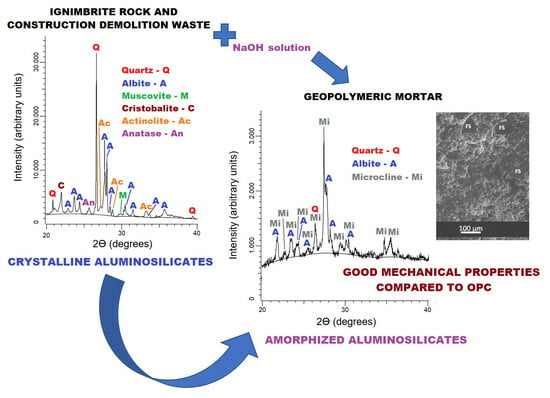
Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Materials and Characterization Techniques
2.2. Preparation of Geopolymer and OPC Mortar
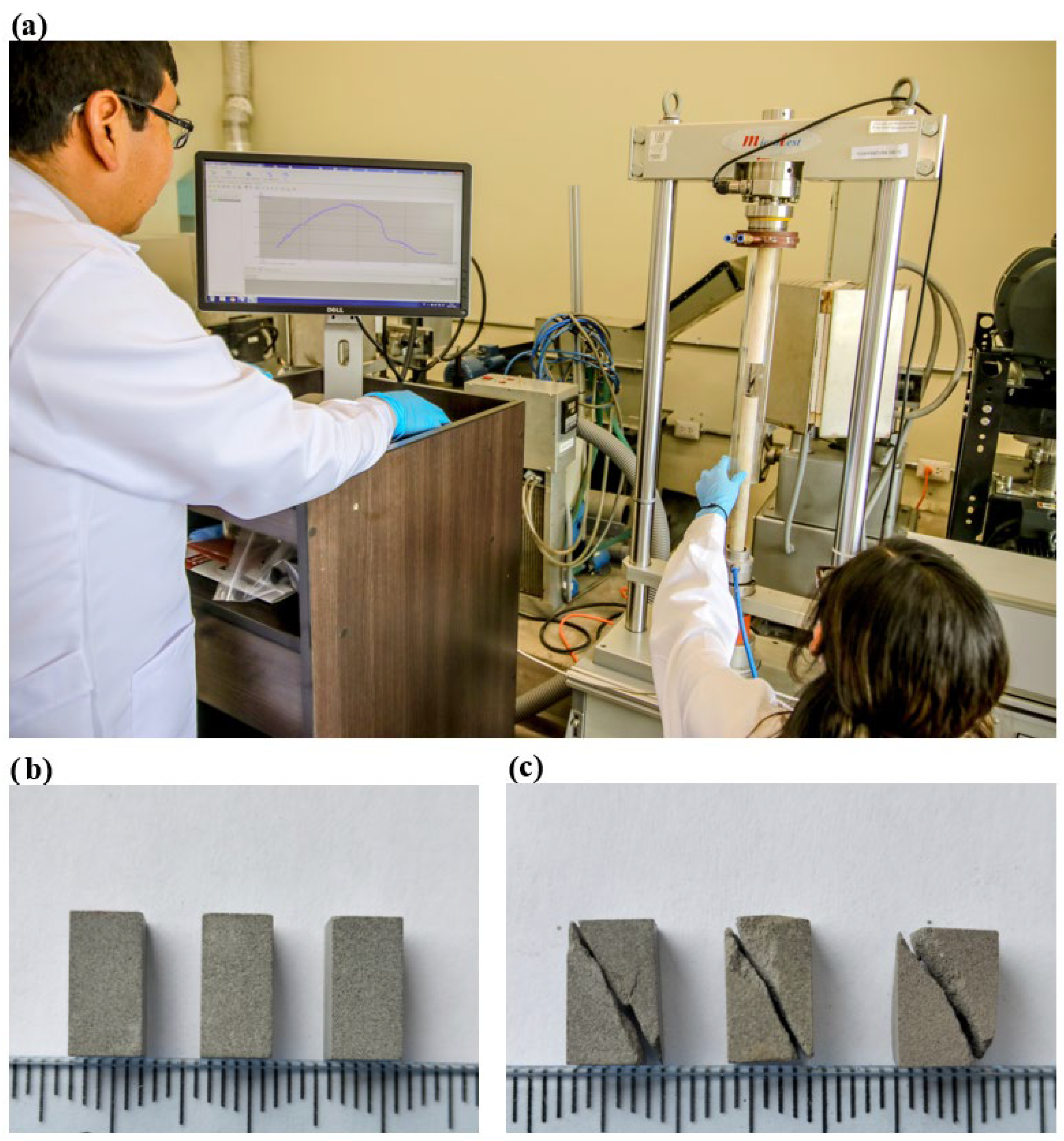
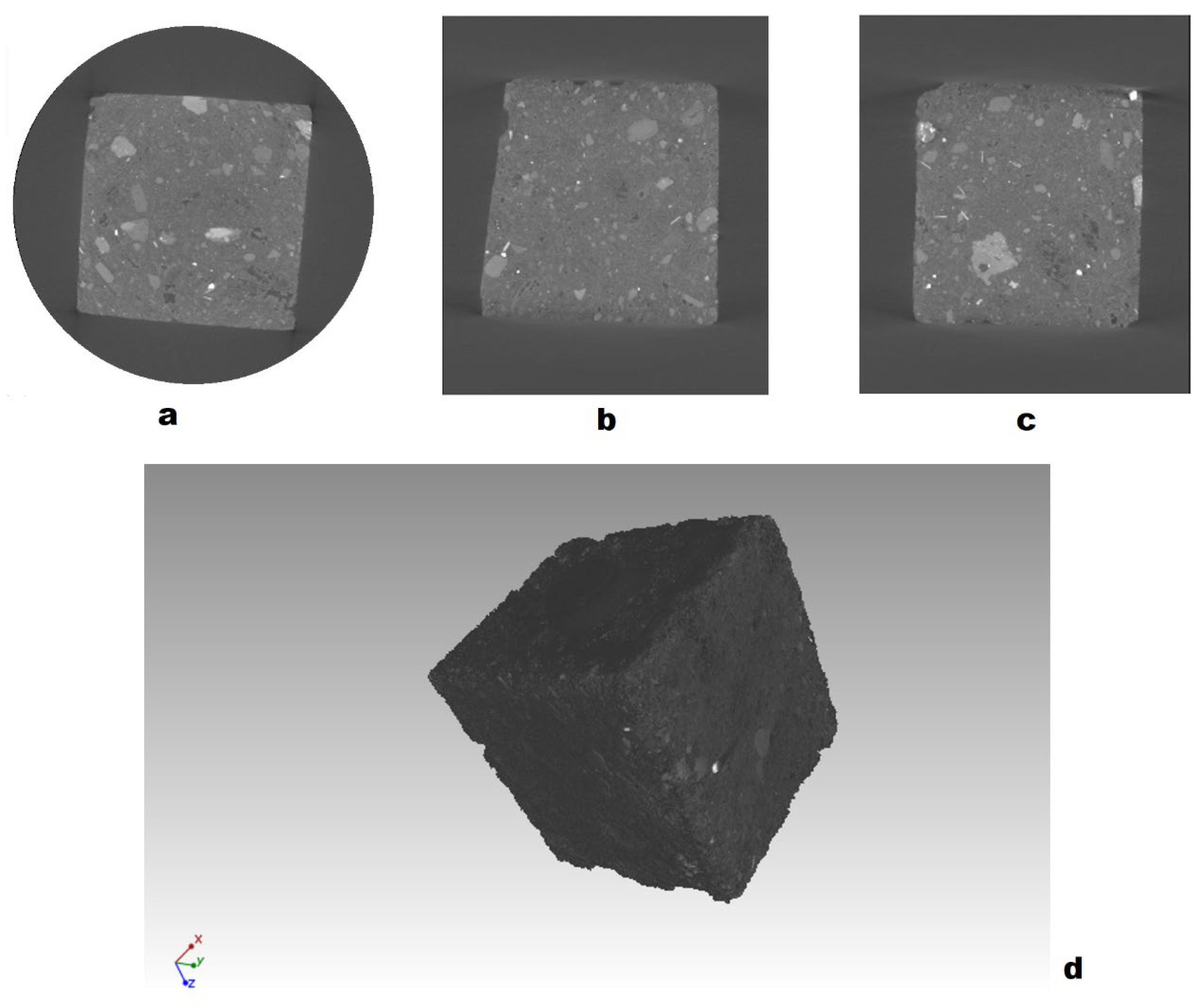
2.3. Structural, Microstructural, and Mechanic Characterization of Geopolymeric and OPC Mortar
3. Results and Discussion
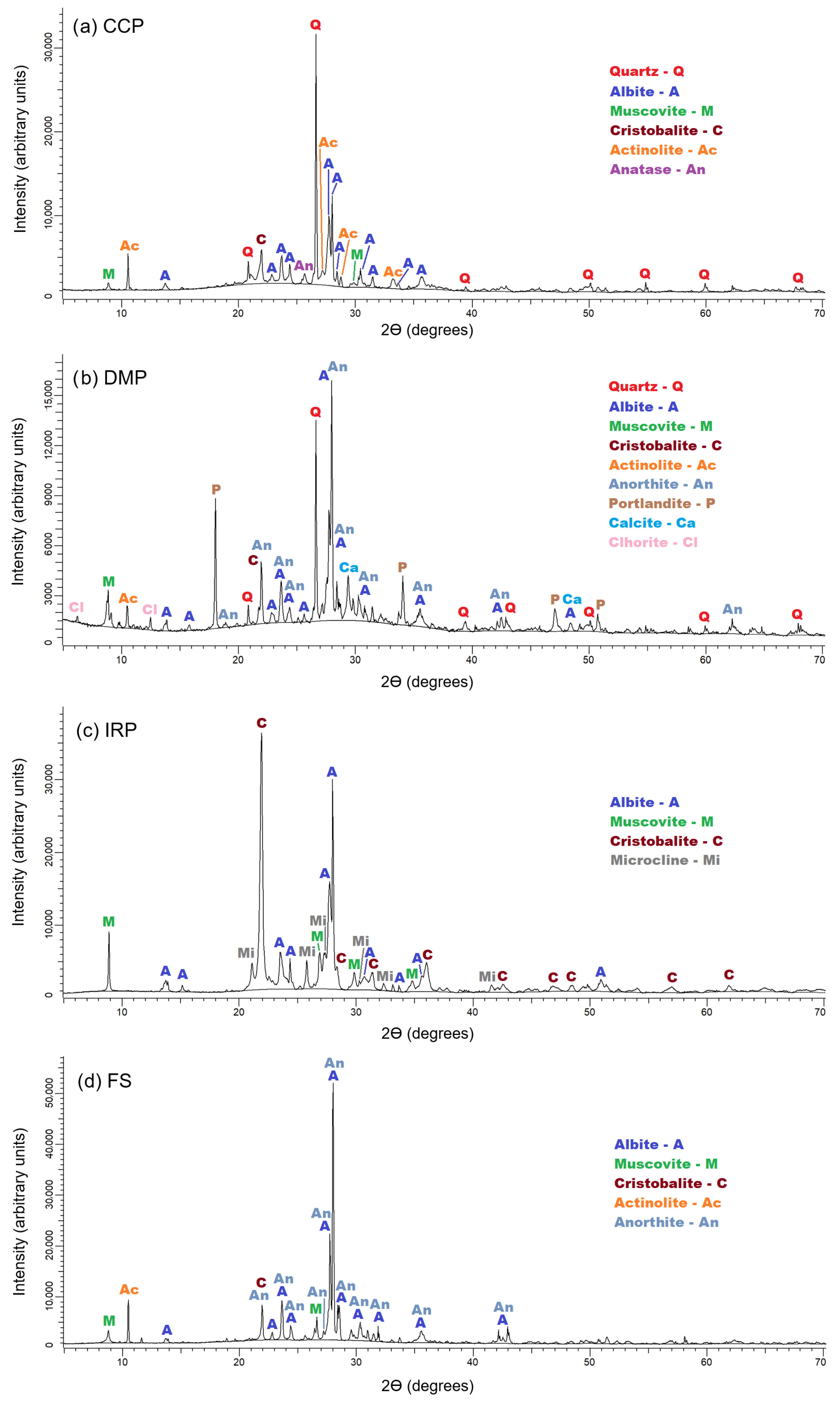
3.1. Raw Materials
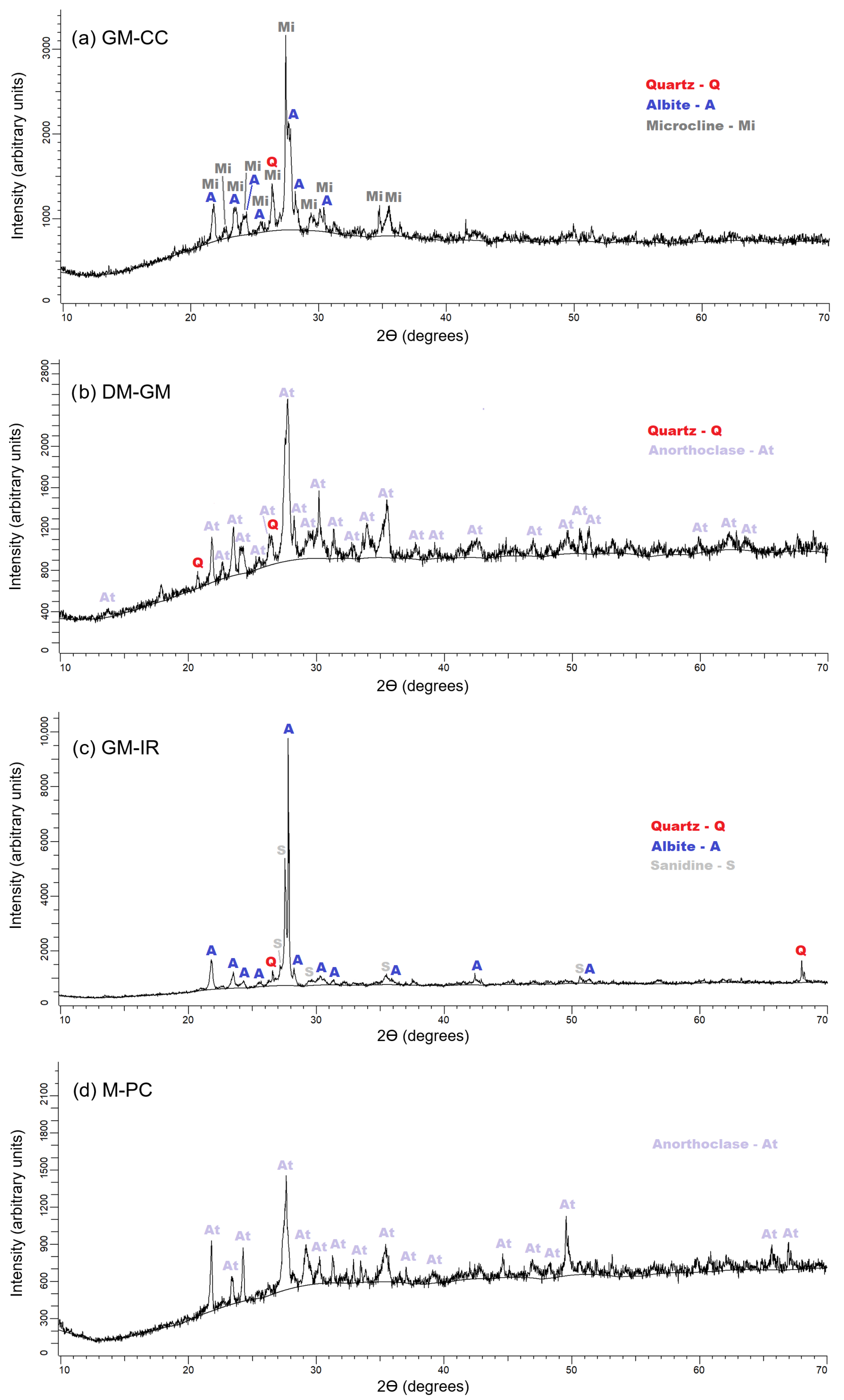
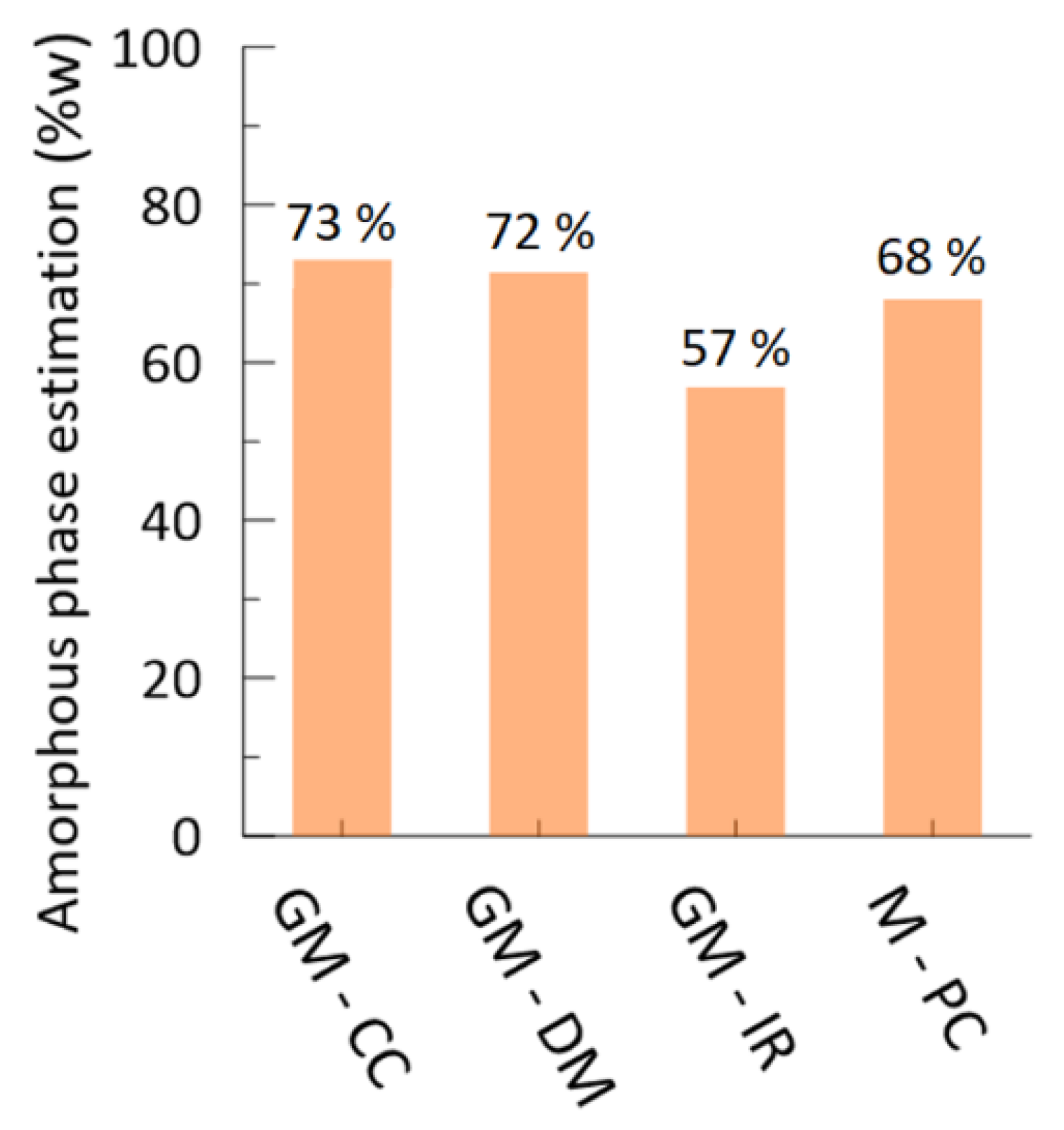
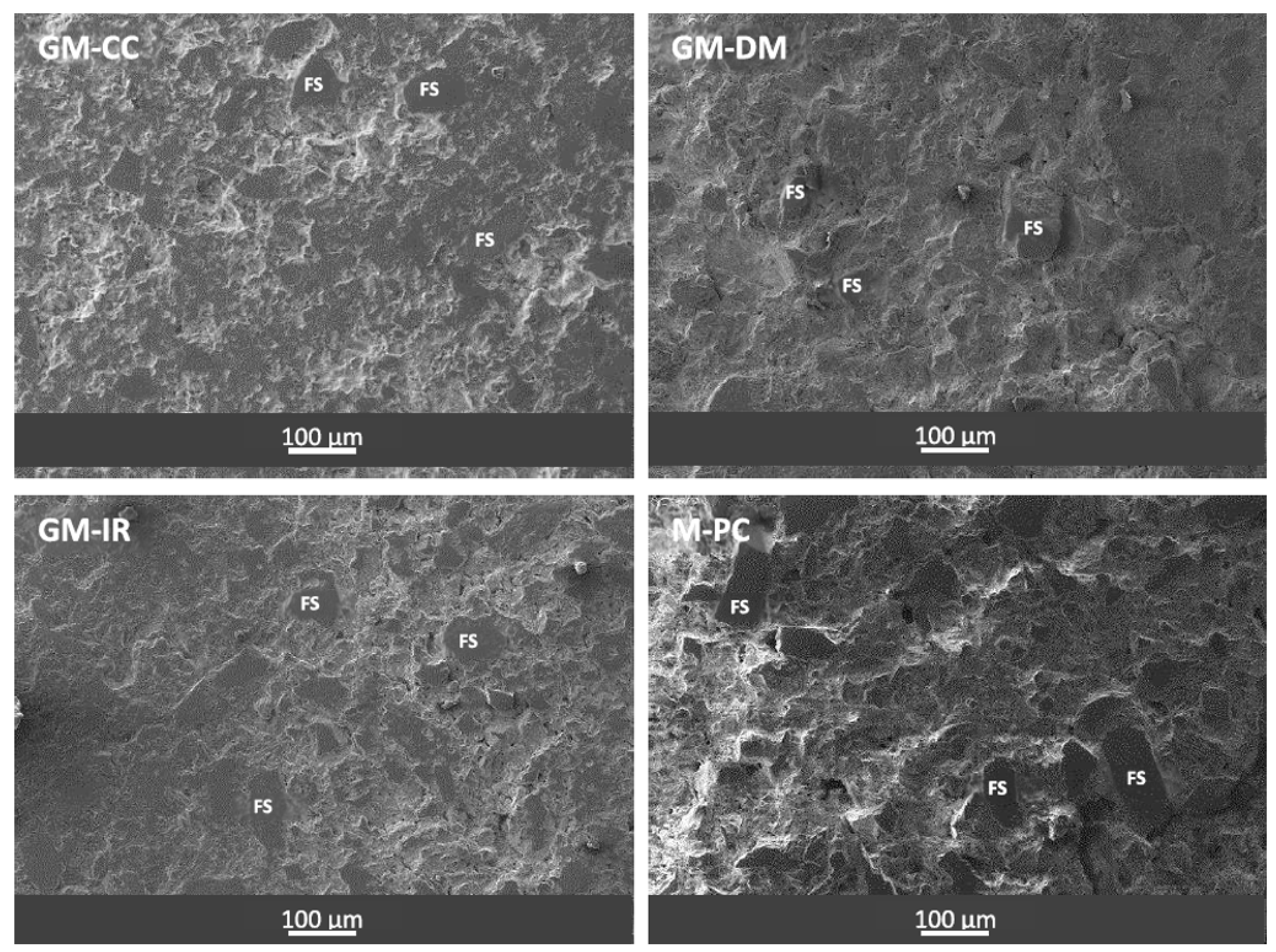
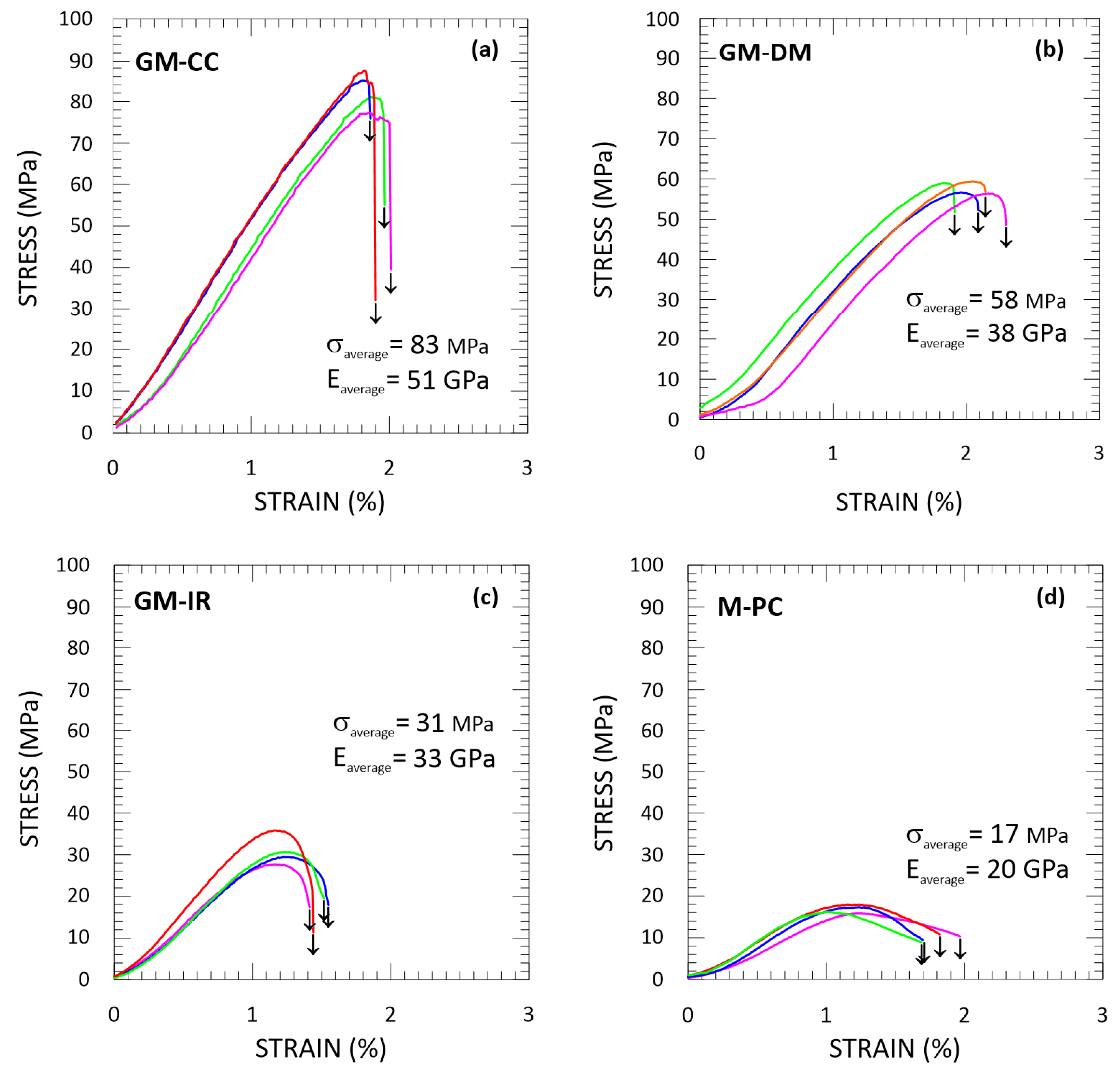
3.2. Geopolymeric and OPC Mortars
- Geopolymeric mortar from calcined clay (GM-CC): quartz (SiO2), albite (NaAlSi3O8), and microcline (KAlSi3O8).
- Geopolymeric mortar from demolition mortar (GM-DM): quartz (SiO2) and anorthoclase ((Na0.85K0.15)AlSi3O8).
- Geopolymeric mortar from ignimbrite rock (GM-IR): quartz (SiO2), albite (NaAlSi3O8) and sanidine (KSi3AlO8).
- Mortar from Portland cement (M-PC): anorthoclase ((Na0.85K0.15)AlSi3O8).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, A.; Manrique, S.; Siancas, L. Compendio de Rocas Ornamentales En El Perú. INGEMMET Bol. Ser. B Geol. Econ. 2020, 70, 1–286. [Google Scholar]
- Lebti, P.P.; Thouret, J.C.; Wörner, G.; Fornari, M. Neogene and Quaternary Ignimbrites in the Area of Arequipa, Southern Peru: Stratigraphical and Petrological Correlations. J. Volcanol. Geotherm. Res. 2006, 154, 251–275. [Google Scholar] [CrossRef]
- Brandmeier, M.; Wörner, G. Compositional Variations of Ignimbrite Magmas in the Central Andes over the Past 26 Ma—A Multivariate Statistical Perspective. Lithos 2016, 262, 713–728. [Google Scholar] [CrossRef]
- Aguilar, R.; Thouret, J.C.; Samaniego, P.; Wörner, G.; Jicha, B.; Paquette, J.L.; Suaña, E.; Finizola, A. Growth and Evolution of Long-Lived, Large Volcanic Clusters in the Central Andes: The Chachani Volcano Cluster, Southern Peru. J. Volcanol. Geotherm. Res. 2022, 426, 107539. [Google Scholar] [CrossRef]
- Manrique Llerena, N.; Arias Salazar, C.; de Vries, B.; Aguilar Contreras, R.; Palacios, C. Ruta Del Sillar: Quebrada de Añashuayco, Arequipa-Perú. Libr. Resúm. IX Foro Int. Peligros Volcánicos-IX FIPVO 2023, 3, 389–393. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries 2019; Tolcin, A.C., Ed.; USGS: Reston, VA, USA, 2019. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2017. Earth Syst. Sci. Data 2018, 10, 2213–2239. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D.; Djobo, J.N.Y. Natural Pozzolan Based Geopolymers: A Review on Mechanical, Microstructural and Durability Characteristics. Constr. Build. Mater. 2018, 190, 1251–1263. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and Material Performance of Geopolymer Concrete: A Review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Asim, N.; Alghoul, M.; Mohammad, M.; Amin, M.H.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Emerging Sustainable Solutions for Depollution: Geopolymers. Constr. Build. Mater. 2019, 199, 540–548. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrialwaste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Koleżyński, A.; Król, M.; Żychowicz, M. The Structure of Geopolymers—Theoretical Studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to Control the Geopolymer Setting Time with the Alkaline Silicate Solution. J. Non. Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH Concentrations on Physical and Electrical Properties of High Calcium Fly Ash Geopolymer Paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Dal Molin, D.C.C.; Vilela, A.C.F.; Da Silva, F.J.; Pavão, B.; Veit, H. The Effects of Na2O/SiO2 Molar Ratio, Curing Temperature and Age on Compressive Strength, Morphology and Microstructure of Alkali-Activated Fly Ash-Based Geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía-Arcila, J.M.; Mejía de Gutiérrez, R. Eco-Efficient Alkali-Activated Cement Based on Red Clay Brick Wastes Suitable for the Manufacturing of Building Materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of Geopolymers Sourced from Construction and Demolition Waste: A Review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status Quo and Future Directions of Construction and Demolition Waste Research: A Critical Review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Provis, J.L.; Duxson, P.; van Deventer, J.S.J. The Role of Particle Technology in Developing Sustainable Construction Materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Kvočka, D.; Lešek, A.; Knez, F.; Ducman, V.; Panizza, M.; Tsoutis, C.; Bernardi, A. Life Cycle Assessment of Prefabricated Geopolymeric Façade Cladding Panels Made from Large Fractions of Recycled Construction and Demolition Waste. Materials 2020, 13, 3931. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, A.; Cárdenas, V.; Robayo, R.A.; de Gutiérrez, R.M. Geopolymer Based on Concrete Demolition Waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Panizza, M.; Natali, M.; Garbin, E.; Tamburini, S.; Secco, M. Assessment of Geopolymers with Construction and Demolition Waste (CDW) Aggregates as a Building Material. Constr. Build. Mater. 2018, 181, 119–133. [Google Scholar] [CrossRef]
- Singh, N.B.; Wali, S.K.; Saxena, S.K.; Kumar, M. Properties of Calcined Clay-Based Geopolymer Mortars in Presence of Alccofine Powder and Recron Fiber. In RILEM Bookseries; RILEM: Champs sur Marne, France, 2020. [Google Scholar] [CrossRef]
- Hattatoglu, F.; Bakis, A. Usability of Ignimbrite Powder in Reactive Powder Concrete Road Pavement. Road Mater. Pavement Des. 2017, 18, 1448–1459. [Google Scholar] [CrossRef]
- Gimeno, D.; Davidovits, J.; Marini, C.; Rocher, P.; Tocco, S.; Cara, S.; Diaz, N.; Segura, C.; Sistu, C. Desarrollo de Un Cemento de Base Silicatada a Partir de Rocas Volcánicas Vítreas Alcalinas: Interpretatión de Los Resultados Preindustriales Basada En La Compositión Químico- Mineralógica de Los Precursores Geológicos. Bol. Soc. Esp. Cerám. Vidr. 2003, 42, 69–78. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Osio-Norgaard, J.; Gevaudan, J.P.; Srubar, W.V. A Review of Chloride Transport in Alkali-Activated Cement Paste, Mortar, and Concrete. Constr. Build. Mater. 2018, 186, 191–206. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Adam, G.; Samali, B. Mechanical Properties of Ambient Cured One-Part Hybrid OPC-Geopolymer Concrete. Constr. Build. Mater. 2018, 186, 330–337. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of Geopolymer Concrete for a Cleaner and Sustainable Environment—A Review of Mechanical Properties and Microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, C.J.; Li, N. Fracture Properties of Slag/Fly Ash-Based Geopolymer Concrete Cured in Ambient Temperature. Constr. Build. Mater. 2018, 190, 787–795. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P. Evaluation of Tension-Stiffening, Crack Spacing and Crack Width of Geopolymer Concretes. Constr. Build. Mater. 2018, 160, 408–414. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. Structural Performance of Reinforced Geopolymer Concrete Members: A Review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Visintin, P.; Mohamed Ali, M.S.; Albitar, M.; Lucas, W. Shear Behaviour of Geopolymer Concrete Beams without Stirrups. Constr. Build. Mater. 2017, 148, 10–21. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Yu, T.; Hadi, M.N.S. Factors Affecting the Bond Strength Between the Fly Ash-Based Geopolymer Concrete and Steel Reinforcement. Structures 2018, 14, 262–272. [Google Scholar] [CrossRef]
- Castel, A.; Foster, S.J. Bond Strength between Blended Slag and Class F Fly Ash Geopolymer Concrete with Steel Reinforcement. Cem. Concr. Res. 2015, 72, 48–53. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Effect of Granulated Lead Smelter Slag on Strength of Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Alzeebaree, R.; Çevik, A.; Nematollahi, B.; Sanjayan, J.; Mohammedameen, A.; Gülşan, M.E. Mechanical Properties and Durability of Unconfined and Confined Geopolymer Concrete with Fiber Reinforced Polymers Exposed to Sulfuric Acid. Constr. Build. Mater. 2019, 215, 1015–1032. [Google Scholar] [CrossRef]
- Bouaissi, A.; Li, L.y.; Al Bakri Abdullah, M.M.; Bui, Q.B. Mechanical Properties and Microstructure Analysis of FA-GGBS-HMNS Based Geopolymer Concrete. Constr. Build. Mater. 2019, 210, 198–209. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, J.H.; Ding, Y.C.; Cheng, T.W. A Study on the Characteristics and Microstructures of GGBS/FA Based Geopolymer Paste and Concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Luhar, S.; Chaudhary, S.; Luhar, I. Development of Rubberized Geopolymer Concrete: Strength and Durability Studies. Constr. Build. Mater. 2019, 204, 740–753. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: Oxford, UK, 1993; p. 312. [Google Scholar]
- Bruker AXS TOPAS V6: General Profile and Structure Analysis Software for Powder Diffraction Data; User’s Manual; Bruker AXS: Karlsruhe, Germany, 2017.
- Zhang, P.; Wang, K.; Li, Q.; Wang, J.; Ling, Y. Fabrication and Engineering Properties of Concretes Based on Geopolymers/Alkali-Activated Binders—A Review. J. Clean. Prod. 2020, 258, 120896. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening Evolution of Geopolymers from Setting to Equilibrium: A Review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Rathnaweera, T.D.; Perera, M.S.A. Recent Advances in Alkaline Cement Binders: A Review. J. Clean. Prod. 2019, 227, 70–87. [Google Scholar] [CrossRef]
- Piault, P.; King, A.; Henry, L.; Rathore, J.S.; Guignot, N.; Deslandes, J.-P.; Itié, J.-P. A Thresholding Based Iterative Reconstruction Method for Limited-Angle Tomography Data. Tomogr. Mater. Struct. 2023, 2, 100008. [Google Scholar] [CrossRef]
- Bossa, N.; Chaurand, P.; Vicente, J.; Borschneck, D.; Levard, C.; Aguerre-Chariol, O.; Rose, J. Micro- and Nano-X-Ray Computed-Tomography: A Step Forward in the Characterization of the Pore Network of a Leached Cement Paste. Cem. Concr. Res. 2015, 67, 138–147. [Google Scholar] [CrossRef]
- Madsen, I.C.; Scarlett, N.V.Y.; Kern, A. Description and Survey of Methodologies for the Determination of Amorphous Content via X-Ray Powder Diffraction. Z. Krist. 2011, 226, 944–955. [Google Scholar] [CrossRef]
- De Azeredo Melo, L.G.; Pereira, R.A.; Pires, E.F.C.; Darwish, F.A.I.; Da Silva, F.J. Physicochemical Characterization of Pulverized Phyllite Rock for Geopolymer Resin Synthesis. Mater. Res. 2017, 20, 236–243. [Google Scholar] [CrossRef]

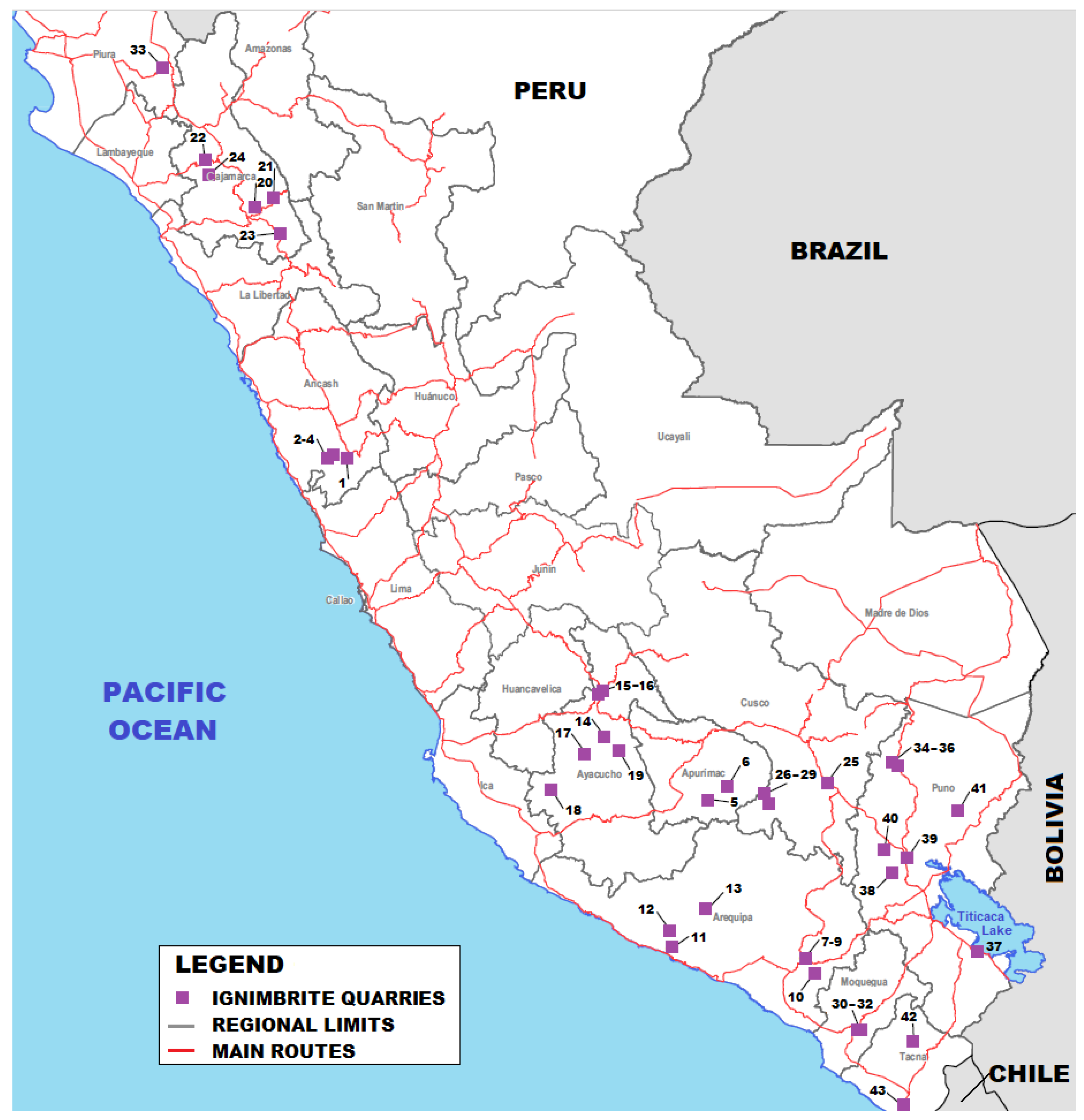
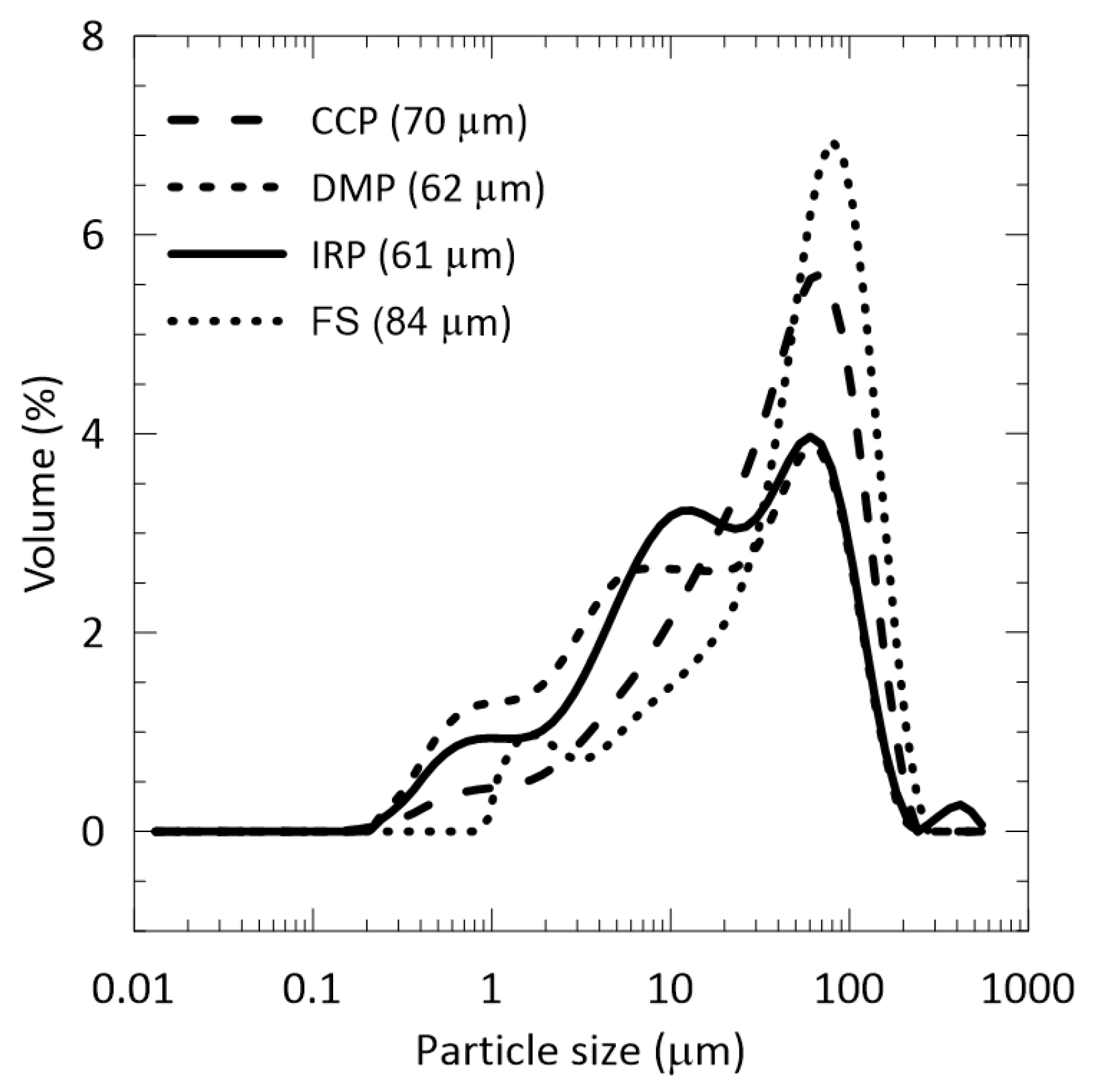
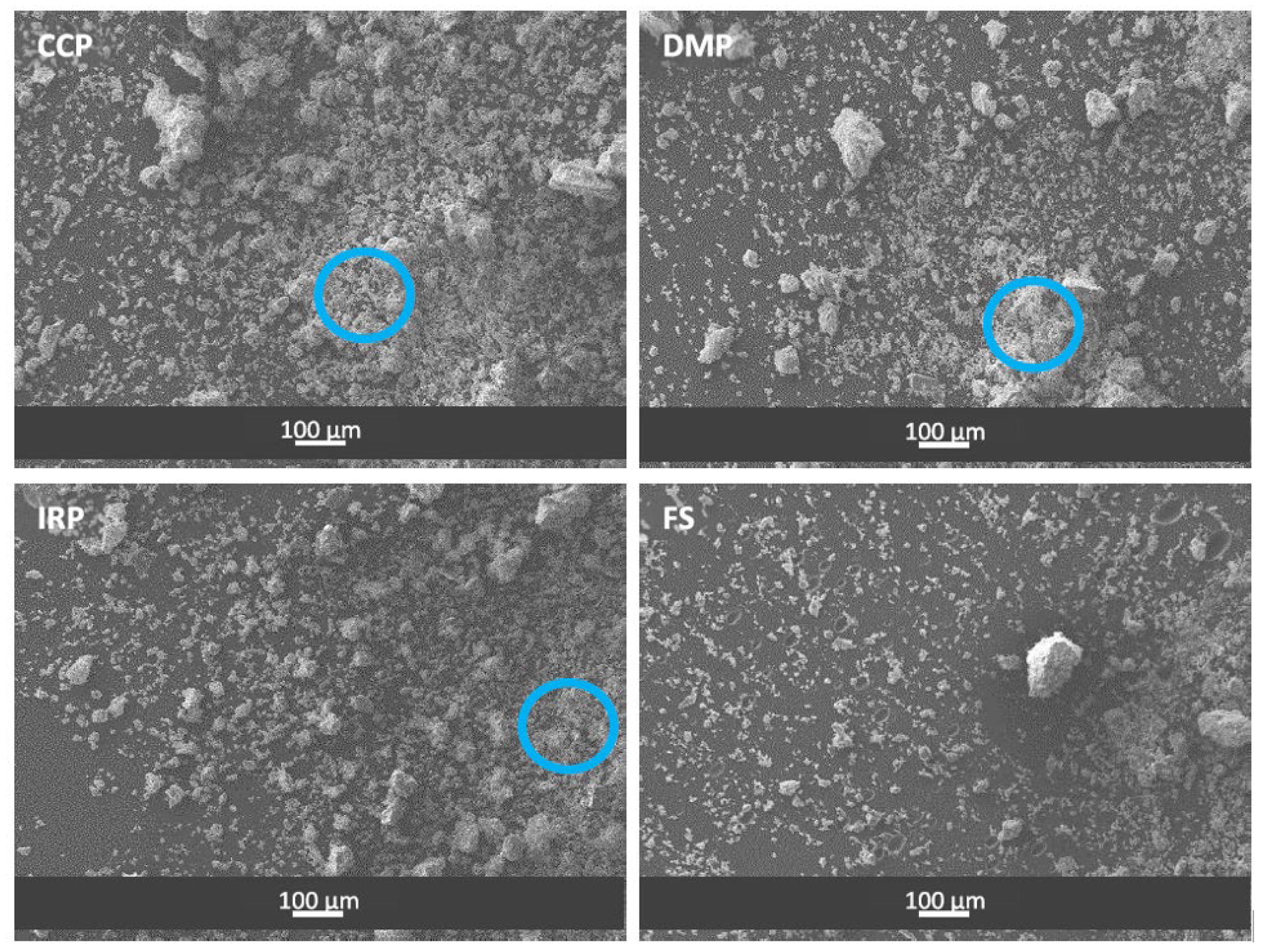


| Code | Region | Province | District | Name | UTM Coordinates | Category | |
|---|---|---|---|---|---|---|---|
| East | North | ||||||
| 1 | Ancash | Bolognesi | Cajacay | Incahuanca | 245241 | 8876224 | Deposit |
| 2 | Ancash | Recuay | Llacllin | Chaucayan | 219806 | 8872650 | Deposit |
| 3 | Ancash | Recuay | Marca | Coricoto | 229027 | 8877271 | Deposit |
| 4 | Ancash | Recuay | Marca | Mogote | 224474 | 8877412 | Deposit |
| 5 | Apurimac | Antabamba | Antabamba | Mollojo (Wilca) | 732995 | 8406657 | Deposit |
| 6 | Apurimac | Grau | Mamara | Mamara | 759554 | 8425354 | Quarry |
| 7 | Arequipa | Arequipa | Cerro Colorado | Flor Blanca | 221000 | 8188994 | Deposit |
| 8 | Arequipa | Arequipa | Cerro Colorado | La Paccha | 222077 | 8191198 | Quarry |
| 9 | Arequipa | Arequipa | Majes | Añashuayco | 220670 | 8191105 | Deposit |
| 10 | Arequipa | Arequipa | Quequeña | El Ingenio II | 234960 | 8169952 | Quarry |
| 11 | Arequipa | Camana | Ocoña | Lomas Agua Blanca | 682514 | 8201524 | Deposit |
| 12 | Arequipa | Caravelí | Caravelí | Cantera | 682641 | 8225304 | Deposit |
| 13 | Arequipa | Caylloma | Huambos | Andaray | 730300 | 8253800 | Deposit |
| 14 | Ayacucho | Cangallo | Cangallo | Cangallo | 593373 | 8492466 | Deposit |
| 15 | Ayacucho | Huamanga | Las Nazarenas | Chacco | 584212 | 8553134 | Deposit |
| 16 | Ayacucho | Huamanga | Pacaycasa | Sillar Ayacuchano | 588500 | 8556620 | Deposit |
| 17 | Ayacucho | Huanca Sancos | Santiago de Lucanamarca | Lucanamarca | 567814 | 8469314 | Deposit |
| 18 | Ayacucho | Lucanas | Laramate | Laramate | 521412 | 8420618 | Deposit |
| 19 | Ayacucho | Víctor Fajardo | Cayara | Mayopampa | 612246 | 8473702 | Deposit |
| 20 | Cajamarca | Cajamarca | Baños del Inca | La mesma | 784674 | 9220370 | Deposit |
| 21 | Cajamarca | Celendín | José Galvez | Cerro Lázaro | 810624 | 9233327 | Deposit |
| 22 | Cajamarca | Chota | Huambos | Pululo | 718968 | 9286276 | Deposit |
| 23 | Cajamarca | San Marcos | Ichocan | Llanupacha | 820181 | 9184347 | Quarry |
| 24 | Cajamarca | Santa Cruz | Santa Cruz | Piedra Blanca | 726237 | 9267049 | Quarry |
| 25 | Cusco | Canchis | San Pedro | Auquisa Dos | 245689 | 8432456 | Deposit |
| 26 | Cusco | Chumbivilcas | Llusco | Llusco I | 809113 | 8413826 | Quarry |
| 27 | Cusco | Chumbivilcas | Llusco | Llusco II | 809848 | 8414374 | Quarry |
| 28 | Cusco | Chumbivilcas | Santo Tomás | Santo Tomás I | 813998 | 8401356 | Quarry |
| 29 | Cusco | Chumbivilcas | Santo Tomás | Santo Tomás II | 815350 | 8402623 | Quarry |
| 30 | Moquegua | Mariscal Nieto | Moquegua | El Calicanto I | 298927 | 8092960 | Deposit |
| 31 | Moquegua | Mariscal Nieto | Moquegua | KAGIBEDA V | 297325 | 8092367 | Deposit |
| 32 | Moquegua | Mariscal Nieto | Moquegua | San Diego 5 | 295596 | 8091797 | Deposit |
| 33 | Piura | Huancabamba | Sondorillo | Cascapampa | 664564 | 9411821 | Deposit |
| 34 | Puno | Carabaya | Corani | Cerro Huillacota | 332446 | 8461578 | Deposit |
| 35 | Puno | Carabaya | Corani | Cerro Huillacota | 332877 | 8461280 | Quarry |
| 36 | Puno | Carabaya | Macusani | Chillicuno | 339363 | 8458070 | Quarry |
| 37 | Puno | Chucuito | Juli | Cruzpata | 453648 | 8207043 | Quarry |
| 38 | Puno | Lampa | Palca | Umpuco | 335360 | 8310123 | Quarry |
| 39 | Puno | Lampa | Pucará | Cerro Llallahua | 35.6062 | 8331301 | Quarry |
| 40 | Puno | Melgar | Ayaviri | Cacapunco | 324817 | 8342147 | Quarry |
| 41 | Puno | San Antonio de Putina | Putina | Moroccarca | 421765 | 8395996 | Quarry |
| 42 | Tacna | Candarave | Quilahuani | Buena Vista | 371012 | 8081711 | Deposit |
| 43 | Tacna | Tacna | Tacna | Hospicio | 361251 | 7990188 | Deposit |
| White Ignimbrite (wt %) | Pink Ignimbrite (wt %) | |
|---|---|---|
| SiO2 | 73.60 | 75.50 |
| Al2O3 | 13.60 | 13.50 |
| K2O | 4.23 | 4.64 |
| Na2O | 3.94 | 3.44 |
| Fe2O3 | 1.41 | 1.60 |
| CaO | 1.20 | 1.14 |
| TiO2 | 0.24 | - |
| MgO | 0.20 | 0.21 |
| MnO | 0.06 | 0.09 |
| SO3 | 0.06 | 0.04 |
| P2O5 | 0.05 | 0.05 |
| ZrO2 | 0.04 | 0.04 |
| SrO | 0.03 | 0.04 |
| Component | GM-CC | GM-DM | GM-IR | M-PC |
|---|---|---|---|---|
| FS (kg/m3) | 1676.4 | 1654.4 | 1634.1 | 1791.7 |
| Raw material (kg/m3) | 1005.9 | 992.7 | 980.4 | 1075.0 |
| Alkaline NaOH solution (kg/m3) | 502.9 | 496.3 | 490.2 | 537.5 |
| Raw Material | Weight (g) | Real Volume (cm3) | Real Density (g/cm3) |
|---|---|---|---|
| CCP | 6.2478 | 2.3502 | 2.6584 |
| DMP | 6.3599 | 2.4802 | 2.5643 |
| IRP | 5.0702 | 2.0466 | 2.4774 |
| FS | 7.1178 | 2.6394 | 2.6967 |
| Elements | CCP | DMP | IRP | FS |
|---|---|---|---|---|
| Si/Al~5:2 | Si/Al~4:1 | Si/Al~5:1 | Si/Al~3:1 | |
| mg/kg | ||||
| Al | 30,180.00 ± 3604.26 | 16,363.00 ± 53.74 | 26,496.67 ± 1086.66 | 27,026.67 ± 1167.79 |
| Si | 78,093.33 ± 7583.89 | 68,435.00 ± 2156.68 | 136,373.33 ± 4622.92 | 90,120.00 ± 3592.51 |
| P | 357.90 ± 41.40 | 1059.80 ± 25.74 | 112.17 ± 16.67 | 479.13 ± 18.53 |
| Cl | 156.55 ± 5.44 | 331.25 ± 2.19 | 240.87 ± 31.26 | 374.27 ± 5.02 |
| K | 7197.33 ± 779.61 | 7175.00 ± 159.81 | 16,586.67 ± 1055.00 | 7958.00 ± 448.02 |
| Ca | 8329.67 ± 1195.03 | 98,380.00 ± 1781.91 | 4995.67 ± 131.20 | 19,018.00 ± 1325.10 |
| Ti | 1933.80 ± 315.46 | 1133.00 ± 32.81 | 921.15 ± 155.92 | 2172.90 ± 181.80 |
| Cr | 19.34 ± 1.98 | 14.38 ± 0.22 | - | 29.46 ± 4.22 |
| Mn | 437.80 ± 52.75 | 270.09 ± 5.39 | 359.80 ± 56.81 | 284.36 ± 25.06 |
| Fe | 24,631.67 ± 3325.44 | 16,697.00 ± 131.52 | 6056.25 ± 75.31 | 17,918.33 ± 1725.66 |
| Ni | 8.79 ± 1.52 | 7.61 ± 0.23 | 12.44 ± 3.28 | 9.93 ± 0.74 |
| Cu | 56.47 ± 9.34 | 49.70 ± 7.23 | 29.65 ± 0.03 | 28.08 ± 2.34 |
| Zn | 62.21 ± 7.24 | 46.89 ± 5.33 | 48.74 ± 1.83 | 41.09 ± 2.54 |
| As | 15.43 ± 1.51 | 7.61 ± 1.17 | - | - |
| Rb | 47.20 ± 7.00 | 25.16 ± 0.45 | 68.38 ± 9.25 | 27.91 ± 2.59 |
| Sr | 223.66 ± 45.51 | 242.66 ± 19.00 | 126.72 ± 13.39 | 445.65 ± 45.43 |
| Ba | 595.53 ± 80.33 | 358.50 ± 22.63 | 941.50 ± 70.63 | 577.30 ± 47.93 |
| Pb | 14.44 ± 2.23 | 10.37 ± 0.10 | 16.35 ± 1.88 | 13.80 ± 2.17 |
| Br | - | 1.42 ± 0.01 | - | 1.06 ± 0.05 |
| Crystalline Phases | CCP | DMP | IRP | FS |
|---|---|---|---|---|
| %Weight | ||||
| Quartz (SiO2) | 14 | 7.9 | - | - |
| Albite (NaAlSi3O8) | 40.5 | 10.8 | 22.3 | 10.9 |
| Muscovite 2M1 (KAl3Si3O10(OH)2) | 28.1 | 9 | 1.6 | 9.1 |
| Cristobalite (SiO2) | 9.6 | 4.8 | 38.9 | 3.4 |
| Actinolite (Ca2(Mg,Fe2+)5Si8O22(OH)2) | 6.2 | 3.5 | - | 5.1 |
| Anatase (TiO2) | 1.6 | - | - | - |
| Anorthite (CaAl2Si2O8) | - | 40.9 | - | 71.5 |
| Portlandite (Ca(OH)2) | - | 8.5 | - | - |
| Calcite (Ca(CO)3) | - | 10.6 | - | - |
| Chlorite ((Mg,Al)6(Si,Al)4O10(OH)8) | - | 4 | - | - |
| Microcline (KAlSi3O8) | - | - | 37.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huamán-Mamani, F.A.; Palomino-Ñaupa, C.K.; Orta Cuevas, M.d.M.; Medina-Carrasco, S. Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru. Geosciences 2024, 14, 80. https://doi.org/10.3390/geosciences14030080
Huamán-Mamani FA, Palomino-Ñaupa CK, Orta Cuevas MdM, Medina-Carrasco S. Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru. Geosciences. 2024; 14(3):80. https://doi.org/10.3390/geosciences14030080
Chicago/Turabian StyleHuamán-Mamani, Fredy Alberto, Cris Katherin Palomino-Ñaupa, María del Mar Orta Cuevas, and Santiago Medina-Carrasco. 2024. "Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru" Geosciences 14, no. 3: 80. https://doi.org/10.3390/geosciences14030080
APA StyleHuamán-Mamani, F. A., Palomino-Ñaupa, C. K., Orta Cuevas, M. d. M., & Medina-Carrasco, S. (2024). Fabrication and Mechanical Evaluation of Eco-Friendly Geopolymeric Mortars Derived from Ignimbrite and Demolition Waste from the Construction Industry in Peru. Geosciences, 14(3), 80. https://doi.org/10.3390/geosciences14030080