4.3. Petrogeochemistry of Kudurits, Rocks of Terrigenous–Carbonate Metamorphic Formation and Granitoid Migmatites
The macroelement compositions of the kudurits, coprolites, and background rocks from Olkhon Island are shown in
Table 4. It can be seen that the background rocks (samples A-n and C-n,
Table 4) belong to the high-carbonate, low-alkaline series. In terms of silica content, most of them correspond to rocks of medium and basic composition. Granitoid migmatites (samples G-n) are characterized by elevated potassium contents. Clayey rocks of the weathering crust (kudurits) are significantly depleted in silica, alkaline, and alkaline–earth elements and partially in manganese compared to the parent rocks, but enriched in Ti, Al, Fe, and P (see samples n-N,
Table 4). In the bottom sediments of the Shara-Nur salt lake (sample OZ), more than half of the mass fraction is composed of Ca and Mg carbonates, with a rather significant fraction of Na and simultaneously low content of Al and Fe and especially K. The characteristics of the chemical composition of coprolites (samples n-NK) should be evaluated in comparison with kudurits only taking into account the proportional variation in hygroscopic water (H
2O) and loss on ignition (LOI) values. This will be carried out in the Discussion section.
Table 4.
Composition of kudurits, coprolites, and the most common rocks on Olkhon Island in percent of elemental oxides.
Table 4.
Composition of kudurits, coprolites, and the most common rocks on Olkhon Island in percent of elemental oxides.
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | H2O− | LOI | Σ |
|---|
| 1–6 | 34.81 | 2.15 | 24.83 | 18.76 | 0.078 | 0.37 | 1.51 | 0.36 | 0.11 | 0.41 | 2.01 | 14.20 | 99.59 |
| 1–7 | 35.03 | 2.42 | 26.50 | 17.14 | 0.06 | 0.53 | 1.56 | 0.65 | 0.07 | 0.56 | 1.27 | 13.98 | 99.76 |
| 1–9 | 34.88 | 2.53 | 26.61 | 18.26 | 0.09 | 0.36 | 1.30 | 0.41 | 0.12 | 0.37 | 1.03 | 13.84 | 99.79 |
| 1–11 | 36.30 | 1.48 | 26.24 | 18.03 | 0.03 | 0.54 | 1.08 | 0.42 | 0.18 | 0.30 | 2.64 | 12.25 | 99.51 |
| 1–12 | 36.34 | 1.42 | 26.71 | 18.23 | 0.027 | 0.39 | 0.85 | 0.41 | 0.16 | 0.30 | 2.31 | 12.64 | 99.78 |
| 2–17 | 50.00 | 0.77 | 13.77 | 6.77 | 0.03 | 1.22 | 9.15 | 0.36 | 0.46 | 0.06 | 3.81 | 13.14 | 99.54 |
| 3–22 | 47.42 | 1.06 | 20.03 | 11.65 | 0.02 | 1.02 | 0.39 | 0.40 | 3.05 | 0.09 | 6.39 | 8.29 | 99.79 |
| 1–2K | 36.50 | 1.35 | 18.36 | 11.85 | 0.213 | 0.81 | 2.03 | 0.27 | 0.99 | 0.42 | 0.10 | 26.88 | 99.77 |
| 1–3K | 25.13 | 1.58 | 19.10 | 13.30 | 0.049 | 0.25 | 1.23 | 0.05 | 0.22 | 0.52 | 3.43 | 34.70 | 99.55 |
| 1–14K | 33.11 | 1.20 | 20.96 | 12.95 | 0.043 | 0.44 | 2.99 | 0.13 | 0.34 | 0.55 | 2.83 | 24.12 | 99.66 |
| 1–15K | 23.67 | 1.27 | 16.47 | 10.04 | 0.05 | 0.50 | 3.14 | 0.11 | 0.48 | 1.01 | 4.96 | 38.07 | 99.78 |
| 3–24K | 33.52 | 0.94 | 17.72 | 10.61 | 0.12 | 1.31 | 1.84 | 0.12 | 1.24 | 0.30 | 4.26 | 27.62 | 99.61 |
| G-3 | 62.45 | 0.32 | 19.58 | 3.82 | 0.03 | 0.91 | 0.36 | 0.21 | 4.01 | 0.07 | 1.94 | 5.82 | 99.52 |
| G-4 | 69.75 | 0.59 | 13.24 | 4.73 | 0.053 | 0.50 | 2.23 | 2.63 | 5.07 | 0.15 | 0.15 | 0.45 | 99.55 |
| G-5 | 75.65 | 0.08 | 15.81 | 0.34 | 0.02 | 0.04 | 0.03 | 0.14 | 3.36 | 0.01 | 0.23 | 4.06 | 99.78 |
| A-O | 52.15 | 1.49 | 14.43 | 11.24 | 0.17 | 5.48 | 10.59 | 2.71 | 0.71 | 0.14 | 0.01 | 0.64 | 99.76 |
| A-1 | 49.02 | 0.18 | 14.86 | 7.31 | 0.122 | 11.34 | 15.27 | 0.95 | 0.08 | 0.02 | 0.19 | 0.48 | 99.82 |
| A-16 | 49.15 | 0.97 | 19.76 | 12.52 | 0.21 | 3.74 | 8.88 | 2.93 | 1.15 | 0.20 | 0.16 | 0.06 | 99.74 |
| A-26 | 62.76 | 0.71 | 14.26 | 6.07 | 0.08 | 3.41 | 7.69 | 1.85 | 0.56 | 0.28 | 0.38 | 1.71 | 99.76 |
| C-4a | 1.34 | 0.01 | 0.09 | 0.09 | 0.003 | 0.14 | 54.62 | 0.01 | 0.01 | 0.01 | 0.10 | 43.09 | 99.51 |
| C-18 | 3.45 | 0.02 | 0.61 | 0.52 | 0.03 | 0.48 | 52.44 | 0.14 | 0.13 | 0.04 | 0.23 | 40.94 | 99.03 |
| OZ | 14.72 | 0.18 | 3.02 | 2.22 | 0.093 | 13.38 | 18.03 | 4.02 | 1.95 | 0.17 | 4.52 | 37.31 | 99.61 |
Table 5.
Comparison of the composition of kudurits and coprolites from Olkhon Island in percent of elemental oxides without H2O and LOI.
Table 5.
Comparison of the composition of kudurits and coprolites from Olkhon Island in percent of elemental oxides without H2O and LOI.
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Σ |
|---|
| 1–6 | 41.74 | 2.58 | 29.79 | 22.5 | 0.09 | 0.44 | 1.81 | 0.43 | 0.13 | 0.49 | 100.00 |
| 1–3K | 40.90 | 2.57 | 31.10 | 21.65 | 0.08 | 0.41 | 2.00 | 0.08 | 0.36 | 0.85 | 100.00 |
| | 0.84 | 0.01 | 1.31 | 0.85 | 0.01 | 0.03 | 0.19 | 0.35 | 0.23 | 0.36 | 2.09–2.09 |
| 1–6 | 41.74 | 2.58 | 29.79 | 22.5 | 0.09 | 0.44 | 1.81 | 0.43 | 0.13 | 0.49 | 100.00 |
| 1–14K | 45.53 | 1.65 | 28.83 | 17.81 | 0.06 | 0.61 | 4.11 | 0.18 | 0.47 | 0.76 | 100.00 |
| | 3.79 | 0.93 | 0.96 | 4.69 | 0.03 | 0.17 | 2.30 | 0.25 | 0.34 | 0.27 | 6.87–6.87 |
| 1–6 | 41.75 | 2.58 | 29.79 | 22.5 | 0.09 | 0.44 | 1.80 | 0.43 | 0.13 | 0.49 | 100.00 |
| 1–15K | 41.75 | 2.23 | 29.03 | 17.69 | 0.09 | 0.88 | 5.52 | 0.19 | 0.84 | 1.78 | 100.00 |
| | 0 | 0.35 | 0.76 | 4.81 | 0 | 0.44 | 3.72 | 0.24 | 0.71 | 1.29 | 6.16–6.16 |
| 1–9 | 41.07 | 2.98 | 31.34 | 21.5 | 0.11 | 0.42 | 1.53 | 0.48 | 0.14 | 0.43 | 100.00 |
| 1–3K | 40.9 | 2.57 | 31.1 | 21.65 | 0.08 | 0.41 | 2 | 0.08 | 0.36 | 0.85 | 100.00 |
| | 0.17 | 0.41 | 0.24 | 0.15 | 0.03 | 0.01 | 0.47 | 0.40 | 0.22 | 0.42 | 1.26–1.26 |
| 1–9 | 41.07 | 2.98 | 31.34 | 21.5 | 0.11 | 0.42 | 1.53 | 0.48 | 0.14 | 0.43 | 100.00 |
| 1–22K | 42.01 | 2.34 | 31.73 | 20.41 | 0.08 | 0.41 | 1.88 | 0.11 | 0.35 | 0.68 | 100.00 |
| | 0.94 | 0.64 | 0.39 | 1.09 | 0.03 | 0.01 | 0.35 | 0.37 | 0.21 | 0.25 | 2.14–2.14 |
| 1–12 | 42.84 | 1.67 | 31.49 | 21.49 | 0.03 | 0.46 | 1.00 | 0.48 | 0.19 | 0.35 | 100.00 |
| 1–3K | 41.00 | 2.56 | 31.10 | 21.65 | 0.08 | 0.41 | 2.00 | 0.08 | 0.36 | 0.85 | 100.00 |
| | 1.84 | 0.89 | 0.39 | 0.16 | 0.05 | 0.05 | 1.00 | 0.40 | 0.17 | 0.50 | 2.72–2.72 |
Among the trace elements in the samples of kudurits (n = 11), the highest values (in ppm) are in Ba (average—311; range—57–692). Then, in descending order: V, 209 (3–315); Sr, 124 (57–336); Zn, 97 (13–186); Cr, 70 (8–141); Zr, 56 (9–89); Cu, 55 (4–123); Ce, 52 (11–141); Rb, 41 (2–178); Ni, 38 (12–61); Sc, 30 (1–48); Nd, 25 (3–74); Ga, 24 (17–29); Li, 21 (8–41); La, 20 (3–60); Y, 14 (2–37); Pb, 12 (5–16); and U, 11 (1–20). Next, in concentrations less than 10 ppm in descending order: Nb, Co, Pr, Sm, Gd, Th, Dy, Mo, As, Hf, Er, Yb, Sn, Be, Ge, Eu, and Cs; in concentrations less than 1 ppm: Tb, Ho, W, Ta, Sb, Lu, Tm, Tl, Se, Cd, Bi, Ag, and Te. The amount of REEs (including Y and Sc) varies from 24 to 420 ppm, with an average of 167 and a median of 134.
Coprolite samples (n = 9) also have the highest Ba values, with an average of 240, ranging from 77 to 624. Other elements in descending order: V, 164 (119–222); Sr, 129 (68–180); Zn, 91 (67–130); Cr, 52 (13–151); Cu, 44 (29–84); Zr, 38 (26–59); Rb, 33 (3–163); Ni, 31 (10–96); Ce, 28 (12–58); Sc, 27 (17–49); Ga, 16 (11–20); Li, 13 (6–27); and Nd, 11 (4–23). Then, in concentrations less than 10 ppm in descending order: Pb, Y, La, Co, U, Nb, Mo, Pr, Sm, Th, Gd, Dy, As, Be, Hf, Yb, Sn, Er, and Cs; in concentrations less than 1 ppm: Ge, Eu, W, Ho, Tb, Cd, Ta, Tl, Sb, Lu, Tm, Se, Bi, Ag, and Te. The amount of REEs ranges from 64 to 141, with an average of 97 and a median of 90.
In two samples of granitoid migmatites at kudur No. 1, the highest values are again in Ba, with an average of 842 ppm, ranging from 772 to 911. Next, in descending order: Sr, 196 (143–248); Ce, 135 (10–260); La, 127 (50–204); Rb, 114 (67–160); Nd, 64 (24–105); Zn, 40 (12–69); V, 32 (5–60); Cr, 25 (3–46); Zr, 24 (23–24); Th, 23 (2–45); Ga, 21. 2 (20–22); Pr, 20.8 (7–35); Pb, 19.2 (9–29); Li, 18.9 (10–28); Y, 17.7 (17–18); Ni, 17.2 (15–19); Cu, 16 (8–25). Then, in descending order, in concentrations less than 10 ppm: Gd, Sm, Sc, U, Nb, Dy, As, Cs, Er, Co, Yb, Ge, Eu, and Be; in concentrations less than 1 ppm: Tl, Tb, Mo, Hf, Ho, Sn, W, Se, Tm, Lu, Sb, Ta, Bi, Cd, Ag, and Te. The amount of REEs ranges from 128 to 666, with an average content of 397.
Rocks of the amphibolite crystalline schist type (n = 5) are characterized by the highest Sr values (average—253 ppm; range 79–450). Accordingly, in descending order: Cr, 253 (9–592); V, 162 (42–261); Ba, 150 (46–298); Ni, 101 (1–182); Zn, 65 (27–97); Cu, 34 (5–60); Co, 32 (18–47); Sc, 29 (15–45); Zr, 21 (5–46); Y, 21 (5–32); Ce, 21 (1–43); Ga, 14 (10–17); Nd, 12 (74–128); Li, 11 (2–18). Furthermore, in concentrations less than 10 ppm in descending order: La, Rb, Nb, Pb, Gd, Dy, Sm, Pr, Er, Yb, Th, and Ge; in concentrations less than 1 ppm: Eu, Ho, Hf, Sn, W, Mo, Tb, Be, U, As, Tm, Lu, Ta, Se, Cd, Sb, Cs, Bi, Tl, and Te. The amounts of REEs range from 57 to 160 ppm, with a mean of 111 and a median of 120.
Terrigenous carbonate rocks were characterized by two samples. They had the highest Sr concentrations (average—5532; range 2130–8934 ppm). Accordingly, in descending order: Ba, 240 (28–453); V, 10 (5–15). Then, in concentrations less than 10 ppm in descending order: Zr, Ce, Zn, Y, Cr, La, Pb, Nd, Rb, Ni, Cu, and U; in concentrations less than 1 ppm: Co, Mo, Lo, Pr, Th, Sc, Gd, As, Sm, Dy, Ga, Er, Cd, Yb, Nb, Sn, Hf, W, Eu, Ho, Ge, Tb, Sb, Cs, Be, Tm, Lu, Te, Ta, Tl, Se, Bi, and Ag. The amount of REEs varies from 9 to 30 ppm, with an average content of 20 ppm.
The muddy sediments of the Shara-Nur salt lake are represented by one sample. Sr (1065), Mo (74.28), U (32.24), and Li (18.66) are prominent in its trace element content. With respect to other trace elements (including REEs), they are generally similar to some varieties of amphibolite schists.
Figure 4 shows REE content profiles normalized to North American shale composite (NASC, according to [
32]) in different rock types on Olkhon: from the marl zone of shale rocks at kudur No. 3 and migmatites at kudur No. 1; from amphibolite crystalline schists; from fine-grained sand and diluvium over amphibolite crystalline schists; and from metamorphic rocks of the carbonate series. As can be seen from the graphs presented, the background rocks of the carbonate series on Olkhon Island are characterized by much lower REE contents compared to the American shale. In the amphibolite crystalline schists, a noticeable difference is observed only in the light REE content. This is particularly pronounced in the sample from the deluvium. The highest REE concentrations are characteristic of new mineral formations in the weathering zones of amphibolite crystalline schists and migmatites, along which clayey kudurits are formed.
4.7. Biogeochemistry
The study of the chemical composition of 14 soil samples showed that, in terms of macro and trace elements, their compositions correspond well to the composition of the parent rocks. Here, it is only necessary to provide data on the content of Cl, Br, I, and Hg (which were not determined in the parent rocks of the island) and Sr, As, Pb, and Cd (since they may be relevant to the problem of geophagy as toxic), as well as on the REEs. The average concentrations of elements in the soils were as follows: Cl, 654 ppm (range 65–1736); Br, 77.1 (8.78–456); I, 8.73 (1.46–36.85); Hg, 0.03 (0.10–0.25); Sr, 74.17 (32.1–272); As, 1.09 (0.25–2.74); Pb, 5.52 (3.24–9.35); and Cd, 0.28 (0.09–0.89).
In eight soil samples collected on background rocks (terrigenous–carbonate and amphibolite schists), the sum of REEs varies from 13.20 to 67.77 ppm (average 43.85). The ranges of average element values in descending order are as follows: Ce, 14.4; Y, 6.50; La, 6.38; Nd, 6.37; Sc, 2.31; Pr, 1.60; Gd, 1.48; Sm, 1.34; Dy, 1.29; Er, 0.70; Yb, 0.67; Eu, 0.34; Ho, 0.26; Tb, 0.22; Tm, 0.10; and Lu, 0.10. In six soil samples collected within kudurs No. 1, 2, and 3, REEs amounts ranged from 73.07 to 285.9 ppm (average, 138.7). The ranges of average element values in descending order are as follows: Ce, 45.13; La, 25.48; Y, 22.18; Nd, 20.20; Pr, 5.31; Gd, 3.84; Sm, 3.74; Sc, 3.26; Dy, 3.15; Er, 2.05; Yb, 1.78; Eu, 0.80; Ho, 0.68; Tb, 0.53; Tm, 0.29; and Lu, 0.28.
The results of determining the concentrations of macroelements in air-dried sedge samples from Olkhon Island are presented in
Table 7. They show that most of the island territory is insufficiently supplied with sodium and rather well supplied with other biophilic elements, although there are deviations from the norm in their ratios. For example, the Ca/Mg ratio in sedges varies from 1/1.1 to 1/4, while the norm is 1/3.
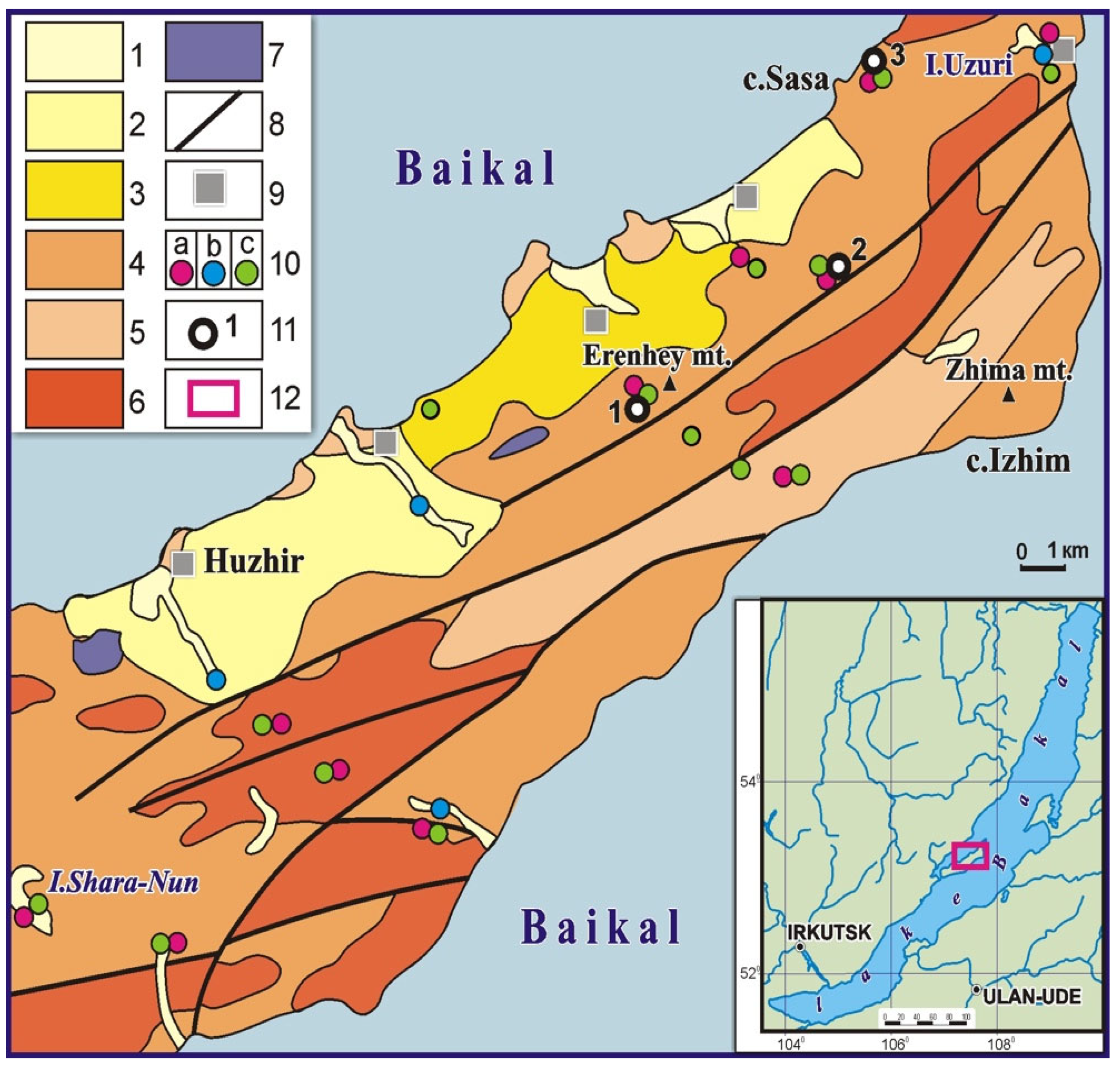
The maximum Na content in sedges up to 1 g/kg (which is close to the index in lucerne hay) was determined in the sample from the coastal part of the Shara-Nur salt lake. The average Na content in sedges in the rest of the island is an order of magnitude lower. Such an uneven sodium supply of the island territory has a significant impact on the distribution of ungulates on it. It is worth remembering that about two-thirds of all recorded encounters of deer on the island, both in winter and in summer, were reported in the area of Shara-Nur Lake. This fact indicates that the most attractive site for animals is the forested area in the southwestern sector of the central part of the island (the area in the lower left corner in
Figure 1). In this area, there is a natural salt water basin and vegetation enriched with this element.
In the composition of trace elements in sedges, the highest values (ppm) are in Ba (from 9 to 31 with an average of 20). They are followed by Zn (12–36/17); Sr (9–20/14); Cu (3–11/5); Rb (1–20/4); Ni (0.8–8/2); Cr (0.4–2.6/2); and Mo (0.5–1.7/1). The remaining trace elements have concentrations of less than 1 ppm.
In terms of the REE content in vegetation, most of the island’s territory (where terrigenous carbonate rocks and most of the amphibolite schists predominate) is characterized by reduced concentrations of these elements. The amounts of lanthanides with scandium and yttrium in the four sedge samples collected on high carbonate rocks range from 0.054 to 0.127 ppm on dry matter (average—0.093). The REE concentrations in three sedge samples collected on amphibolite crystalline schists range from 0.179 to 0.235 (0.200). The REE concentrations (ppb) in the vegetation are presented in decreasing order as follows: Ce, La, Nd, Y, Sc, Pr, Gd, Sm, Dy, Eu, Er, Yb, Ho, Tb, Lu, and Tm.
The profiles of NASC-normalized and seawater-normalized REE concentrations in sedge samples collected from background rocks on Olkhon Island are shown in
Figure 9.
Higher concentrations of REEs (by at least three times) were detected in the sedge sample from Lake Shara-Nur (0.75 ppm), as well as in the sample from the area where granitoid migmatites are distributed (0.64 ppm).
Figure 10A–C shows the profiles of lanthanide and yttrium concentrations in the tail gland of red deer from areas with evidence of mass geophagy in the Altai Mountains (1, 2) and on Olkhon Island (3). The profiles are presented in three variants: in ppb per dry matter (
Figure 10A) and normalized to North American shale composite (
Figure 10B) and to seawater (
Figure 10C). As can be seen, the REE concentrations in the tail gland are a good reflection of the background content of the elements in question in the landscape components of a given region. At the same time, the range of concentration variations by region can be up to two orders of magnitude.