Petrographic Analysis of Mafic and Ultramafic Rocks in Northern Thailand: Implications for CO2 Mineralization and Enhanced Rock Weathering Approach
Abstract
:1. Introduction
2. Geological Background
3. Materials and Methods
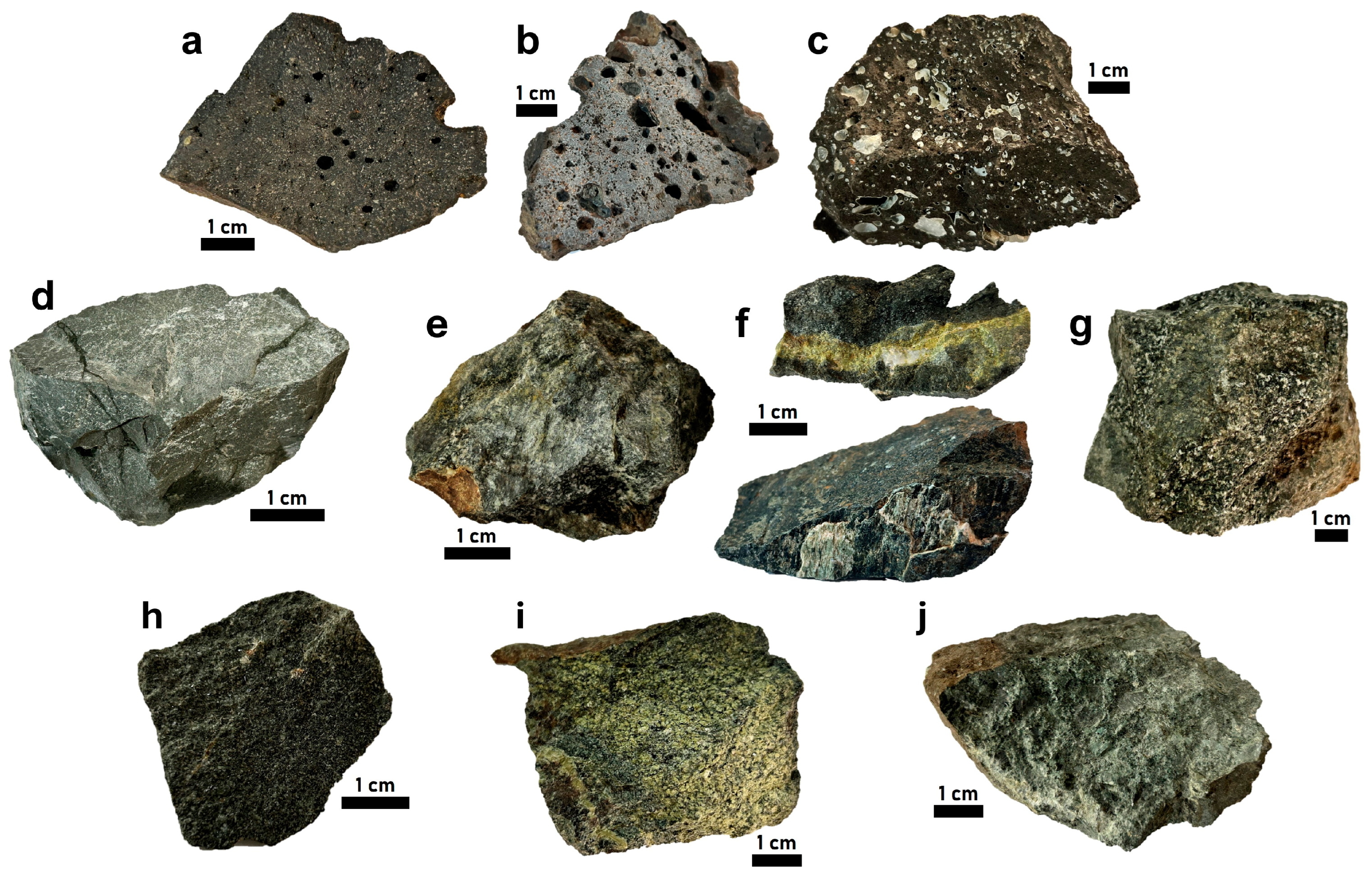
3.1. Rock Sampling and Lithologic Descriptions
3.2. Sample Preparation and Optical Mineral Analysis
3.3. Modal Analyses
4. Results
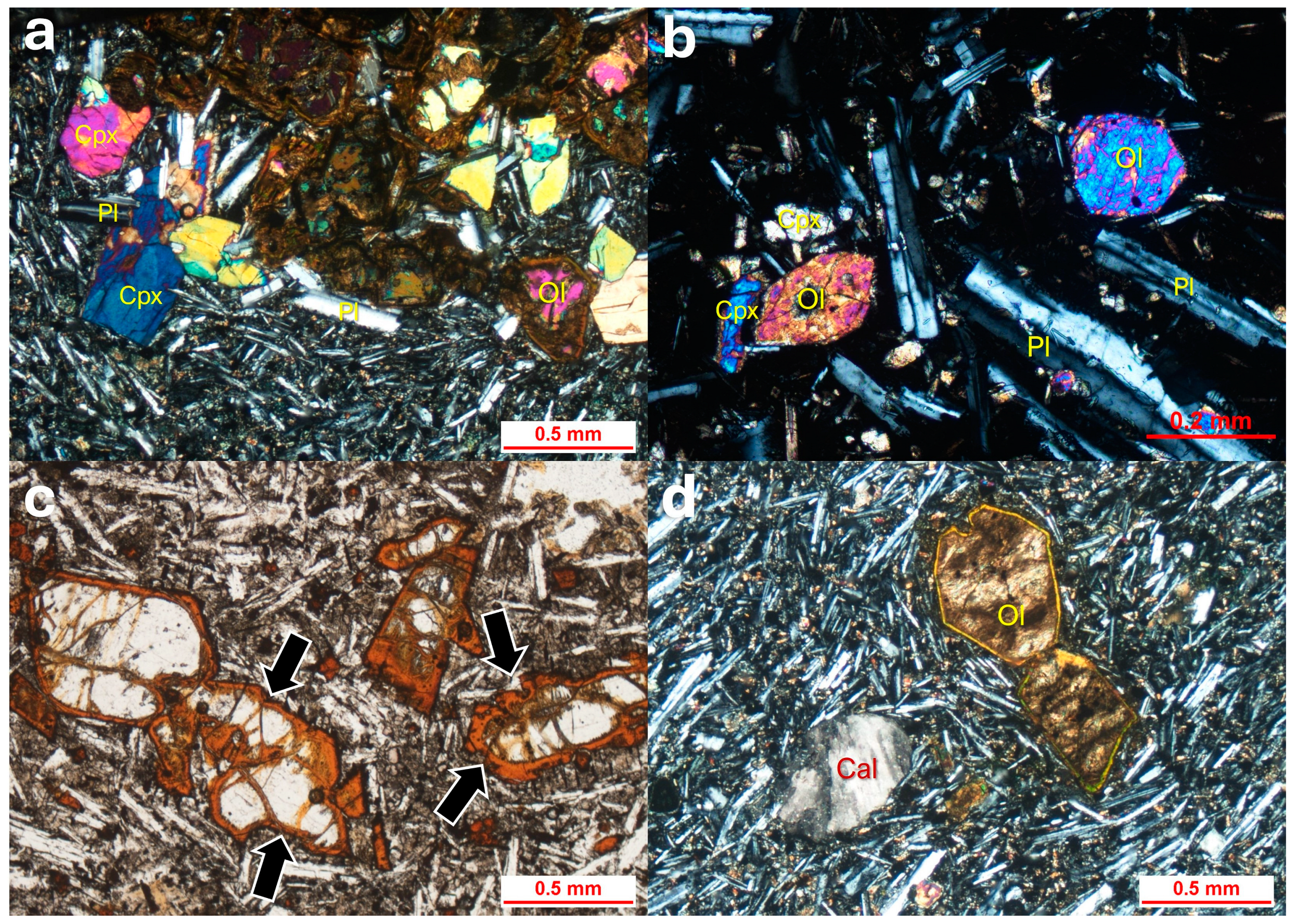
4.1. Vesicular Olivine Basalt
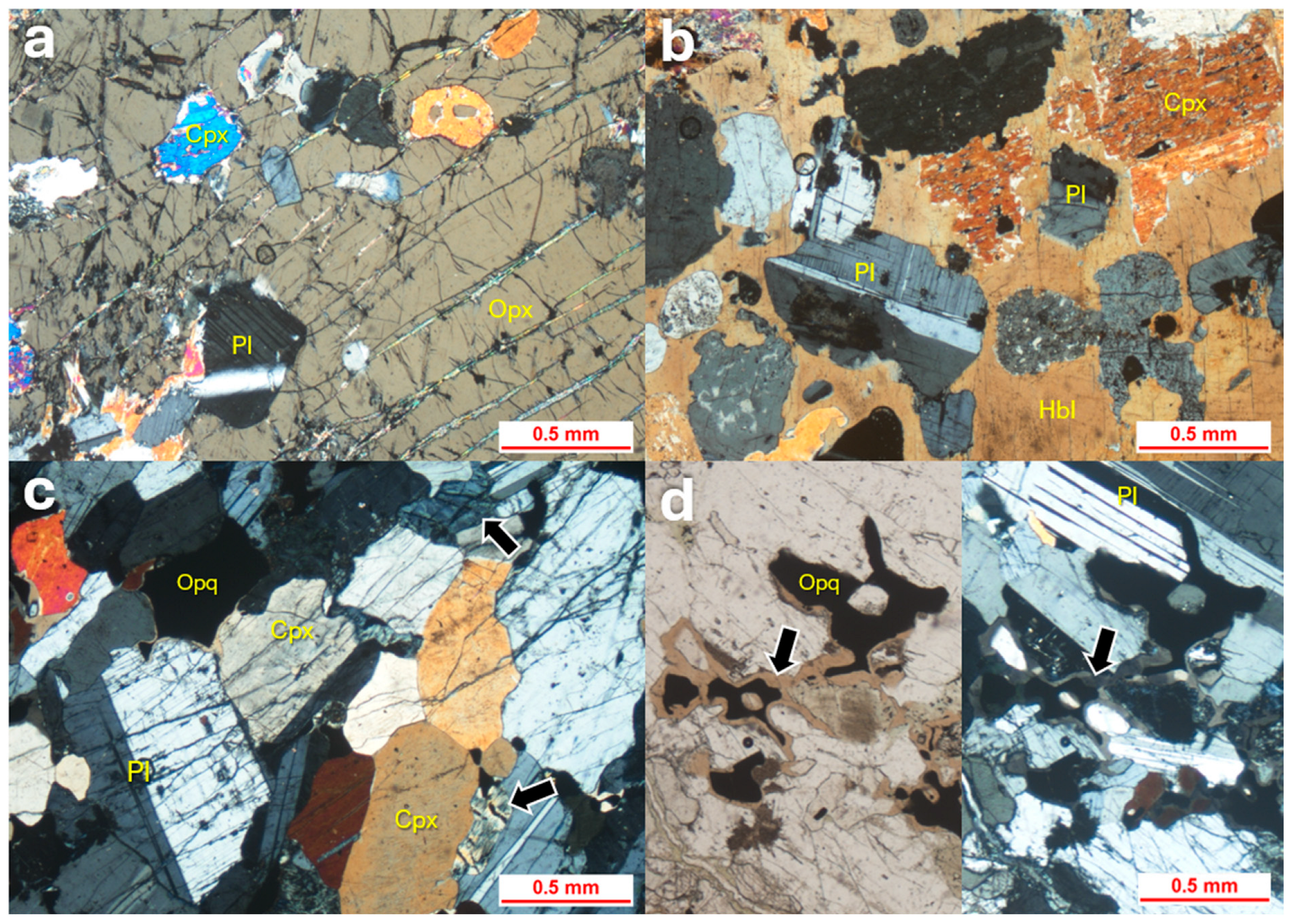
4.2. Gabbro
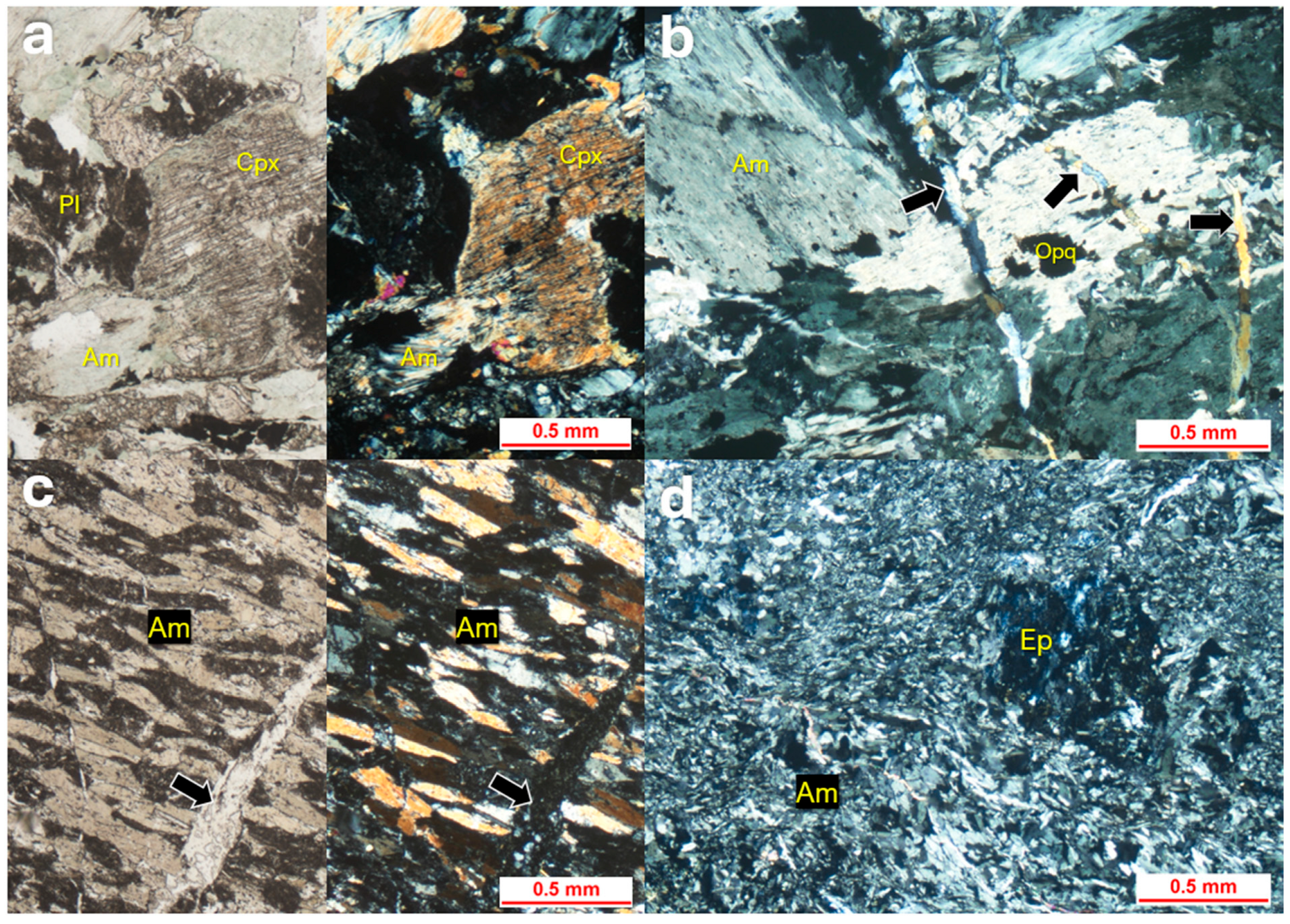
4.3. Metabasite
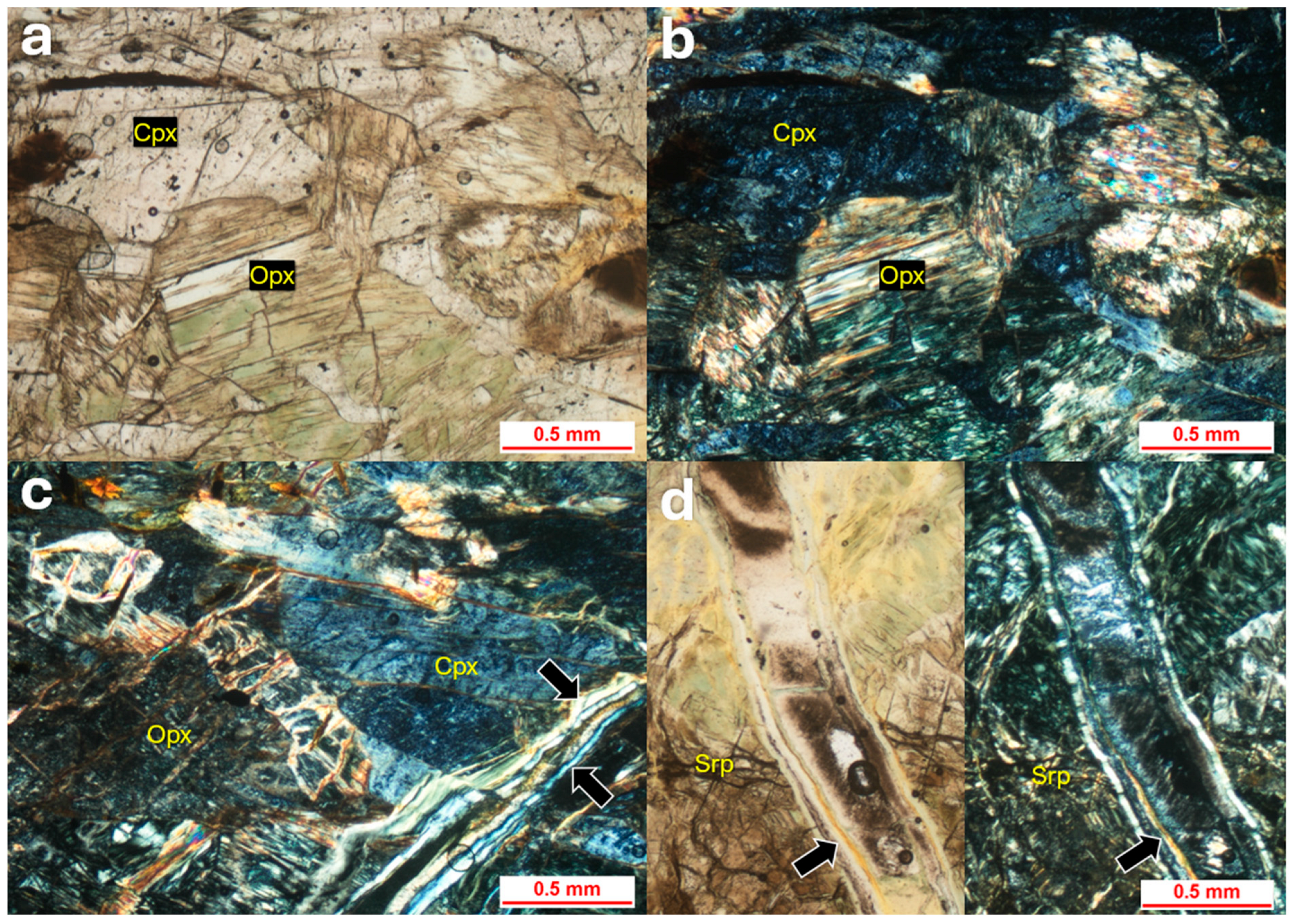
4.4. Peridotite
4.5. Altered Pyroxenite
4.6. Basaltic Tuff
5. Discussion
5.1. CO2-Reactive Minerals in Mafic and Ultramafic Rocks
5.2. Primary Assessment of Potential CO2 Uptake
5.3. Implications for Enhanced Rock Weathering Technology
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Holloway, S. Carbon dioxide capture and geological storage. Philos. Trans. A Math. Phys. Eng. Sci. 2007, 365, 1095–1107. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Larachi, F.; Daldoul, I.; Beaudoin, G. Fixation of CO2 by chrysotile in low-pressure dry and moist carbonation: Ex-situ and in-situ characterizations. Geochim. Cosmochim. Acta 2010, 74, 3051–3075. [Google Scholar] [CrossRef]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Con. 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Santos, R.; Van Audenaerde, A.; Chiang, Y.; Iacobescu, R.; Knops, P.; Van Gerven, T. Nickel extraction from olivine: Effect of carbonation pre-treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; Defries, R.; Galloway, J.; Heimann, M. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Ocean Studies Board; Board on Chemical Sciences and Technology; Board on Earth Sciences and Resources; Board on Agriculture and Natural Resources; Board on Energy and Environmental Systems; Board on Atmospheric Sciences and Climate; Committee on Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An overview of the status and challenges of CO2 storage in minerals and geological formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth-Sci. Rev. 2022, 229, 104036. [Google Scholar] [CrossRef]
- Béarat, H.; McKelvy, M.J.; Chizmeshya, A.V.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: Role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Mwenketishi, G.; Benkreira, H.; Rahmanian, N. Carbon dioxide sequestration methodothologies—A review. Am. J. Clim. Change 2023, 12, 579–627. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and mechanisms of mineral carbonation in peridotite: Natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Ulrich Mayer, K.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Con. 2014, 25, 121–140. [Google Scholar] [CrossRef]
- Sigfusson, B.; Gislason, S.R.; Matter, J.M.; Stute, M.; Gunnlaugsson, E.; Gunnarsson, I.; Aradottir, E.S.; Sigurdardottir, H.; Mesfin, K.; Alfredsson, H.A.; et al. Solving the carbon-dioxide buoyancy challenge: The design and field testing of a dissolved CO2 injection system. Int. J. Greenh. Gas Con. 2015, 37, 213–219. [Google Scholar] [CrossRef]
- Hariharan, S.; Mazzotti, M. Kinetics of flue gas CO2 mineralization processes using partially dehydroxylated lizardite. Chem. Eng. J. 2017, 324, 397–413. [Google Scholar] [CrossRef]
- Liu, R.; Heinemann, N.; Liu, J.; Zhu, W.; Wilkinson, M.; Xie, Y.; Wang, Z.; Wen, T.; Hao, F.; Haszeldine, R.S. CO2 sequestration by mineral trapping in natural analogues in the Yinggehai Basin, South China Sea. Mar. Pet. Geol. 2019, 104, 190–199. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J. In situ carbonation of peridotite for CO2 storage. Proc. Natl. Acad. Sci. USA 2008, 105, 17295–17300. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R.; Matter, J. Mineral carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Dipple, G.M. Serpentinite Carbonation for CO2 Sequestration. Elements 2013, 9, 115–121. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snaebjornsdottir, S.O.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, I.; Aradóttir, E.S.; Oelkers, E.H.; Clark, D.E.; Arnarson, M.Þ.; Sigfússon, B.; Snæbjörnsdóttir, S.Ó.; Matter, J.M.; Stute, M.; Júlíusson, B.M.; et al. The rapid and cost-effective capture and subsurface mineral storage of carbon and sulfur at the CarbFix2 site. Int. J. Greenh. Gas Con. 2018, 79, 117–126. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization technology for carbon capture, utilization, and storage. Front. Energy Res. 2020, 8, 142. [Google Scholar] [CrossRef]
- Sandalow, D.; Aines, R.; Friedmann, J.; Kelemen, P.; McCormick, C.; Power, I.M.; Schmidt, B.; Wilson, S.A.S. Carbon Mineralization Roadmap (ICEF Innovation Roadmap Project); Lawrence Livermore National Laboratory: California, USA, 2021. [Google Scholar]
- Goff, F.; Lackner, K.S. Carbon dioxide sequestering using ultramafic rocks. Environ. Geosci. 1998, 5, 89–102. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Ho, A.M.; Chien, Y.J.; Dooley, J.J.; Davidson, C.L. Potential for carbon dioxide sequestration in flood basalts. J. Geophys. Res. Solid Earth 2006, 111, B12201. [Google Scholar] [CrossRef]
- Styles, M.T.; Sanna, A.; Lacinska, A.M.; Naden, J.; Maroto-Valer, M. The variation in composition of ultramafic rocks and the effect on their suitability for carbon dioxide sequestration by mineralization following acid leaching. Greenh. Gases Sci. Technol. 2014, 4, 440–451. [Google Scholar] [CrossRef]
- Mervine, E.M.; Wilson, S.; Power, I.M.; Dipple, G.M.; Turvey, C.C.; Hamilton, J.L.; Vanderzee, S.; Raudsepp, M.; Southam, C.; Matter, J.M.; et al. Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: An assessment of De Beers mine sites in South Africa and Canada. Mineral. Petrol. 2018, 112, 755–765. [Google Scholar] [CrossRef]
- Nisbet, H.; Buscarnera, G.; Carey, J.W.; Chen, M.A.; Detournay, E.; Huang, H.; Hyman, J.D.; Kang, P.K.; Kang, Q.; Labuz, J.F.; et al. Carbon mineralization in fractured mafic and ultramafic rocks: A review. Rev. Geophys. 2024, 62, e2023RG000815. [Google Scholar] [CrossRef]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P. (Eds.) Igneous Rocks: A Classification and Glossary of Terms: Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Brady, P.V. The effect of silicate weathering on global temperature and atmospheric CO2. J. Geophys. Res. Solid Earth 1991, 96, 18101–18106. [Google Scholar] [CrossRef]
- Raymo, M.E.; Ruddiman, W.F. Tectonic forcing of late Cenozoic climate. Nature 1992, 359, 117–122. [Google Scholar] [CrossRef]
- Berner, R.A.; Berner, E.K. Silicate weathering and climate. In Tectonic Uplift and Climate Change; Ruddiman, W.F., Ed.; Springer: Boston, MA, USA, 1997; pp. 353–365. [Google Scholar]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hirth, G. Reaction-driven cracking during retrograde metamorphism: Olivine hydration and carbonation. Earth Planet. Sci. Lett. 2012, 345–348, 81–89. [Google Scholar] [CrossRef]
- Beaulieu, E.; Goddéris, Y.; Donnadieu, Y.; Labat, D.; Roelandt, C. High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nat. Clim. Change 2012, 2, 346–349. [Google Scholar] [CrossRef]
- Streit, E.; Kelemen, P.; Eiler, J. Coexisting serpentine and quartz from carbonate-bearing serpentinized peridotite in the Samail Ophiolite, Oman. Contribut. Mineral. Petrol. 2012, 164, 821–837. [Google Scholar] [CrossRef]
- Falk, E.S.; Kelemen, P.B. Geochemistry and petrology of listvenite in the Samail ophiolite, Sultanate of Oman: Complete carbonation of peridotite during ophiolite emplacement. Geochim. Cosmochim. Acta. 2015, 160, 70–90. [Google Scholar] [CrossRef]
- Penman, D.E.; Caves Rugenstein, J.K.; Ibarra, D.E.; Winnick, M.J. Silicate weathering as a feedback and forcing in Earth’s climate and carbon cycle. Earth-Sci. Rev. 2020, 209, 103298. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Krijgsman, P. Enhanced weathering: An effective and cheap tool to sequester CO2. Clim. Change 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Hartmann, J.; West, A.J.; Renforth, P.; Köhler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Moosdorf, N.; Renforth, P.; Hartmann, J. Carbon dioxide efficiency of terrestrial enhanced weathering. Environ. Sci. Technol. 2014, 48, 4809–4816. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13, 034010. [Google Scholar] [CrossRef]
- Power, I.M.; Paulo, C.; Rausis, K. The mining industry’s role in enhanced weathering and mineralization for CO2 removal. Environ. Sci. Technol. 2024, 58, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.L.; Quirk, J.; Thorley, R.M.S.; Kharecha, P.A.; Hansen, J.; Ridgwell, A.; Lomas, M.R.; Banwart, S.A.; Beerling, D.J. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 2016, 6, 402–406. [Google Scholar] [CrossRef]
- Edwards, D.P.; Lim, F.; James, R.H.; Pearce, C.R.; Scholes, J.; Freckleton, R.P.; Beerling, D.J. Climate change mitigation: Potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 2017, 13, 20160715. [Google Scholar] [CrossRef]
- Montserrat, F.; Renforth, P.; Hartmann, J.; Leermakers, M.; Knops, P.; Meysman, F.J. Olivine dissolution in seawater: Implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 2017, 51, 3960–3972. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Co-benefits of wollastonite weathering in agriculture: CO2 sequestration and promoted plant growth. ACS Omega 2019, 4, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas Con. 2020, 97, 103017. [Google Scholar] [CrossRef]
- Cong, L.; Lu, S.; Jiang, P.; Zheng, T.; Yu, Z.; Lü, X. CO2 sequestration and soil improvement in enhanced rock weathering: A review from an experimental perspective. Greenh. Gases Sci. Technol. 2024, 14, 1122–1138. [Google Scholar] [CrossRef]
- Medeiros, F.P.; Theodoro, S.H.; Carvalho, A.M.X.; Oliveira, V.S.; Oliveira, L.C.; Almeida, R.M.P.; Viana, M.B.; Gomide, C.S. The combination of crushed rock and organic matter enhances the capture of inorganic carbon in tropical soils. J. S. Am. Earth Sci. 2025, 152, 105254. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Bradshaw, P.M.D.; Harrison, A.L. Prospects for CO2 mineralization and enhanced weathering of ultramafic mine tailings from the Baptiste nickel deposit in British Columbia, Canada. Int. J. Greenh. Gas Con. 2020, 94, 102895. [Google Scholar] [CrossRef]
- Vienne, A.; Poblador, S.; Portillo-Estrada, M.; Hartmann, J.; Ijiehon, S.; Wade, P.; Vicca, S. Enhanced weathering using basalt rock powder: Carbon sequestration, co-benefits and risks in a mesocosm study with Solanum tuberosum. Front. Clim. 2022, 4, 869456. [Google Scholar] [CrossRef]
- Paulo, C.; Power, I.M.; Zeyen, N.; Wang, B.; Wilson, S. Geochemical modeling of CO2 sequestration in ultramafic mine wastes from Australia, Canada, and South Africa: Implications for carbon accounting and monitoring. Appl. Geochem. 2023, 152, 105630. [Google Scholar] [CrossRef]
- Taksavasu, T.; Arin, P.; Khatecha, T.; Kojinok, S. Microtextural characteristics of ultramafic rock-forming minerals and their effects on carbon sequestration. Minerals 2024, 14, 597. [Google Scholar] [CrossRef]
- Barr, S.M.; Tantisukrit, C.; Yaowanoiyothin, W.; Macdonald, A.S. Petrology and tectonic implications of Upper Paleozoic volcanic rocks of the Chiang Mai belt, northern Thailand. J. Southeast Asian Earth Sci. 1990, 4, 37–47. [Google Scholar] [CrossRef]
- DMR. Geology of Thailand, 1st ed.; Department of Mineral Resources, Ministry of Natural Resources and Environment: Bangkok, Thailand, 2014.
- Metcalfe, I. Permian tectonic framework and palaeogeography of SE Asia. J. Asian Earth Sci. 2002, 20, 551–566. [Google Scholar] [CrossRef]
- Phajuy, B.; Panjasawatwong, Y.; Osataporn, P. Preliminary geochemical study of volcanic rocks in the Pang Mayao area, Phrao, Chiang Mai, northern Thailand: Tectonic setting of formation. J. Asian Earth Sci. 2005, 24, 765–776. [Google Scholar] [CrossRef]
- Bandibas, J.; Takarada, S. Geoinformation sharing system for East and Southeast Asia using SDI, OGC web services and FOSS. Int. J. Geosci. 2019, 10, 209–224. [Google Scholar] [CrossRef]
- DMR. Geological Map of Thailand on 1:50,000 Scale. 2007. Available online: https://www.dmr.go.th/wp-content/uploads/2022/10/INDEXgeo50k_8_60-Model.pdf (accessed on 5 September 2024).
- Promneewat, K.; Taksavasu, T.; Mankhemthong, N.; Siritongkham, N. Offline interactive map from hybrid app development: A case from geologic map app. In Proceedings of the 27th International Computer Science and Engineering Conference (ICSEC), Samui, Thailand, 14–15 September 2023; pp. 331–334. [Google Scholar]
- DMR. Thailand WEBGIS Geoinformation System: Geoinformation Sharing System for East and Southeast Asia. Available online: https://geohazards-info.gsj.jp/gsi/thailand/index.php (accessed on 16 August 2024).
- Koukouzas, N.; Koutsovitis, P.; Tyrologou, P.; Karkalis, C.; Arvanitis, A. Potential for mineral carbonation of CO2 in Pleistocene basaltic rocks in Volos Region (Central Greece). Minerals 2019, 9, 627. [Google Scholar] [CrossRef]
- Kwon, S. Mineralization for CO2 Sequestration Using Olivine Sorbent in the Presence of Water Vapor. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2011. [Google Scholar]
- Rasool, M.H.; Ahmad, M. Reactivity of basaltic minerals for CO2 sequestration via in situ mineralization: A review. Minerals 2023, 13, 1154. [Google Scholar] [CrossRef]
- Garcia, B.; Beaumont, V.; Perfetti, E.; Rouchon, V.; Blanchet, D.; Oger, P.; Dromart, G.; Huc, A.Y.; Haeseler, F. Experiments and geochemical modelling of CO2 sequestration by olivine: Potential, quantification. Appl. Geochem. 2010, 25, 1383–1396. [Google Scholar] [CrossRef]
- Munz, I.A.; Brandvoll, Ø.; Haug, T.A.; Iden, K.; Smeets, R.; Kihle, J.; Johansen, H. Mechanisms and rates of plagioclase carbonation reactions. Geochim. Cosmochim. Acta 2012, 77, 27–51. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Slotte, M.; Koivisto, E.; Erlund, R. Serpentinite carbonation process routes using ammonium sulfate and integration in industry. Energy Technol. 2017, 5, 945–954. [Google Scholar] [CrossRef]
- Daval, D.; Martinez, I.; Corvisier, J.; Findling, N.; Goffé, B.; Guyot, F. Carbonation of Ca-bearing silicates, the case of wollastonite: Experimental investigations and kinetic modeling. Chem. Geol. 2009, 265, 63–78. [Google Scholar] [CrossRef]
- Gerdemann, S.J.; Dahlin, D.C.; O’Connor, W.K.; Penner, L.R.; Rush, G.E. Ex-situ and in-situ mineral carbonation as a means to sequester carbon dioxide. In Proceedings of the 21st Annual International Pittsburgh Coal Conference, Osaka, Japan, 13–17 September 2004; p. 17. [Google Scholar]
- Zevenhoven, R.; Kavaliauskaite, I. Mineral carbonation for long-term CO2 storage: An exergy analysis. Int. J. Thermodyn. 2004, 7, 23–31. [Google Scholar]
- Monasterio-Guillot, L.; Fernandez-Martinez, A.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Carbonation of calcium-magnesium pyroxenes: Physical-chemical controls and effects of reaction-driven fracturing. Geochim. Cosmochim. Acta 2021, 304, 258–280. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Petrol. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals, 2nd ed.; The Geological Society: London, UK, 1997. [Google Scholar]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Mineral. Mag. 1998, 52, 535–550. [Google Scholar] [CrossRef]
- Ding, W.; Fu, L.; Ouyang, J.; Yang, H. CO2 mineral sequestration by wollastonite carbonation. Phys. Chem. Miner. 2014, 41, 489–496. [Google Scholar] [CrossRef]
- Min, Y.; Li, Q.; Voltolini, M.; Kneafsey, T.; Jun, Y.S. Wollastonite carbonation in water-bearing supercritical CO2: Effects of particle size. Environ. Sci. Technol. 2017, 51, 13044–13053. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, X.; Li, R.; Zhang, Y.; Yan, S.; Guan, X.; Yang, Q.; Chen, L.; Fang, Y.; Zhang, W.; et al. Wollastonite addition stimulates soil organic carbon mineralization: Evidences from 12 land-use types in subtropical China. Catena 2023, 225, 107031. [Google Scholar] [CrossRef]
- Lackner, K.S. Carbonate chemistry for sequestering fossil carbon. Annu. Rev. Energy Environ. 2002, 27, 193–232. [Google Scholar] [CrossRef]
- Cao, X.; Li, Q.; Xu, L.; Tan, Y. Experiments of CO2-basalt-fluid interactions and micromechanical alterations: Implications for carbon mineralization. Energy Fuels 2024, 38, 6205–6214. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Sheen, J.-D.; Chen, S.-Y.; Fan, Y.-C. Feasibility of CO2 fixation via artificial rock weathering. Ind. Eng. Chem. Res. 2001, 40, 3902–3905. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetic evaluation of mineral carbonation of natural silicate samples. Chem. Eng. J. 2021, 404, 126522. [Google Scholar] [CrossRef]
- Wilson, S.; Dipple, G.M.; Power, I.M.; Barker, S.L.; Fallon, S.J.; Southam, G. Subarctic weathering of mineral wastes provides a sink for atmospheric CO2. Environ. Sci. Technol. 2011, 45, 7727–7736. [Google Scholar] [CrossRef]
- Amann, T.; Hartmann, J.; Hellmann, R.; Pedrosa, E.T.; Malik, A. Enhanced weathering potentials—The role of in situ CO2 and grain size distribution. Front. Clim. 2022, 4, 929268. [Google Scholar] [CrossRef]
- Stubbs, A.R.; Paulo, C.; Power, I.M.; Wang, B.; Zeyen, N.; Wilson, S. Direct measurement of CO2 drawdown in mine wastes and rock powders: Implications for enhanced rock weathering. Int. J. Greenh. Gas Con. 2022, 113, 103554. [Google Scholar] [CrossRef]
- Reershemius, T.; Kelland, M.E.; Jordan, J.S.; Davis, I.R.; D’Ascanio, R.; Kalderon-Asael, B.; Asael, D.; Suhrhoff, T.J.; Epihov, D.Z.; Beerling, D.J.; et al. Initial validation of a soil-based mass-balance approach for empirical monitoring of enhanced rock weathering rates. Environ. Sci. Technol. 2023, 57, 19497–19507. [Google Scholar] [CrossRef]
- Lewis, A.L.; Sarkar, B.; Wade, P.; Kemp, S.J.; Hodson, M.E.; Taylor, L.L.; Yeong, K.L.; Davies, K.; Nelson, P.N.; Bird, M.I.; et al. Effects of mineralogy, chemistry and physical properties of basalts on carbon capture potential and plant-nutrient element release via enhanced weathering. Appl. Geochem. 2021, 132, 105023. [Google Scholar] [CrossRef]
- Renforth, P. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Con. 2012, 10, 229–243. [Google Scholar] [CrossRef]
- Lefebvre, D.; Goglio, P.; Williams, A.; Manning, D.A.C.; de Azevedo, A.C.; Bergmann, M.; Meersmans, J.; Smith, P. Assessing the potential of soil carbonation and enhanced weathering through Life Cycle Assessment: A case study for Sao Paulo State, Brazil. J. Clean. Prod. 2019, 233, 468–481. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
- Zhou, Q. Potential CO2 capture via enhanced weathering by basaltic sand spreading on golf courses in the U.S. Int. J. Greenh. Gas Con. 2024, 131, 104032. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.E.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef] [PubMed]
- Vanderkloot, E.; Ryan, P. Quantifying the effect of grain size on weathering of basaltic powders: Implications for negative emission technologies via soil carbon sequestration. Appl. Geochem. 2023, 155, 105728. [Google Scholar] [CrossRef]
- Power, I.M.; Hatten, V.N.J.; Guo, M.; Schaffer, Z.R.; Rausis, K.; Klyn-Hesselink, H. Are enhanced rock weathering rates overestimated? A few geochemical and mineralogical pitfalls. Front. Clim. 2025, 6, 1510747. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, K.U. Influence of surface passivation and water content on mineral reactions in unsaturated porous media: Implications for brucite carbonation and CO2 sequestration. Geochim. Cosmochim. Acta 2015, 148, 477–495. [Google Scholar] [CrossRef]
- Jerden, J.; Mejbel, M.; Filho, A.N.Z.; Carroll, M.; Campe, J. The impact of geochemical and life-cycle variables on carbon dioxide removal by enhanced rock weathering: Development and application of the Stella ERW model. Appl. Geochem. 2024, 167, 106002. [Google Scholar] [CrossRef]



| Rock Unit | Sample Site [Sample No.] | Province | Latitude | Longitude | Lithologic Description |
|---|---|---|---|---|---|
| DCv | Phrao [1] | Chiang Mai | 1,656,777.346 N | 71,786.47703 W | Black to greenish-black, Dense, Phaneritic-to-cumulated texture; Ol, Px |
| Cb | Pha Luat [2] | Uttaradit | 1,502,454.345 N | 30,388.22661 E | Greenish gray, pale gray, yellowish brown, Phaneritic texture with low-grade deformed (foliated) features; Crosscut by Chl-Qz veins |
| CPu | Mae Chan [3] | Chiang Rai | 1,721,148.906 N | 5085.97194 E | Dark green to dark gray, Phaneritic texture; Pl, Px |
| CPu | Mae Fah Luang [4] | Chiang Rai | 1,709,346.880 N | 8392.25100 W | Dark gray, greenish black, and pale green, Phaneritic texture with low-grade deformed (schistose) features; Crosscut by Cal-Ep veins |
| CPu | Huay Lao [5] | Nan | 1,557,746.874 N | 77,298.03278 E | Greenish black-to-green, Phaneritic texture with weakly foliated features, high-degree alteration; Srp, Px |
| CPu | Na Noi [6] | Nan | 1,554,609.250 N | 80,359.61868 E | Greenish gray to dark gray, Phaneritic texture with low-grade deformed (weakly foliated to schistose) features; Crosscut by Chl-Qz veinlets |
| PTrv | Mae On [7] | Chiang Mai | 1,598,001.988 N | 63,354.72923 W | Dark green to pale green, Medium-to-fine-grained pyroclastic texture; volcanic fragments |
| bs | Mae Tha [8] | Lampang | 1,553,343.767 N | 30,707.00548 W | Dark gray to black, Porphyritic-vesicular texture; Ol, Px phenocrysts, Px, Pl groundmass; 1–2 mm vesicles |
| bs | Sobprab [9] | Lampang | 1,535,108.371 N | 54,232.37178 W | Dark gray-to-reddish brown, Aphanitic-to-glassy vesicular texture; Ol, Px, Pl; 1–5 mm vesicles |
| bs | Den Chai [10] | Phrae | 1,526,421.533 N | 5843.656028 W | Black to dark gray, Aphanitic-to-glassy vesicular texture; vesicles are filled with white Ze, Cal; 1–2 mm vesicles |
| Rock Type TS ID † | Mineral Assemblage (modal%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am | Cal | Chl | Ep | Gls | Ol | Opg | Pl | Prh | Px | Qz | Sm | Spl | Srp | Ttn | Zeo | |
| Basalts DC | – | – | 6.00 | – | – | 10.63 | 11.00 | 50.00 | – | 20.25 | – | 2.12 | – | – | – | |
| MT | – | – | – | – | 35.90 | 8.71 | – | 41.03 | – | 12.31 | – | – | – | 2.05 | – | – |
| SP | – | – | 2.31 | – | – | 10.63 | 23.75 | 38.00 | – | 14.12 | – | 11.19 | – | – | – | – |
| Gabbronorite MC-1 | 28.25 | – | 0.60 | – | – | 1.00 | 0.75 | 29.25 | 0.58 | 39.00 | – | – | – | – | 0.57 | – |
| MC-2 | 3.75 | – | 3.50 | – | – | 3.25 | 11.50 | 54.75 | – | 23.25 | – | – | – | – | – | – |
| Metabasites PL | 45.25 | – | 3.00 | 3.25 | – | – | 3.00 | 45.25 | – | – | 0.25 | – | – | – | – | – |
| NN | – | – | 9.00 | 17.50 | – | – | 0.75 | 30.00 | – | 42.25 | 0.50 | – | – | – | – | – |
| MF | 67.00 | 1.00 | – | 13.50 | – | – | 3.50 | 5.50 | – | – | 9.50 | – | – | – | – | – |
| Peridotites PR-1 | 0.75 | – | – | – | – | 62.75 | – | 9.00 | – | 9.00 | – | – | 1.25 | 17.25 | – | – |
| PR-2 | 2.00 | 0.25 | – | – | – | 67.00 | 1.25 | 12.50 | – | 6.75 | – | – | 3.00 | 7.25 | – | – |
| PR-3 | 2.00 | – | – | – | – | 67.25 | 0.50 | 12.50 | – | 9.25 | – | – | 1.75 | 6.75 | – | – |
| PR-4 | 3.50 | – | – | – | – | 62.00 | 0.25 | 10.25 | – | 7.50 | – | – | 2.50 | 14.00 | – | – |
| Pyroxenite HL | – | – | 15.89 | – | – | – | 2.52 | – | – | – | – | – | 2.02 | 79.57 | – | – |
| Basaltic tuff MO | – | <0.01 | 8.50 | – | 46.13 | – | 0.25 | 32.50 | – | 2.75 | – | 4.37 | – | 0.50 | – | 5.00 |
| Rock Type: ID † | Unit | Mineral Phase * | |||
|---|---|---|---|---|---|
| Major [>30%] | Moderate [10–30%] | Minor [2–10%] | Trace [<2%] | ||
| Olivine basalt: MT | bs | Lab | Act, Aug | n/d | n/d |
| Olivine basalt: SP | bs | Ol | Lab, Aug | Sa | n/d |
| Olivine basalt: DC | bs | Lab | Aug, Chl, Ctl | Act | n/d |
| Gabbronorite: MC | CPu | Act | Lab, Chl, Sa | Aug, Qz, Cal | n/d |
| Metabasite: PL | Cb | Act | Lab, Mc, Ctl | Py, Aug, Chl | n/d |
| Metabasite: MF | CPu | Lab | Aug, Ctl, Act | Chl | n/d |
| Metabasite: NN | CPu | Ctl | Act, Lab | Chl, Aug | n/d |
| Peridotite: PR | DCv | - | Atg, Aug, Ol, Ab, Chl | Ill, Cal | n/d |
| Altered pyroxenite: HL | CPu | Ctl, Aug | Act | Chl | n/d |
| Basaltic tuff: MO | PTrv | Ctl | Cal, Chl | Lab, Act, Aug | n/d |
| Mineral Group | End Member | Chemical Formula | Atomic Weight (g/mol) | * Reactive CO2 (mol) | Calculated CO2 Uptake (%wt/wt) |
|---|---|---|---|---|---|
| Olivine | Forsterite | Mg2SiO4 | 140.691 | 2 | 62.56 |
| Fayalite | Fe2SiO4 | 203.771 | 2 | 43.19 | |
| Pyroxene | Augite | (Ca,Na)(Mg,Fe,Al,Ti)Si2O6 | 222.448 | 2 | 39.57 |
| Diopside | CaMgSi2O6 | 216.547 | 2 | 40.65 | |
| Enstatite | MgSiO3 | 100.387 | 1 | 43.84 | |
| Hypersthene | (Mg,Fe)SiO3 | 116.157 | 1 | 37.89 | |
| Ferrosilite | FeSiO3 | 131.927 | 1 | 33.36 | |
| Wollastonite | CaSiO3 | 116.160 | 1 | 37.89 | |
| Plagioclase | Albite | NaAlSi3O8 | 262.220 | 1 | 16.78 |
| Labradorite | (Ca,Na)(Al,Si)4O8 | 269.659 | 1 | 16.32 | |
| Anorthite | CaAl2Si2O8 | 278.203 | 1 | 15.82 | |
| Serpentine | Antigorite | Mg3(Si2O5)(OH)4 | 277.108 | 3 | 47.64 |
| Chrysotile | Mg3(Si2O5)(OH)4 | 277.108 | 3 | 47.64 | |
| Lizardite | Mg3(Si2O5)(OH)4 | 277.108 | 3 | 47.64 |
| Rock Type | Olivine | Pyroxene | Plagioclase | Serpentine | Total CO2 Uptake | ||||
|---|---|---|---|---|---|---|---|---|---|
| Modal% | %CO2 Uptake | Modal% | %CO2 Uptake | Modal% | %CO2 Uptake | Modal% | %CO2 Uptake | ||
| Olivine basalts | 10.63 | 6.65 | 20.25 a | 8.01 | 50.00 | 8.16 | − | − | 22.82% 1 |
| 8.71 | 5.45 | 12.31 a | 4.87 | 41.03 | 6.49 | − | − | 16.81% 2 | |
| 10.63 | 6.65 | 14.13 a | 5.59 | 38.00 | 6.20 | − | − | 18.44% 3 | |
| Gabbronorite | 3.25 | 2.03 | 17.25 a 6.00 b | 6.83 2.63 | 54.75 | 8.94 | − | − | 20.43% |
| Px-Hbl gabbronorite | 1.00 | 0.63 | 21.25 a 17.75 b | 8.41 7.78 | 29.25 | 4.77 | − | − | 21.59% |
| Metabasite | − | − | − | − | 45.25 | 7.38 | − | − | 7.38% 4 |
| − | − | − | − | 5.50 | 0.90 | − | − | 0.90% 5 | |
| − | − | 42.25 a | 16.72 | 30.00 | 4.90 | − | − | 21.62% 6 | |
| Peridotite | 62.75 | 39.26 | 9.00 a | 3.56 | 9.00 | 1.47 | 17.25 d | 8.22 | 52.51% |
| 67.00 | 41.92 | 6.75 a | 2.67 | 12.50 | 2.04 | 7.25 d | 3.45 | 50.08% | |
| 67.25 | 42.07 | 9.25 a | 3.66 | 12.50 | 2.04 | 6.75 d | 3.22 | 50.99% | |
| 62.00 | 38.79 | 7.50 a | 2.97 | 10.25 | 1.67 | 14.00 d | 6.67 | 50.10% | |
| Altered pyroxenite | − | − | 28.00 a 60.00 b | 11.08 26.30 | − | − | 8.00 e | 3.81 | 41.19% |
| Basaltic tuff | − | − | 2.75 a | 1.09 | 32.50 | 5.30 | 0.5 d | 0.24 | 6.63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taksavasu, T. Petrographic Analysis of Mafic and Ultramafic Rocks in Northern Thailand: Implications for CO2 Mineralization and Enhanced Rock Weathering Approach. Geosciences 2025, 15, 89. https://doi.org/10.3390/geosciences15030089
Taksavasu T. Petrographic Analysis of Mafic and Ultramafic Rocks in Northern Thailand: Implications for CO2 Mineralization and Enhanced Rock Weathering Approach. Geosciences. 2025; 15(3):89. https://doi.org/10.3390/geosciences15030089
Chicago/Turabian StyleTaksavasu, Tadsuda. 2025. "Petrographic Analysis of Mafic and Ultramafic Rocks in Northern Thailand: Implications for CO2 Mineralization and Enhanced Rock Weathering Approach" Geosciences 15, no. 3: 89. https://doi.org/10.3390/geosciences15030089
APA StyleTaksavasu, T. (2025). Petrographic Analysis of Mafic and Ultramafic Rocks in Northern Thailand: Implications for CO2 Mineralization and Enhanced Rock Weathering Approach. Geosciences, 15(3), 89. https://doi.org/10.3390/geosciences15030089






