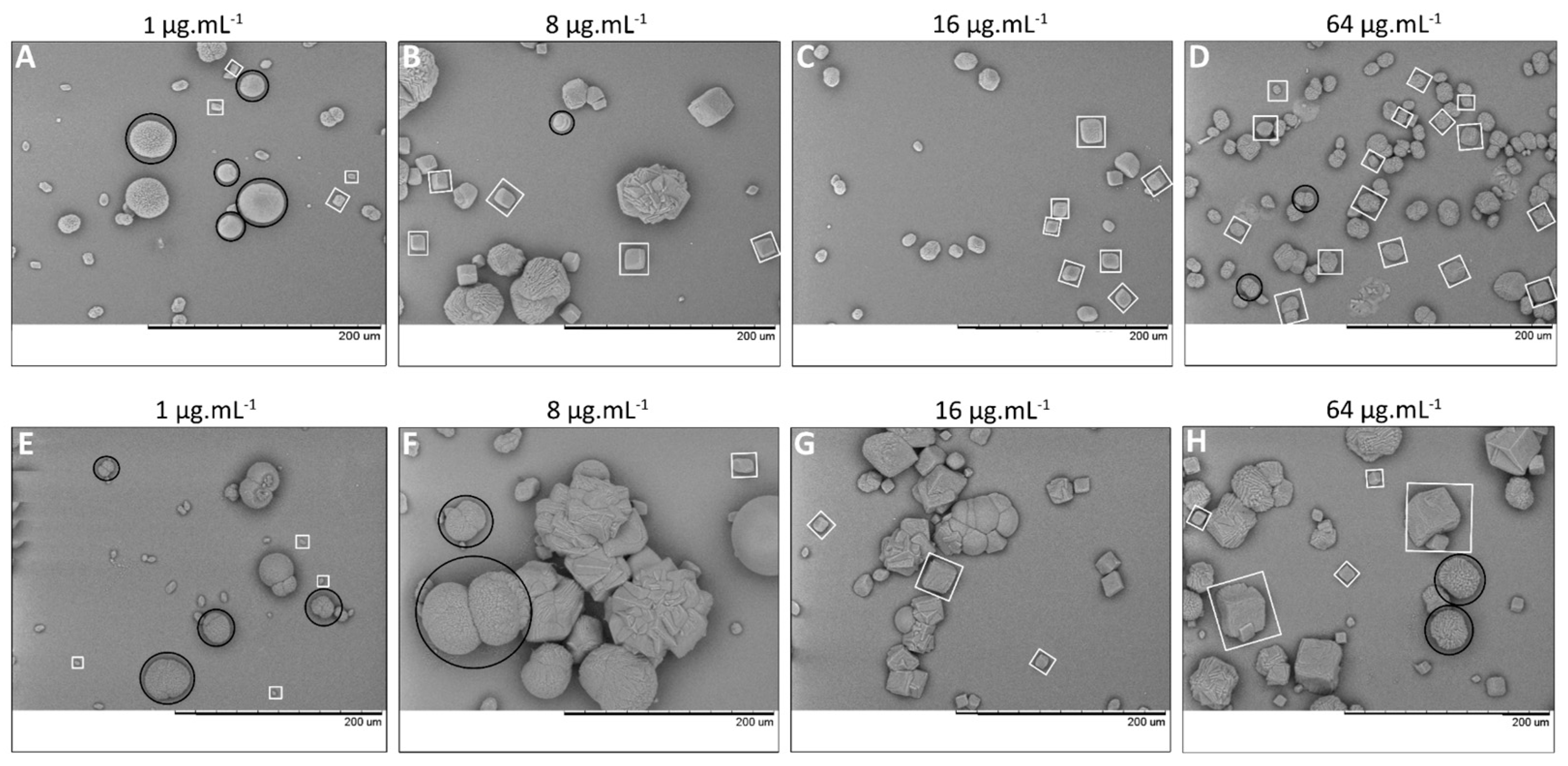
2.3.6. Morphology and Mineralogy of Carbonate Crystals
2.3.7. Crystal Count and Size Distribution
3. Results
3.1. Cell Growth and pH Evolution
3.2. EPS Production
3.3. EPS Characteristics
3.4. Forced Precipitation and Mineral Properties of Calcium Carbonate
4. Discussion
From the Lab to the Field–Potential Importance of pH Effects in Bloom Formation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raven, J. The Twelfth Tansley Lecture. Small Is Beautiful: The Picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Ridgwell, A.; Zeebe, R.E. The Role of the Global Carbonate Cycle in the Regulation and Evolution of the Earth System. Earth Planet. Sci. Lett. 2005, 234, 299–315. [Google Scholar] [CrossRef]
- Ivanikova, N.V.; Popels, L.C.; McKay, R.M.L.; Bullerjahn, G.S. Lake Superior Supports Novel Clusters of Cyanobacterial Picoplankton. Appl. Environ. Microbiol. 2007, 73, 4055–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkowski, P.G. The Role of Phytoplankton Photosynthesis in Global Biogeochemical Cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agawin, N.S.; Duarte, C.M.; Agustí, S. Nutrient and Temperature Control of the Contribution of Picoplankton to Phytoplankton Biomass and Production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Agustí, S. Picophytoplankton Niche Partitioning in the Warmest Oligotrophic Sea. Front. Mar. Sci. 2021, 8, 429. [Google Scholar] [CrossRef]
- Pomar, L.; Hallock, P. Carbonate Factories: A Conundrum in Sedimentary Geology. Earth-Sci. Rev. 2008, 87, 134–169. [Google Scholar] [CrossRef]
- Kranz, S.A.; Gladrow, D.W.-; Nehrke, G.; Langer, G.; Rosta, B. Calcium Carbonate Precipitation Induced by the Growth of the Marine Cyanobacteria Trichodesmium. Limnol. Oceanogr. 2010, 55, 2563–2569. [Google Scholar] [CrossRef] [Green Version]
- Buick, R. When Did Oxygenic Photosynthesis Evolve? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, R.E.; Hartman, H. The Origin and Evolution of Oxygenic Photosynthesis. Trends Biochem. Sci. 1998, 23, 94–97. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Lundgren, P.; Falkowski, P. Nitrogen Fixation and Photosynthetic Oxygen Evolution in Cyanobacteria. Res. Microbiol. 2003, 154, 157–164. [Google Scholar] [CrossRef]
- Knoll, A.H. Cyanobacteria and Earth History. Cyanobacteria Mol. Biol. Genom. Evol. 2008, 484, 1–19. [Google Scholar]
- Hoehler, T.M.; Bebout, B.M.; Des Marais, D.J. The Role of Microbial Mats in the Production of Reduced Gases on the Early Earth. Nature 2001, 412, 324–327. [Google Scholar] [CrossRef]
- Uyeda, J.C.; Harmon, L.J.; Blank, C.E. A Comprehensive Study of Cyanobacterial Morphological and Ecological Evolutionary Dynamics through Deep Geologic Time. PLoS ONE 2016, 11, e0162539. [Google Scholar] [CrossRef]
- Arrigo, K.R. Marine Microorganisms and Global Nutrient Cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Litchman, E.; de Tezanos Pinto, P.; Edwards, K.F.; Klausmeier, C.A.; Kremer, C.T.; Thomas, M.K. Global Biogeochemical Impacts of Phytoplankton: A Trait-based Perspective. J. Ecol. 2015, 103, 1384–1396. [Google Scholar] [CrossRef] [Green Version]
- Buitenhuis, E.T.; Li, W.K.; Vaulot, D.; Lomas, M.; Landry, M.; Partensky, F.; Karl, D.; Ulloa, O.; Campbell, L.; Jacquet, S. Picophytoplankton Biomass Distribution in the Global Ocean. Earth Syst. Sci. Data 2012, 4, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Robbins, L.; Tao, Y.; Evans, C. Temporal and Spatial Distribution of Whitings on Great Bahama Bank and a New Lime Mud Budget. Geology 1997, 25, 947–950. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Schultze-Lam, S.; Beveridge, T.J.; Des Marais, D.J. Whiting Events: Biogenic Origin Due to the Photosynthetic Activity of Cyanobacterial Picoplankton. Limnol. Oceanogr. 1997, 42, 133–141. [Google Scholar] [CrossRef]
- Thompson, J.B.; Ferris, F.G. Cyanobacterial Precipitation of Gypsum, Calcite, and Magnesite from Natural Alkaline Lake Water. Geology 1990, 18, 995. [Google Scholar] [CrossRef]
- Danckwerts, P.; Melkersson, K.-A. Kinetics of the Conversion of Bicarbonate to Carbon Dioxide. Trans. Faraday Soc. 1962, 58, 1832–1838. [Google Scholar] [CrossRef]
- Heywood, B.R.; Mann, S. Template-directed Nucleation and Growth of Inorganic Materials. Adv. Mater. 1994, 6, 9–20. [Google Scholar] [CrossRef]
- Heywood, B.R.; Mann, S. Molecular Construction of Oriented Inorganic Materials: Controlled Nucleation of Calcite and Aragonite under Compressed Langmuir Monolayers. Chem. Mater. 1994, 6, 311–318. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The Influence of Bacillus Pasteurii on the Nucleation and Growth of Calcium Carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of Carbonate Precipitation in Modern Microbial Mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Kamennaya, N.; Ajo-Franklin, C.; Northen, T.; Jansson, C. Cyanobacteria as Biocatalysts for Carbonate Mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric Substances (EPS) from Bacillus Subtilis: Polymers and Genes Encoding Their Synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, M.; Sibler, S. Calcium Carbonate Precipitation by Cyanobacterial Polysaccharides. Geol. Soc. Lond. Spec. Publ. 2010, 336, 51–63. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbial Lithification in Marine Stromatolites and Hypersaline Mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichet, J.; Defarge, C. Non-Biologically Supported Organomineralization, Bulletin de l’Institut Océanographique (Monaco) Numéro Spécial 14; de l’Institut Oceanographique (Monaco): Monaco City, Monaco, 1995; 203–236.
- Decho, A.W. Microbial Exopolymer Secretions in Ocean Environments: Their Role (s) in Food Webs and Marine Processes. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 73–153. [Google Scholar]
- Moreno, J.; Vargas, M.A.; Olivares, H.; Rivas, J.; Guerrero, M.G. Exopolysaccharide Production by the Cyanobacterium Anabaena sp. ATCC 33047 in Batch and Continuous Culture. J. Biotechnol. 1998, 60, 175–182. [Google Scholar] [CrossRef]
- Simon, M.; Grossart, H.-P.; Schweitzer, B.; Ploug, H. Microbial Ecology of Organic Aggregates in Aquatic Ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef] [Green Version]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Aikawa, H.; Hayashi, H.; Yuasa, A.; Hirata, A. Extracellular Polymeric Substances Responsible for Bacterial Adhesion onto Solid Surface. FEMS Microbiol. Lett. 2003, 223, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Wang, L.-F.; Ren, X.-M.; Ye, X.-D.; Li, W.-W.; Yuan, S.-J.; Sun, M.; Sheng, G.-P.; Yu, H.-Q.; Wang, X.-K. PH Dependence of Structure and Surface Properties of Microbial EPS. Environ. Sci. Technol. 2012, 46, 737–744. [Google Scholar] [CrossRef]
- Santschi, P.H.; Chin, W.-C.; Quigg, A.; Xu, C.; Kamalanathan, M.; Lin, P.; Shiu, R.-F. Marine Gel Interactions with Hydrophilic and Hydrophobic Pollutants. Gels 2021, 7, 83. [Google Scholar] [CrossRef]
- Moore, K.R.; Gong, J.; Pajusalu, M.; Skoog, E.J.; Xu, M.; Feliz Soto, T.; Sojo, V.; Matreux, T.; Baldes, M.J.; Braun, D. A New Model for Silicification of Cyanobacteria in Proterozoic Tidal Flats. Geobiology 2021, 19, 438–449. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [Green Version]
- Bertilsson, S.; Jones Jr, J.B. Supply of Dissolved Organic Matter to Aquatic Ecosystems: Autochthonous Sources. In Aquatic Ecosystems; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3–24. [Google Scholar]
- Fisher, M.L.; Allen, R.; Luo, Y.; Curtiss III, R. Export of Extracellular Polysaccharides Modulates Adherence of the Cyanobacterium Synechocystis. PLoS ONE 2013, 8, e74514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoiczyk, E. A Molecular Motor for Gliding Motility in Cyanobacteria. J. Phycol. 2000, 36, 30–31. [Google Scholar] [CrossRef]
- Billi, D.; Potts, M. Life and Death of Dried Prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Scherer, S. UV Protection in Cyanobacteria. Eur. J. Phycol. 1999, 34, 329–338. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular Polysaccharides from Cyanobacteria and Their Possible Applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Deng, W.; Cruz, B.N.; Neuer, S. Effects of Nutrient Limitation on Cell Growth, TEP Production and Aggregate Formation of Marine Synechococcus. Aquat. Microb. Ecol. 2016, 78, 39–49. [Google Scholar] [CrossRef]
- Pannard, A.; Pedrono, J.; Bormans, M.; Briand, E.; Claquin, P.; Lagadeuc, Y. Production of Exopolymers (EPS) by Cyanobacteria: Impact on the Carbon-to-Nutrient Ratio of the Particulate Organic Matter. Aquat. Ecol. 2016, 50, 29–44. [Google Scholar] [CrossRef]
- Bemal, S.; Anil, A.C. Effects of Salinity on Cellular Growth and Exopolysaccharide Production of Freshwater Synechococcus Strain CCAP1405. J. Plankton Res. 2018, 40, 46–58. [Google Scholar] [CrossRef]
- Li, D.; Wu, N.; Tang, S.; Su, G.; Li, X.; Zhang, Y.; Wang, G.; Zhang, J.; Liu, H.; Hecker, M. Factors Associated with Blooms of Cyanobacteria in a Large Shallow Lake, China. Environ. Sci. Eur. 2018, 30, 27. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Gann, E.R.; Martin, R.M.; Pound, H.L.; Krausfeldt, L.E.; Chaffin, J.D.; Wilhelm, S.W. Elevated PH Conditions Associated With Microcystis spp. Blooms Decrease Viability of the Cultured Diatom Fragilaria Crotonensis and Natural Diatoms in Lake Erie. Front. Microbiol. 2021, 12, 188. [Google Scholar] [CrossRef]
- Passow, U. Transparent Exopolymer Particles (TEP) in Aquatic Environments. Prog. Oceanogr. 2002, 55, 287–333. [Google Scholar] [CrossRef] [Green Version]
- Iuculano, F.; Mazuecos, I.P.; Reche, I.; Agustí, S. Prochlorococcus as a Possible Source for Transparent Exopolymer Particles (TEP). Front. Microbiol. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, A. Distribution of Transparent Exopolymer Particles (TEP) in the Northeast Atlantic Ocean and Their Potential Significance for Aggregation Processes. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Callieri, C. Picophytoplankton in Freshwater Ecosystems: The Importance of Small-Sized Phototrophs. Freshw. Rev. 2008, 1, 1–28. [Google Scholar] [CrossRef]
- Cruz, B.N.; Neuer, S. Heterotrophic Bacteria Enhance the Aggregation of the Marine Picocyanobacteria Prochlorococcus and Synechococcus. Front. Microbiol 2019, 10, 1864. [Google Scholar] [CrossRef] [Green Version]
- Robbins, L.; Blackwelder, P. Biochemical and Ultrastructural Evidence for the Origin of Whitings: A Biologically Induced Calcium Carbonate Precipitation Mechanism. Geology 1992, 20, 464–468. [Google Scholar] [CrossRef]
- Larson, E.B.; Mylroie, J.E. A Review of Whiting Formation in the Bahamas and New Models. Carbonates Evaporites 2014, 29, 337–347. [Google Scholar] [CrossRef]
- Dittrich, M.; Obst, M. Are Picoplankton Responsible for Calcite Precipitation in Lakes? AMBIO 2004, 33, 6. [Google Scholar] [CrossRef]
- Ohlendorf, C.; Sturm, M. Precipitation and Dissolution of Calcite in a Swiss High Alpine Lake. Arct. Antarct. Alp. Res. 2001, 33, 410–417. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Harauz, G.; Beveridge, T. Participation of a Cyanobacterial S Layer in Fine-Grain Mineral Formation. J. Bacteriol. 1992, 174, 7971–7981. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, M.; Müller, B.; Mavrocordatos, D.; Wehrli, B. Induced Calcite Precipitation by Cyanobacterium Synechococcus. Acta Hydrochim. Hydrobiol. 2003, 31, 162–169. [Google Scholar] [CrossRef]
- Lee, B.D.; Apel, W.A.; Walton, M.R. Screening of Cyanobacterial Species for Calcification. Biotechnol. Prog. 2004, 20, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Dynes, J.J.; Lawrence, J.R.; Swerhone, G.D.W.; Benzerara, K.; Karunakaran, C.; Kaznatcheev, K.; Tyliszczak, T.; Hitchcock, A.P. Precipitation of Amorphous CaCO3 (Aragonite-like) by Cyanobacteria: A STXM Study of the Influence of EPS on the Nucleation Process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar] [CrossRef]
- Liang, A.; Paulo, C.; Zhu, Y.; Dittrich, M. CaCO3 Biomineralization on Cyanobacterial Surfaces: Insights from Experiments with Three Synechococcus Strains. Colloids Surf. B Biointerfaces 2013, 111, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.K.; Robbins, L.L. Production of Carbonate Sediments by a Unicellular Green Alga. Am. Mineral. 1998, 83, 1503–1509. [Google Scholar] [CrossRef]
- Allen, M.M. SIMPLE CONDITIONS FOR GROWTH OF UNICELLULAR BLUE-GREEN ALGAE ON PLATES 1, 2. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; p. a5606. ISBN 978-0-470-02731-8. [Google Scholar]
- de Moreno, M.R.; Smith, J.F.; Smith, R.V. Silver Staining of Proteins in Polyacrylamide Gels: Increased Sensitivity through a Combined Coomassie Blue-Silver Stain Procedure. Anal. Biochem. 1985, 151, 466–470. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Oudot, M.; Shir, I.B.; Schmidt, A.; Plasseraud, L.; Broussard, C.; Neige, P.; Marin, F. A Nature’s Curiosity: The Argonaut “Shell” and Its Organic Content. Crystals 2020, 10, 839. [Google Scholar] [CrossRef]
- Morrissey, J.H. Silver Stain for Proteins in Polyacrylamide Gels: A Modified Procedure with Enhanced Uniform Sensitivity. Anal. Biochem. 1981, 117, 307–310. [Google Scholar] [CrossRef]
- Wall, R.S.; Gyi, T.J. Alcian Blue Staining of Proteoglycans in Polyacrylamide Gels Using the “Critical Electrolyte Concentration” Approach. Anal. Biochem. 1988, 175, 298–299. [Google Scholar] [CrossRef]
- Blake, D.C.; Russell, R.G. Demonstration of Lipopolysaccharide with O-Polysaccharide Chains among Different Heat-Stable Serotypes of Campylobacter Jejuni by Silver Staining of Polyacrylamide Gels. Infect. Immun 1993, 61, 5384–5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korenevsky, A.A.; Vinogradov, E.; Gorby, Y.; Beveridge, T.J. Characterization of the Lipopolysaccharides and Capsules of Shewanella spp. Appl. Environ. Microbiol. 2002, 68, 4653–4657. [Google Scholar] [CrossRef] [Green Version]
- Marin, F.; Pereira, L.; Westbroek, P. Large-Scale Fractionation of Molluscan Shell Matrix. Protein Expr. Purif. 2001, 23, 175–179. [Google Scholar] [CrossRef]
- Wheeler, A.; George, J.W.; Evans, C. Control of Calcium Carbonate Nucleation and Crystal Growth by Soluble Matrx of Oyster Shell. Science 1981, 212, 1397–1398. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Decho, A.W. ISOLATION AND BIOCHEMICAL CHARACTERIZATION OF EXTRACELLULAR POLYMERIC SECRETIONS (EPS) FROM MODERN SOFT MARINE STROMATOLITES (BAHAMAS) AND ITS INHIBITORY EFFECT ON CACO3 PRECIPITATION. Prep. Biochem. Biotechnol. 2002, 32, 51–63. [Google Scholar] [CrossRef]
- Marin, F.; Corstjens, P.; de Gaulejac, B.; de Vrind-De Jong, E.; Westbroek, P. Mucins and Molluscan Calcification: Molecular Characterization of Mucoperlin, a Novel Mucin-like Protein from the Nacreous Shell Layer of the Fan Mussel Pinna Nobilis (Bivalvia, Pteriomorphia). J. Biol. Chem. 2000, 275, 20667–20675. [Google Scholar] [CrossRef] [Green Version]
- Albeck, S.; Aizenberg, J.; Addadi, L.; Weiner, S. Interactions of Various Skeletal Intracrystalline Components with Calcite Crystals. J. Am. Chem. Soc. 1993, 115, 11691–11697. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Kallas, T.; Castenholz, R.W. Rapid Transient Growth at Low PH in the Cyanobacterium Synechococcus sp. J. Bacteriol 1982, 149, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldez-Ruiz, N.; Mateo, P.; Bonilla, I.; Fernandez-Piñas, F. The Relationship between Intracellular PH, Growth Characteristics and Calcium in the Cyanobacterium Anabaena sp. Strain PCC7120 Exposed to Low PH. New Phytol. 1997, 137, 599–605. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of High PH on Growth of Synechocystis sp. PCC 6803 Cultures and Their Contamination by Golden Algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurian, D.; Phadwal, K.; Mäenpää, P. Proteomic Characterization of Acid Stress Response in Synechocystis sp. PCC 6803. Proteomics 2006, 6, 3614–3624. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Yang, H.-M.; Cui, S.-X.; Hu, J.; Wang, J.; Kuang, T.-Y.; Norling, B.; Huang, F. Proteomic Analysis of Plasma Membranes of Cyanobacterium Synechocystis sp. Strain PCC 6803 in Response to High PH Stress. J. Proteome Res. 2009, 8, 2892–2902. [Google Scholar] [CrossRef]
- Dogsa, I.; Kriechbaum, M.; Stopar, D.; Laggner, P. Structure of Bacterial Extracellular Polymeric Substances at Different PH Values as Determined by SAXS. Biophys. J. 2005, 89, 2711–2720. [Google Scholar] [CrossRef] [Green Version]
- De Philippis, R.; Sili, C.; Paperi, R.; Vincenzini, M. Exopolysaccharide-Producing Cyanobacteria and Their Possible Exploitation: A Review. J. Appl. Phycol. 2001, 13, 293–299. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of Cyanobacterial Exopolysaccharides: Composition, Structures, Inducing Factors and Putative Genes Involved in Their Biosynthesis and Assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Pereira, S.B.; Mota, R.; Vieira, C.P.; Vieira, J.; Tamagnini, P. Phylum-Wide Analysis of Genes/Proteins Related to the Last Steps of Assembly and Export of Extracellular Polymeric Substances (EPS) in Cyanobacteria. Sci. Rep. 2015, 5, 14835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoenix, V.R.; Martinez, R.E.; Konhauser, K.O.; Ferris, F.G. Characterization and Implications of the Cell Surface Reactivity of Calothrix sp. Strain KC97. Appl. Environ. Microbiol. 2002, 68, 4827–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fein, J.B.; Boily, J.-F.; Yee, N.; Gorman-Lewis, D.; Turner, B.F. Potentiometric Titrations of Bacillus Subtilis Cells to Low PH and a Comparison of Modeling Approaches. Geochim. Cosmochim. Acta 2005, 69, 1123–1132. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric Substances of Sulfate-Reducing Bacteria: Interactions with Calcium at Alkaline PH and Implication for Formation of Carbonate Minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- d’Abzac, P.; Bordas, F.; Joussein, E.; van Hullebusch, E.D.; Lens, P.N.; Guibaud, G. Metal Binding Properties of Extracellular Polymeric Substances Extracted from Anaerobic Granular Sludges. Environ. Sci. Pollut. Res. 2013, 20, 4509–4519. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, C.; Benedetti, M.; Wilkinson, K.J.; Slaveykova, V.I. Characterization of H+ and Cd2+ Binding Properties of the Bacterial Exopolysaccharides. Chemosphere 2006, 65, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H. Characterization of Electrostatic Binding Sites of Extracellular Polymers by Linear Programming Analysis of Titration Data. Biotechnol. Bioeng. 2002, 80, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0-470-01113-0. [Google Scholar]
- Parikh, A.; Madamwar, D. Partial Characterization of Extracellular Polysaccharides from Cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic De-Regulation in Cyanobacteria in Response to Abiotic Stresses. Front. Microbiol. 2019, 10, 1315. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Beardall, J.; Sánchez-Baracaldo, P. The Possible Evolution and Future of CO2-Concentrating Mechanisms. J. Exp. Bot. 2017, 68, 3701–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, G.D.; Badger, M.R.; Box, P.O. Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO2-Requiring Phenotype. Plant Physiol. 1989, 91, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, G.D.; Pengelly, J.J.; Forster, B.; Du, J.; Whitney, S.M.; von Caemmerer, S.; Badger, M.R.; Howitt, S.M.; Evans, J.R. The Cyanobacterial CCM as a Source of Genes for Improving Photosynthetic CO2 Fixation in Crop Species. J. Exp. Bot. 2013, 64, 753–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badger, M.R.; Price, G.D. The CO2concentrating Mechanism in Cyanobacteria and Microalgae. Physiol. Plant. 1992, 84, 606–615. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Bedbenov, V.S.; Pronina, N.A.; Los, D.A. Putative Extracellular α-Class Carbonic Anhydrase, EcaA, of Synechococcus Elongatus PCC 7942 Is an Active Enzyme: A Sequel to an Old Story. Microbiology 2018, 164, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Chrachri, A.; Hopkinson, B.M.; Flynn, K.; Brownlee, C.; Wheeler, G.L. Dynamic Changes in Carbonate Chemistry in the Microenvironment around Single Marine Phytoplankton Cells. Nat. Commun. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badger, M.R.; Price, G.D. The Role of Carbonic Anhydrase in Photosynthesis. Annu. Rev. Plant Biol. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. Carbonic Anhydrase Activity Associated with the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989, 89, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Lee, C.; Lim, S.W.; Adhikari, A.; Andring, J.T.; McKenna, R.; Ghim, C.-M.; Kim, C.U. Elucidating the Role of Metal Ions in Carbonic Anhydrase Catalysis. Nat. Commun. 2020, 11, 4557. [Google Scholar] [CrossRef]
- Jong, E.W.; Bosch, L.; Westbroek, P. Isolation and Characterization of a Cat 2+-Binding Polysaccharide Associated with Coccoliths of Emiliania Huxleyi (Lohmann) Kamptner. Eur. J. Biochem. 1976, 70, 611–621. [Google Scholar] [CrossRef]
- Gunthorpe, M.; Sikes, C.; Wheeler, A. Promotion and Inhibition of Calcium Carbonate Crystallization in Vitro by Matrix Protein from Blue Crab Exoskeleton. Biol. Bull. 1990, 179, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Stipp, S.L.S.; Harding, J. Biological Control on Calcite Crystallization by Polysaccharides. Cryst. Growth Des. 2008, 8, 4066–4074. [Google Scholar] [CrossRef]
- Walker, J.M.; Marzec, B.; Lee, R.B.Y.; Vodrazkova, K.; Day, S.J.; Tang, C.C.; Rickaby, R.E.M.; Nudelman, F. Polymorph Selectivity of Coccolith-Associated Polysaccharides from Gephyrocapsa Oceanica on Calcium Carbonate Formation In Vitro. Adv. Funct. Mater. 2019, 29, 1807168. [Google Scholar] [CrossRef] [Green Version]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-Induced Biofilm Calcification and Calcium Concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of Model Globular Proteins with Different Isoelectric Points on the Precipitation of Calcium Carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Verrecchia, E.P.; Freytet, P.; Verrecchia, K.E.; Dumont, J.-L. Spherulites in Calcrete Laminar Crusts; Biogenic CaCO3 Precipitation as a Major Contributor to Crust Formation. J. Sediment. Res. 1995, 65, 690–700. [Google Scholar]
- Saraya, M.E.-S.I.; Rokbaa, H.H.A.E.-L. Formation and Stabilization of Vaterite Calcium Carbonate by Using Natural Polysaccharide. Adv. Nanopart. 2017, 6, 158. [Google Scholar]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- McEwen, J.T.; Machado, I.M.; Connor, M.R.; Atsumi, S. Engineering Synechococcus Elongatus PCC 7942 for Continuous Growth under Diurnal Conditions. Appl. Environ. Microbiol. 2013, 79, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Morales, E.; Rodríguez, M.; García, D.; Loreto, C.; Marco, E. Crecimiento, Producción de Pigmentos y Exopolisacáridos de La Cianobacteria Anabaena sp. PCC 7120 En Función Del PH y CO2. Interciencia 2002, 27, 373–378. [Google Scholar]
- De Farias Silva, C.E.; Sforza, E.; Bertucco, A. Effects of PH and Carbon Source on Synechococcus PCC 7002 Cultivation: Biomass and Carbohydrate Production with Different Strategies for PH Control. Appl. Biochem. Biotechnol. 2017, 181, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Kurian, N.; Hall, C.J.; Wilkinson, G.F.; Sullivan, M.; Tobin, A.B.; Willars, G.B. Full and Partial Agonists of Muscarinic M3 Receptors Reveal Single and Oscillatory Ca2+ Responses by Β2-Adrenoceptors. J. Pharmacol. Exp. Ther. 2009, 330, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, A.; Schwarz, R.; Lieman-Hurwitz, J.; Reinhold, L. Physiological and Molecular Aspects of the Inorganic Carbon-Concentrating Mechanism in Cyanobacteria. Plant Physiol. 1991, 97, 851–855. [Google Scholar] [CrossRef]
- Maeda, S.; Price, G.D.; Badger, M.R.; Enomoto, C.; Omata, T. Bicarbonate Binding Activity of the CmpA Protein of the Cyanobacterium Synechococcus sp. Strain PCC 7942 Involved in Active Transport of Bicarbonate. J. Biol. Chem. 2000, 275, 20551–20555. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, K.; Dupraz, C.; Braissant, O.; Norman, R.; Decho, A.; Visscher, P. Mineralization of Sedimentary Biofilms: Modern Mechanistic Insights. In Biofilm: Formation, Development and Properties.Bailley; Columbus, F., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 227–258. [Google Scholar]
- Ibelings, B.W.; Maberly, S.C. Photoinhibition and the Availability of Inorganic Carbon Restrict Photosynthesis by Surface Blooms of Cyanobacteria. Limnol. Oceanogr. 1998, 43, 408–419. [Google Scholar] [CrossRef]
- Verspagen, J.M.; Van de Waal, D.B.; Finke, J.F.; Visser, P.M.; Van Donk, E.; Huisman, J. Rising CO2 Levels Will Intensify Phytoplankton Blooms in Eutrophic and Hypertrophic Lakes. PLoS ONE 2014, 9, e104325. [Google Scholar]
- Liao, J.; Zhao, L.; Cao, X.; Sun, J.; Gao, Z.; Wang, J.; Jiang, D.; Fan, H.; Huang, Y. Cyanobacteria in Lakes on Yungui Plateau, China Are Assembled via Niche Processes Driven by Water Physicochemical Property, Lake Morphology and Watershed Land-Use. Sci. Rep. 2016, 6, 36357. [Google Scholar] [CrossRef] [Green Version]
- Hoiczyk, E.; Hansel, A. Cyanobacterial Cell Walls: News from an Unusual Prokaryotic Envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- De Philippis, R.; Sili, C.; Vincenzini, M. Response of an Exopolysaccharide-Producing Heterocystous Cyanobacterium to Changes in Metabolic Carbon Flux. J. Appl. Phycol. 1996, 8, 275–281. [Google Scholar] [CrossRef]
- Sohm, J.A.; Webb, E.A.; Capone, D.G. Emerging Patterns of Marine Nitrogen Fixation. Nat. Rev. Microbiol. 2011, 9, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.; Bhosle, N.B. Microbial Extracellular Polymeric Substances in Marine Biogeochemical Processes. Curr. Sci. 2005, 88, 45–53. [Google Scholar]
- Dam, H.G.; Drapeau, D.T. Coagulation Efficiency, Organic-Matter Glues and the Dynamics of Particles during a Phytoplankton Bloom in a Mesocosm Study. Deep Sea Res. Part II: Top. Stud. Oceanogr. 1995, 42, 111–123. [Google Scholar] [CrossRef]
- Passow, U. Formation of Transparent Exopolymer Particles, TEP, from Dissolved Precursor Material. Mar. Ecol. Prog. Ser. 2000, 192, 1–11. [Google Scholar] [CrossRef]
- Callieri, C. Synechococcus Plasticity under Environmental Changes. FEMS Microbiol. Lett. 2017, 364, fnx229. [Google Scholar] [CrossRef]
- Polowczyk, I.; Bastrzyk, A.; Fiedot, M. Protein-Mediated Precipitation of Calcium Carbonate. Materials 2016, 9, 944. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S.; Oliver, R.L.; Walsby, A.E. Cyanobacterial Dominance: The Role of Buoyancy Regulation in Dynamic Lake Environments. N. Z. J. Mar. Freshw. Res. 1987, 21, 379–390. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1999; ISBN 0-07-016384-7. [Google Scholar]
- Azetsu-Scott, K.; Passow, U. Ascending Marine Particles: Significance of Transparent Exopolymer Particles (TEP) in the Upper Ocean. Limnol. Oceanogr. 2004, 49, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Thornton, D.C. Phytoplankton Mucilage Production in Coastal Waters: A Dispersal Mechanism in a Front Dominated System? Ethol. Ecol. Evol. 1999, 11, 179–185. [Google Scholar] [CrossRef]
- Bach, L.T.; Stange, P.; Taucher, J.; Achterberg, E.P.; Algueró-Muñiz, M.; Horn, H.; Esposito, M.; Riebesell, U. The Influence of Plankton Community Structure on Sinking Velocity and Remineralization Rate of Marine Aggregates. Glob. Biogeochem. Cycles 2019, 33, 971–994. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, I.; Wagermaier, W. Tailoring Calcium Carbonate to Serve as Optical Functional Material: Examples from Biology and Materials Science. Adv. Mater. Interfaces 2017, 4, 1600250. [Google Scholar] [CrossRef] [Green Version]
- Grossart, H.; Simon, M. Limnetic Macroscopic Organic Aggregates (Lake Snow): Occurrence, Characteristics, and Microbial Dynamics in Lake Constance. Limnol. Oceanogr. 1993, 38, 532–546. [Google Scholar] [CrossRef]






| Growth Conditions | ||
|---|---|---|
| Non-Buffered | Buffered | |
| Cell yield (×1011 L−1) | 5.1 ± 0.2 | 2.1 ± 0.1 |
| Total EPS (HMW and LMW fractions) (mg·L−1) | 14.2 ± 1.5 | 15.3 ± 2.0 |
| High Molecular Weight EPS * (mg·L−1) | 12.7 ± 1.0 | 9.3 ± 1.7 |
| Low Molecular Weight EPS ** (mg·L−1) | 1.5 ± 0.5 | 6.0 ± 0.3 |
| Cell-specific EPS production (mg·106 cells−1) | (2.3 ± 0.84) × 10−5 | (7.5 ± 0.98) × 10−5 |
| Growth Conditions | ||
|---|---|---|
| Non-Buffered | Buffered | |
| Sugar (µg xanthan equivalents·mg−1 EPS) | 665 ± 65 | 400 ± 10 |
| Sugar (µg dextran equivalents·mg−1 EPS) | 775 ± 85 | 465 ± 15 |
| Protein (µg·mg−1 EPS) | 343 ± 132 | 32 ± 0.5 |
| Glycosaminoglycan (µg GAGs·mg−1 EPS) | 3.0 ± 1.7 | 3.1 ± 0.1 |
| Growth Conditions | ||
|---|---|---|
| Non-Buffered | Buffered | |
| Sugar (µg glucose equivalents·mg−1 EPS) | 620 ± 180 | 565 ± 115 |
| Protein (µg·mg−1 EPS) | 150 ± 30 | 58 ± 32 |
| Glycosaminoglycan (µg GAGs·mg−1 EPS) | 2.2 ± 0.5 | 1.9 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho de Brito, M.; Bundeleva, I.; Marin, F.; Vennin, E.; Wilmotte, A.; Plasseraud, L.; Visscher, P.T. Effect of Culture pH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation. Geosciences 2022, 12, 210. https://doi.org/10.3390/geosciences12050210
Martinho de Brito M, Bundeleva I, Marin F, Vennin E, Wilmotte A, Plasseraud L, Visscher PT. Effect of Culture pH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation. Geosciences. 2022; 12(5):210. https://doi.org/10.3390/geosciences12050210
Chicago/Turabian StyleMartinho de Brito, Marlisa, Irina Bundeleva, Frédéric Marin, Emmanuelle Vennin, Annick Wilmotte, Laurent Plasseraud, and Pieter T. Visscher. 2022. "Effect of Culture pH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation" Geosciences 12, no. 5: 210. https://doi.org/10.3390/geosciences12050210
APA StyleMartinho de Brito, M., Bundeleva, I., Marin, F., Vennin, E., Wilmotte, A., Plasseraud, L., & Visscher, P. T. (2022). Effect of Culture pH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation. Geosciences, 12(5), 210. https://doi.org/10.3390/geosciences12050210








