The Role of Fluids in Melting the Continental Crust and Generating Granitoids: An Overview
Abstract
:1. Introduction
2. Fluid-Absent Partial Melting
3. Fluid-Assisted Partial Melting
4. Effect of Dissolved Components in Fluids
5. Challenges and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust; Treatise on Geochemistry; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; pp. 1–64. [Google Scholar]
- Rudnick, R.L. Making continental crust. Nature 1995, 378, 571–578. [Google Scholar] [CrossRef]
- Sun, W.D.; Ling, M.X.; Yang, X.Y.; Fan, W.M.; Ding, X.; Liang, H.Y. Ridge subduction and porphyry copper-gold mineralization: An overview. Sci. China-Earth Sci. 2010, 10, 475–484. [Google Scholar] [CrossRef]
- Sun, S.J.; Yang, X.Y.; Wang, G.J.; Sun, W.D.; Zhang, H.Z.; Li, C.Y.; Ding, X. In situ elemental and Sr-O isotopic studies on apatite from the Xu-Huai intrusion at the southern margin of the North China Craton: Implications for petrogenesis and metallogeny. Chem. Geol. 2019, 510, 200–214. [Google Scholar] [CrossRef]
- Mao, J.W.; Cheng, Y.B.; Chen, M.H.; Franco, P. Major types and time–space distribution of Mesozoic ore deposits in South China and their geodynamic settings. Miner. Depos. 2013, 48, 267–294. [Google Scholar] [CrossRef]
- Ballouard, C.; Massuyeau, M.; Elburg, M.A.; Tappe, S.; Viljoen, F.; Brandenburg, J.T. The magmatic and magmatic-hydrothermal evolution of felsic igneous rocks as seen through Nb-Ta geochemical fractionation, with implications for the origins of rare-metal mineralizations. Earth-Sci. Rev. 2020, 203, 103115. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.X.; Guo, L.; Zhang, L.; Tong, Y. Granitoid and tectonics. Acta Petrol. Sin. 2017, 33, 1459–1478. (In Chinese) [Google Scholar]
- Arndt, N.T. The formation and evolution of the continental crust. Geochem. Perspect. 2013, 2, 405–533. [Google Scholar] [CrossRef]
- Fyfe, W.S. Granulite facies partial melting and Archaean crust. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1973, 273, 457–461. [Google Scholar] [CrossRef]
- Brown, M.; Rushmer, T. Evolution and Differentiation of the Continental Crust; Cambridge University Press: Cambridge, UK, 2008; p. 553. [Google Scholar]
- Chen, C.; Ding, X.; Li, R.; Zhang, W.Q.; Ouyang, D.J.; Yang, L.; Sun, W.D. Crystal fractionation of granitic magma during its non-transport processes: A physics-based perspective. Sci. China-Earth Sci. 2018, 61, 190–204. [Google Scholar] [CrossRef]
- Ding, X.; Lundstrom, C.; Huang, F.; Li, J.; Zhang, Z.M.; Sun, X.M.; Liang, J.L.; Sun, W.D. Natural and experimental constraints on formation of the continental crust based on niobium-tantalum fractionation. Int. Geol. Rev. 2009, 51, 473–501. [Google Scholar] [CrossRef]
- Sun, W.D.; Binns, R.A.; Fan, A.C.; Kamenetsky, V.S.; Wysoczanski, R.; Wei, G.J.; Hu, Y.H.; Arculus, R.J. Chlorine in submarine volcanic glasses from the eastern Manus basin. Geochim. Cosmochim. Acta 2007, 71, 1542–1552. [Google Scholar] [CrossRef]
- Winkler, H.G.F.; Platen, H.V. Experimentelle gesteinsmetamorphose—V: Experimentelle anatektische schmelzen und ihre petrogenetische bedeutung. Geochim. Cosmochim. Acta 1961, 24, 250–259. [Google Scholar] [CrossRef]
- Wyllie, P.J. Crustal anatexis: An experimental review. Tectonophysics 1977, 43, 41–71. [Google Scholar] [CrossRef]
- Rushmer, T. Partial melting of two amphibolites—Contrasting experimental results under fluid-absent conditions. Contrib. Mineral. Petrol. 1991, 107, 41–59. [Google Scholar] [CrossRef]
- Rogers, G.; Hawkesworth, C.J. A geochemical traverse across the north chilean andes—Evidence for crust generation from the mantle wedge. Earth Planet. Sci. Lett. 1989, 91, 271–285. [Google Scholar] [CrossRef]
- Atherton, M.P. Granite magmatism. J. Geol. Soc. 1993, 150, 1009–1023. [Google Scholar] [CrossRef]
- Sawyer, E.W. Formation and evolution of granite magmas during crustal reworking: The significance of diatexites. J. Petrol. 1998, 39, 1147–1167. [Google Scholar] [CrossRef]
- Sawyer, E.W.; Cesare, B.; Brown, M. When the continental crust melts. Elements 2011, 7, 229–233. [Google Scholar] [CrossRef]
- Douce, A.E.P. What do experiments tell us about the relative contributions of crust and mantle to the origin of granitic magmas? Underst. Granites Integr. New Class. Tech. 1999, 168, 55–75. [Google Scholar] [CrossRef]
- Ding, X.; Su, K.L.; Yan, H.B.; Liang, J.L.; Sun, W.D. Effect of F-rich fluids on the A-type magmatism and related rare metal mobilities: New insights from the Fogang-Nankunshan-Yajishan igneous rocks in Southeast China. J. Earth Sci. 2022, 33, 591–608. [Google Scholar] [CrossRef]
- Luo, Z.H.; Huang, Z.M.; Ke, S. An overview of granitoid. Geol. Rev. 2007, 558, 180–226. (In Chinese) [Google Scholar]
- Tuttle, O.F.; Bowen, N.L. Origin of Granite in the Light of Experimental Studies in the System NaAlSi3O8-KAlSi3O8-SiO2-H2O; Geological Society of America: Boulder, CO, USA, 1958; Volume 74. [Google Scholar] [CrossRef]
- Sen, C.; Dunn, T. Dehydration melting of a basaltic composition amphibolite at 1.5 and 2.0 GPa: Implications for the origin of adakites. Contrib. Mineral. Petrol. 1994, 117, 394–409. [Google Scholar] [CrossRef]
- Baker, M.B.; Stolper, E.M. Determining the composition of high-pressure mantle melts using diamond aggregates. Geochim. Cosmochim. Acta 1994, 58, 2811–2827. [Google Scholar] [CrossRef]
- Falloon, T.J.; Green, D.H.; Danyushevsky, L.V.; Faul, U.H. Peridotite melting at 1.0 and 1.5 GPa: An experimental evaluation of techniques using diamond aggregates and mineral mixes for determination of near-solidus melts. J. Petrol. 1999, 40, 1343–1375. [Google Scholar] [CrossRef]
- Hirose, K.; Kushiro, I. Partial melting of dry peridotites at high-pressures: Determination of compositions of melts segregated from peridotite using aggregates of diamond. Earth Planet. Sci. Lett. 1993, 114, 477–489. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Kushiro, I. Segregation of high-pressure partial melts from peridotite using aggregates of diamond—A new experimental approach. Geophys. Res. Lett. 1992, 19, 1703–1706. [Google Scholar] [CrossRef]
- Xiong, X.L.; Adam, J.; Green, T.H.; Niu, H.C. Characteristics of trace elements in partial melting of metabasalt and generation conditions of adakite melt. Sci. China Ser. D Earth Sci. 2005, 35, 837–846. [Google Scholar]
- Gao, P.; Zheng, Y.F.; Zhao, Z.F. Experimental melts from crustal rocks: A lithochemical constraint on granite petrogenesis. Lithos 2016, 266, 133–157. [Google Scholar] [CrossRef]
- Chen, G.N. Advances in the study of genesis and metallogeny of granite: A brief introduction of the melting in situ hypothesis and geochemical field of the elements. Adv. Earth Sci. 1998, 13, 29–33. (In Chinese) [Google Scholar] [CrossRef]
- Gardien, V.; Thompson, A.B.; Ulmer, P. Melting of biotite+plagioclase+quartz gneisses: The role of H2O in the stability of amphibole. J. Petrol. 2000, 41, 651–666. [Google Scholar] [CrossRef]
- Yakymchuk, C. On Granites. J. Geol. Soc. India 2019, 94, 9–22. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gao, P. The production of granitic magmas through crustal anatexis at convergent plate boundaries. Lithos 2021, 402–403, 106232. [Google Scholar] [CrossRef]
- Annen, C.; Blundy, J.D.; Sparks, R.S.J. The sources of granitic melt in deep hot zones. Trans. R. Soc. Edinb.-Earth Sci. 2006, 97, 297–309. [Google Scholar] [CrossRef]
- Ding, X.; Sun, W.D.; Chen, W.F.; Chen, P.R.; Sun, T.; Sun, S.J.; Lin, C.T.; Chen, F.K. Multiple Mesozoic magma processes formed the 240-185 Ma composite Weishan pluton, South China: Evidence from geochronology, geochemistry, and Sr-Nd isotopes. Int. Geol. Rev. 2015, 57, 1189–1217. [Google Scholar] [CrossRef]
- Sun, S.J.; Zhang, R.Q.; Cong, Y.N.; Zhang, L.P.; Sun, W.D.; Li, C.Y.; Ding, X. Analogous diagenetic conditions of dark enclave and its host granite derived by magma mixing: Evidence for a post-mixing magmatic process. Lithos 2020, 356, 105373. [Google Scholar] [CrossRef]
- Liu, J.Z.; Li, S.Z. Dehydration melting experiment and the influence factors. World Geol. 1995, 14, 10–16. (In Chinese) [Google Scholar]
- Wyllie, P.J.; Tuttle, O.F. Experimental investigation of silicate systems containing two volatile components—Part 3, The effects of SO3, P2O5, HCl, and Li2O, in addition to H2O, on the melting temperatures of albite and granite. Am. J. Sci. 1964, 262, 930–939. [Google Scholar] [CrossRef]
- Xiong, X.L.; Zhao, Z.H.; Zhu, J.C.; Rao, B. Phase relations in albite granite-H2O-HF system and their petrogenetic application. Geochem. J. 1999, 33, 199–214. [Google Scholar] [CrossRef]
- Yardley, B.W.D.; Valley, J.W. The petrologic case for a dry lower crust. J. Geophys. Res.-Solid Earth 1997, 102, 12173–12185. [Google Scholar] [CrossRef]
- Clemens, J.D.; Vielzeuf, D. Constraints on melting and magma production in the crust. Earth Planet. Sci. Lett. 1987, 86, 287–306. [Google Scholar] [CrossRef]
- Aranovich, L.Y.; Makhluf, A.R.; Manning, C.E.; Newton, R.C. Dehydration melting and the relationship between granites and granulites. Precambrian Res. 2014, 253, 26–37. [Google Scholar] [CrossRef]
- He, J.J.; Ding, X.; Wang, Y.R.; Sun, W.D.; Fu, B. The effect of temperature and concentration on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids: Constraints on titanium mobility in deep geological processes. Acta Petrol. Sin. 2015, 31, 802–810. (In Chinese) [Google Scholar]
- Su, K.L.; Ding, X.; Huang, Y.G.; Zheng, X.Z.; Wu, K.; Hu, Y.B. Compositional differentiation of Early Cretaceous Yajishan syenitic complex and its petrogenesis. Acta Petrol. Sin. 2015, 31, 829–845. (In Chinese) [Google Scholar]
- Yan, H.B.; Sun, W.D.; Liu, J.F.; Tu, X.L.; Ding, X. Thermodynamic properties of ruthenium (IV) chloride complex and the transport of ruthenium in magmatic-hydrothermal fluids. Ore Geol. Rev. 2021, 131, 104043. [Google Scholar] [CrossRef]
- Fyfe, W.S. Fluids in the Earth’s Crust; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Dong, Y.S. A review of researches on fluids in Arehean lower crust. World Geol. 2001, 20, 237–241. (In Chinese) [Google Scholar]
- Zheng, Y.F.; Chen, R.X.; Xu, Z.; Zhang, S.B. The transport of water in subduction zones. Sci. China-Earth Sci. 2016, 59, 651–682. [Google Scholar] [CrossRef]
- Clemens, J.D.; Droop, G.T.R. Fluids, P-T paths and the fates of anatectic melts in the Earth’s crust. Lithos 1998, 44, 21–36. [Google Scholar] [CrossRef]
- Mjelde, R.; Sellevoll, M.A.; Shimamura, H.; Iwwasaki, T.; Kanazawa, T. S-wave anisotropy off Lofoten, Norway, indicative of fluids in the lower continental crust? Geophys. J. Int. 1995, 120, 87–96. [Google Scholar] [CrossRef]
- Schwindinger, M.; Weinberg, R.F.; Clos, F. Wet or dry? The difficulty of identifying the presence of water during crustal melting. J. Metamorph. Geol. 2019, 37, 339–358. [Google Scholar] [CrossRef]
- Weinberg, R.F.; Hasalova, P. Water-fluxed melting of the continental crust: A review. Lithos 2015, 212, 158–188. [Google Scholar] [CrossRef]
- Aranovich, L.Y.; Newton, R.C.; Manning, C.E. Brine-assisted anatexis: Experimental melting in the system haplogranite-H2O-NaCl-KCl at deep-crustal conditions. Earth Planet. Sci. Lett. 2013, 374, 111–120. [Google Scholar] [CrossRef]
- Collins, W.J.; Huang, H.Q.; Jiang, X.Y. Water-fluxed crustal melting produces Cordilleran batholiths. Geology 2016, 44, 143–146. [Google Scholar] [CrossRef]
- Newton, R.C.; Touret, J.L.R.; Aranovich, L.Y. Fluids and H2O activity at the onset of granulite facies metamorphism. Precambrian Res. 2014, 253, 17–25. [Google Scholar] [CrossRef]
- Manning, C.E.; Aranovich, L.Y. Brines at high pressure and temperature: Thermodynamic, petrologic and geochemical effects. Precambrian Res. 2014, 253, 6–16. [Google Scholar] [CrossRef]
- Aranovich, L.Y. The role of brines in high-temperature metamorphism and granitization. Petrology 2017, 25, 486–497. [Google Scholar] [CrossRef]
- Pichavant, M. An experimental study of the effect of B on a water-saturated haplogranite at 1 kbar, 500–800 °C. J. Geol. Soc. 1981, 138, 214. [Google Scholar] [CrossRef]
- Safonov, O.G.; Kosova, S.A. Fluid-mineral reactions and melting of orthopyroxene-cordierite-biotite gneiss in the presence of H2O-CO2-NaCl and H2O-CO2-KCl fluids under parameters of granulite-facies metamorphism. Petrology 2017, 25, 458–485. [Google Scholar] [CrossRef]
- Safonov, O.G.; Kosova, S.A.; Van Reenen, D.D. Interaction of biotite-amphibole gneiss with H2O-CO2-(K, Na) Cl fluids at 550 MPa and 750 and 800 degrees C: Experimental study and applications to dehydration and partial melting in the middle crust. J. Petrol. 2014, 55, 2419–2455. [Google Scholar] [CrossRef]
- Patino Douce, A.E. Effects of pressure and H2O content on the compositions of primary crustal melts. Trans. R. Soc. Edinb.-Earth Sci. 1996, 87, 11–21. [Google Scholar] [CrossRef]
- Shmulovich, K.I.; Graham, C.M. Melting of albite and dehydration of brucite in H2O-NaCl fluids to 9 kbars and 700–900 degrees C: Implications for partial melting and water activities during high pressure metamorphism. Contrib. Mineral. Petrol. 1996, 124, 370–382. [Google Scholar] [CrossRef]
- Tsay, A.; Zajacz, Z.; Sanchez-Valle, C. Efficient mobilization and fractionation of rare-earth elements by aqueous fluids upon slab dehydration. Earth Planet. Sci. Lett. 2014, 398, 101–112. [Google Scholar] [CrossRef]
- Wood, B.J.; Blundy, J.A.D. The effect of H₂O on crystal-melt partitioning of trace elements. Geochim. Cosmochim. Acta 2002, 66, 3647–3656. [Google Scholar] [CrossRef]
- Clemens, J.D. Water contents of silicic to intermediate magmas. Lithos 1984, 17, 273–287. [Google Scholar] [CrossRef]
- Petö, P. An experimental investigation of melting relations involving muscovite and paragonite in the silica-saturated portion of the system K2O-Na2O-Al2O3-SiO2-H2O to 15 kb total pressure. Prog. Exp. Petrol. 1976, 6, 41–45. [Google Scholar]
- Patino Douce, A.E.; Harris, N. Experimental constraints on Himalayan anatexis. J. Petrol. 1998, 39, 689–710. [Google Scholar] [CrossRef]
- Vielzeuf, D.; Holloway, J.R. Experimental-determination of the fluid-absent melting relations in the pelitic system: Consequences for crustal differentiation. Contrib. Mineral. Petrol. 1988, 98, 257–276. [Google Scholar] [CrossRef]
- Conrad, W.K.; Nicholls, I.A.; Wall, V.J. Water-saturated and -undersaturated melting of metaluminous and peraluminous crustal compositions at 10 kb: Evidence for the origin of silicic magmas in the Taupo volcanic zone, New Zealand, and Other occurrences. J. Petrol. 1988, 29, 765–803. [Google Scholar] [CrossRef]
- Beard, J.S.; Lofgren, G.E. Dehydration melting and water-saturated melting of basaltic and andesitic greenstones and amphibolites at 1, 3, and 6.9 kb. J. Petrol. 1991, 32, 365–401. [Google Scholar] [CrossRef]
- Wolf, M.B.; Wyllie, P.J. Dehydration-melting of amphibolite at 10 kbar: The effects of temperature and time. Contrib. Mineral. Petrol. 1994, 115, 369–383. [Google Scholar] [CrossRef]
- Zhou, W.G.; Xie, H.S.; Liu, Y.G.; Zheng, X.G.; Zhao, Z.D.; Zhou, H. Dehydration melting of solid amphibolite at 2.0 GPa: Effects of time and temperature. Sci. China Ser. D-Earth Sci. 2005, 48, 1120–1133. [Google Scholar] [CrossRef]
- Thompson, A.B.; Algor, J.R. Model systems for anataxis of pelitic rocks. Contrib. Mineral. Petrol. 1977, 63, 247–269. [Google Scholar] [CrossRef]
- Zheng, H.F.; Xie, H.S.; Xu, Y.S. Experimental study of the influence of hydrous minerals on the melting behaviour of rocks at high temperatures and pressures. Acta Geol. Sin. 1995, 69, 326–336. (In Chinese) [Google Scholar]
- Dyck, B.; Waters, D.J.; St-Onge, M.R.; Searle, M.P. Muscovite dehydration melting: Reaction mechanisms, microstructures, and implications for anatexis. J. Metamorph. Geol. 2020, 38, 29–52. [Google Scholar] [CrossRef]
- Chen, G.N.; Grapes, R. Granite Genesis: In-Situ Melting and Crustal Evolution; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wei, C.J. Granulite facies metamorphism and petrogenesis of granite (II): Quantitative modeling of the HT-UHT phase equilibria for metapelites and the petrogenesis of S-type granite. Acta Petrol. Sin. 2016, 588, 1611–1624. (In Chinese) [Google Scholar]
- Burnham, C.W. Water and magmas-a mixing model. Geochim. Cosmochim. Acta 1975, 39, 1077–1084. [Google Scholar] [CrossRef]
- Watkins, J.M.; Clemens, J.D.; Treloar, P.J. Archaean TTGs as sources of younger granitic magmas: Melting of sodic metatonalites at 0.6–1.2 GPa. Contrib. Mineral. Petrol. 2007, 154, 91–110. [Google Scholar] [CrossRef]
- Droop, G.T.R.; Brodie, K.H. Anatectic melt volumes in the thermal aureole of the Etive Complex, Scotland: The roles of fluid-present and fluid-absent melting. J. Metamorph. Geol. 2012, 30, 843–864. [Google Scholar] [CrossRef]
- Holtz, F.; Johannes, W. Genesis of peraluminous granites I. experimental investigation of melt compositions at 3 and 5 kb and various H2O activities. J. Petrol. 1991, 32, 935–958. [Google Scholar] [CrossRef]
- Stevens, G.; Clemens, J.D.; Droop, G.T.R. Melt production during granulite-facies anatexis: Experimental data from “primitive” metasedimentary protoliths. Contrib. Mineral. Petrol. 1997, 128, 352–370. [Google Scholar] [CrossRef]
- Hacker, B.R.; Liou, J.G. Influence of fluid and deformation on metamorphism of the deep crust and consequences for the geodynamics of collision zones. Petrol. Struct. Geol. 1998, 10, 297–323. [Google Scholar] [CrossRef]
- Markl, G.; Bucher, K. Composition of fluids in the lower crust inferred from metamorphic salt in lower crustal rocks. Nature 1998, 391, 781–783. [Google Scholar] [CrossRef]
- Audetat, A.; Gunther, D.; Heinrich, C.A. Formation of a magmatic-hydrothermal ore deposit: Insights with LA-ICP-MS analysis of fluid inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.L. Hydrothermal processes: The development of geochemical concepts in the latter half of the twentieth century. Geochem. Perspect. 2015, 4, 1–93. [Google Scholar] [CrossRef]
- Newton, R.C. Fluid and shear zones in the deep crust. World Geol. 1990, 182, 21–37. [Google Scholar] [CrossRef]
- Li, Z.L.; Yang, R.Y.; Sun, X.M.; Li, Y.S. Formation, evolution and mineralization of fluid during geological process. Earth Sci. Front. 1996, 3, 237–244. (In Chinese) [Google Scholar]
- Yan, H.B.; Di, J.; Li, J.H.; Liu, Z.Y.; Liu, J.F.; Ding, X. Synthesis of zirconia micro-nanoflakes with highly exposed (001) facets and their crystal growth. Crystals 2021, 11, 871. [Google Scholar] [CrossRef]
- Johannes, W.; Holtz, F. Formation and ascent of granitic magmas. Geol. Rundsch. 1991, 80, 225–231. [Google Scholar] [CrossRef]
- Grant, T.B.; Harlov, D.E. The influence of NaCl-H2O fluids on reactions between olivine and plagioclase: An experimental study at 0.8 GPa and 800–900 degrees C. Lithos 2018, 323, 78–90. [Google Scholar] [CrossRef]
- Ebadi, A.; Johannes, W. Beginning of melting and composition of 1st melts in the system Qz-Ab-Or-H2O-CO2. Contrib. Mineral. Petrol. 1991, 106, 286–295. [Google Scholar] [CrossRef]
- Xu, Q.D. Fluid-absent melting experiment and modeling of crustal rocks: Major results and implication for study of granitoids. Adv. Earth Sci. 1997, 12, 35–42. (In Chinese) [Google Scholar]
- Johannes, W.; Holtz, F. Petrogenesis and Experimental Petrology of Granitic Rocks; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Acosta-Vigil, A.; London, D.; Morgan, G.B.; Dewers, T.A. Solubility of excess alumina in hydrous granitic melts in equilibrium with peraluminous minerals at 700–800 degrees C and 200 MPa, and applications of the aluminum saturation index. Contrib. Mineral. Petrol. 2003, 146, 100–119. [Google Scholar] [CrossRef]
- Ding, X.; Harlov, D.E.; Chen, B.; Sun, W.D. Fluids, metals, and mineral/ore deposits. Geofluids 2018, 2018, 1452409. [Google Scholar] [CrossRef]
- Manning, D.A.C. An experimental study of the effect of fluorine on the crystallization of granitic melts. J. Geol. Soc. 1981, 138, 213–214. [Google Scholar]
- Winkler, H.G.F.; Platen, H.V. Experimentelle gesteinsmetamorphose—II: Bildung von anatektischen granitischen schmelzen bei der metamorphose von NaCl-führenden kalkfreien Tonen. Geochim. Cosmochim. Acta 1958, 15, 91–112. [Google Scholar] [CrossRef]
- Acosta-Vigil, A.; Pereira, M.D.; Shaw, D.M.; London, D. Contrasting behaviour of boron during crustal anatexis. Lithos 2001, 56, 15–31. [Google Scholar] [CrossRef]
- Li, F.C.; Zhu, J.C.; Rao, B.; Jin, Z.D. Genesis of Li-F-rich granites: Experimental evidence from high temperature and high pressure. Sci. China Ser. D-Earth Sci. 2003, 33, 841–851. [Google Scholar]
- Wise, M.A.; Francis, C.A.; Cerny, P. Compositional and structural variations in columbite-group minerals from granitic pegmatites of the Brunswick and Oxford fields, Maine: Differential trends in F-poor and F-rich environments. Can. Mineral. 2012, 50, 1515–1530. [Google Scholar] [CrossRef]
- Wang, Y. Brine-assisted anatexis: A new model of post-orogenic A-type granites genesis. In Proceedings of the 2016th Annual Meeting of Chinese Geoscience Union, Beijing, China, 15 October 2016; p. 3. (In Chinese). [Google Scholar]
- Li, H.J.; Hermann, J. The effect of fluorine and chlorine on trace element partitioning between apatite and sediment melt at subduction zone conditions. Chem. Geol. 2017, 473, 55–73. [Google Scholar] [CrossRef]
- Wang, Y. Genesis of brine-induced melting of rare element granites in South China. In Proceedings of the 8th National Symposium on Metallogenic Theory and Prospecting Methods, Nanchang, China, 9 December 2017; pp. 243–244. (In Chinese). [Google Scholar]
- Tropper, P.; Manning, C.E.; Harlov, D.E. Solubility of CePO4 monazite and YPO4 xenotime in H2O and H2O-NaCl at 800 degrees C and 1 GPa: Implications for REE and Y transport during high-grade metamorphism. Chem. Geol. 2011, 282, 58–66. [Google Scholar] [CrossRef]
- Mair, P.; Tropper, P.; Harlov, D.E.; Manning, C.E. The solubility of CePO4 monazite and YPO4 xenotime in KCl-H2O fluids at 800 degrees C and 1.0 GPa: Implications for REE transport in high-grade crustal fluids. Am. Mineral. 2017, 102, 2457–2466. [Google Scholar] [CrossRef]
- Finch, E.G.; Tomkins, A.G. Fluorine and chlorine behaviour during progressive dehydration melting: Consequences for granite geochemistry and metallogeny. J. Metamorph. Geol. 2017, 35, 739–757. [Google Scholar] [CrossRef]
- Tropper, P.; Manning, C.E.; Harlov, D.E. Experimental determination of CePO4 and YPO4 solubilities in H2O-NaF at 800 degrees C and 1 GPa: Implications for rare earth element transport in high-grade metamorphic fluids. Geofluids 2013, 13, 372–380. [Google Scholar] [CrossRef]
- Beard, C.D.; van Hinsberg, V.J.; Stix, J.; Wilke, M. The effect of fluorine on clinopyroxene/melt trace-element partitioning. Contrib. Mineral. Petrol. 2020, 175, 44. [Google Scholar] [CrossRef]
- Aseri, A.A.; Linnen, R.L.; Che, X.D.; Thibault, Y.; Holtz, F. Effects of fluorine on the solubilities of Nb, Ta, Zr and Hf minerals in highly fluxed water-saturated haplogranitic melts. Ore Geol. Rev. 2015, 64, 736–746. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Yan, H.B.; He, J.J.; Liu, X.W.; Wang, H.B.; Liu, J.F.; Ding, X. Thermodynamic investigation of the hydrolysis behavior of fluorozirconate complexes at 423.15–773.15 K and 100 MPa. J. Solut. Chem. 2020, 49, 836–848. [Google Scholar] [CrossRef]
- Louvel, M.; Etschmann, B.; Guan, Q.S.; Testemale, D.; Brugger, J. Carbonate complexation enhances hydrothermal transport of rare earth elements in alkaline fluids. Nat. Commun. 2022, 13, 1456. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Zhang, L. The role of sulfate-, alkali-, and halogen-rich fluids in mobilization and mineralization of rare earth elements: Insights from bulk fluid compositions in the Mianning-Dechang carbonatite-related REE belt, southwestern China. Lithos 2021, 386, 106008. [Google Scholar] [CrossRef]
- Garcia-Arias, M.; Guillermo Corretge, L.; Castro, A. Trace element behavior during partial melting of Iberian orthogneisses: An experimental study. Chem. Geol. 2012, 292, 1–17. [Google Scholar] [CrossRef]
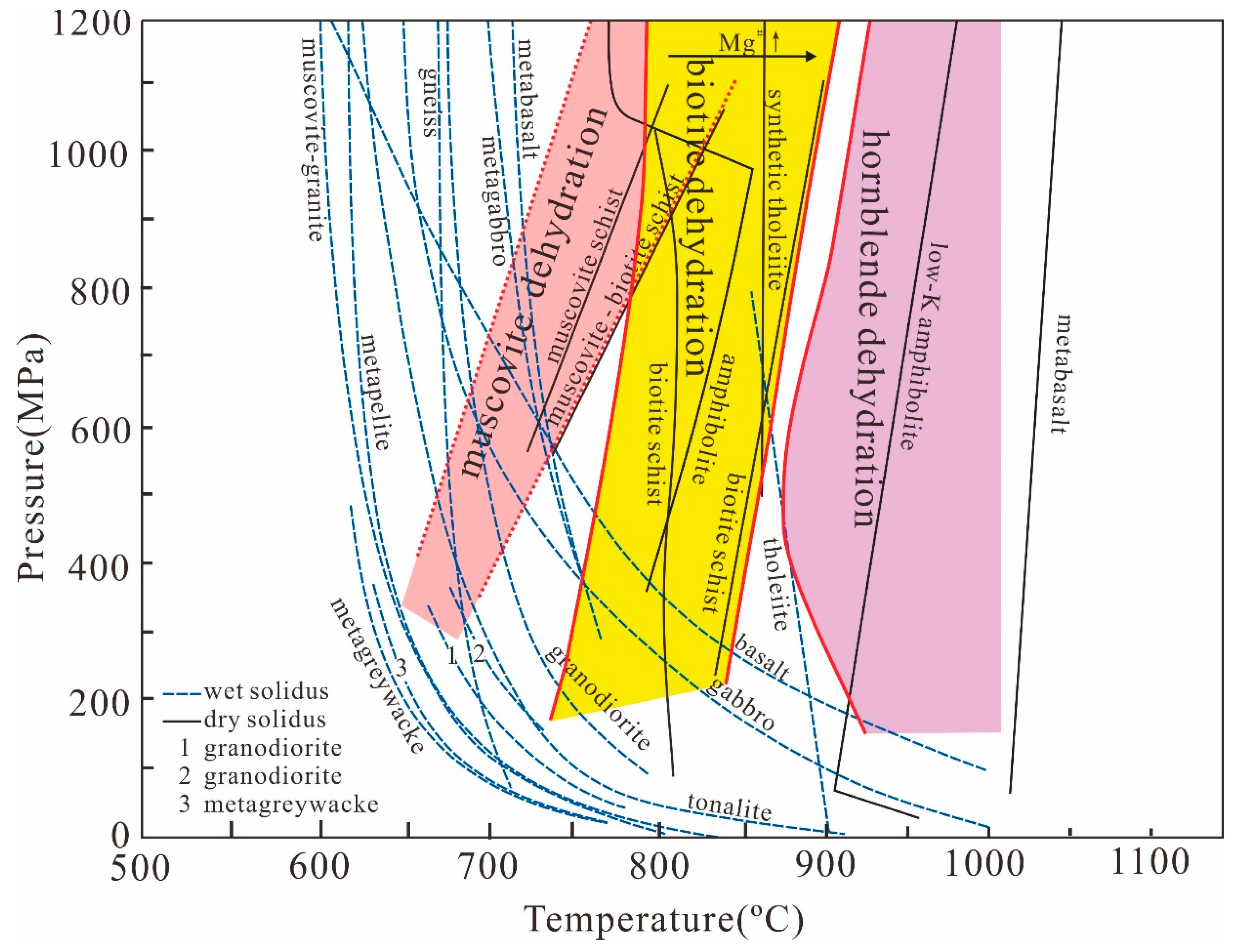
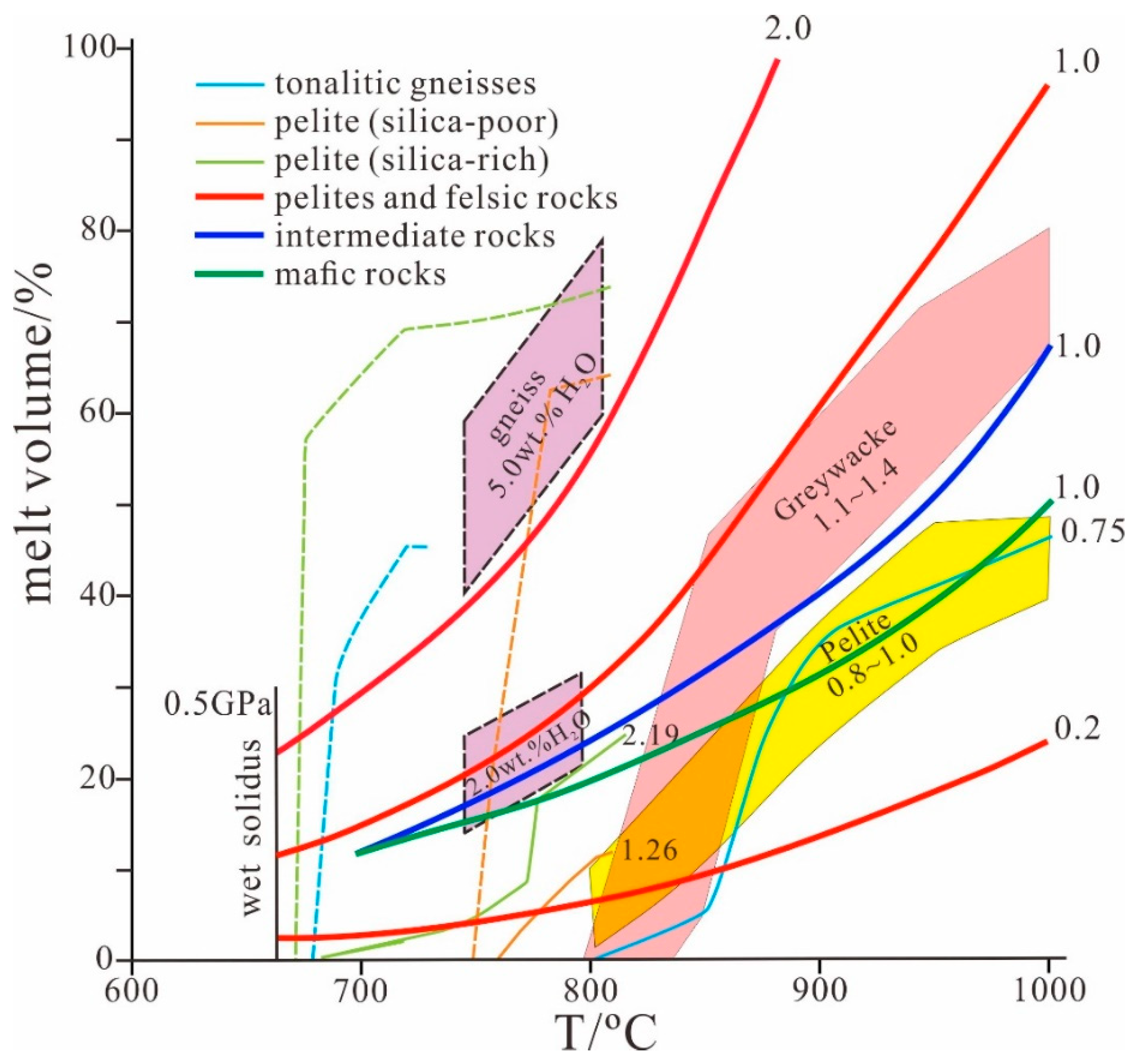
| Melting Reaction | Rock Type | References |
|---|---|---|
| Ms + Ab + Qz = Kfs + Als + melt | pelite | Petö (1976) [68] |
| Ms + Pl + Qz = Kfs + Als + Bt + melt | pelite | Patino Douce and Harris (1998) [69] |
| Bt + Pl + Q = Opx + Crd + melt (lower pressure) Bt + Pl + Q = Opx + Grt + melt (higher pressure) | metagreywacke | Vielzeuf and Holloway (1988) [70] |
| Bt + Pl + Q = Hb +Grt + melt | greywacke | Conrad (1988) [71] |
| Bt + Pl + Q = Hb + melt | dacite | |
| Amp + Pl + Qz = Cpx + Opx + Mag + melt | basalt | Beard and Lofgren (1991) [72] |
| Amp + Pl = Cpx + Opx + Grt + melt (1 GPa) | amphibolite | Wolf and Wyllie (1994) [73] |
| Amp + Pl = Grt + Cpx + Melt (2 Gpa) | amphibolite | Zhou (2005) [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ding, X.; Liu, J. The Role of Fluids in Melting the Continental Crust and Generating Granitoids: An Overview. Geosciences 2022, 12, 285. https://doi.org/10.3390/geosciences12080285
Li J, Ding X, Liu J. The Role of Fluids in Melting the Continental Crust and Generating Granitoids: An Overview. Geosciences. 2022; 12(8):285. https://doi.org/10.3390/geosciences12080285
Chicago/Turabian StyleLi, Jiahao, Xing Ding, and Junfeng Liu. 2022. "The Role of Fluids in Melting the Continental Crust and Generating Granitoids: An Overview" Geosciences 12, no. 8: 285. https://doi.org/10.3390/geosciences12080285
APA StyleLi, J., Ding, X., & Liu, J. (2022). The Role of Fluids in Melting the Continental Crust and Generating Granitoids: An Overview. Geosciences, 12(8), 285. https://doi.org/10.3390/geosciences12080285








