Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling
Abstract
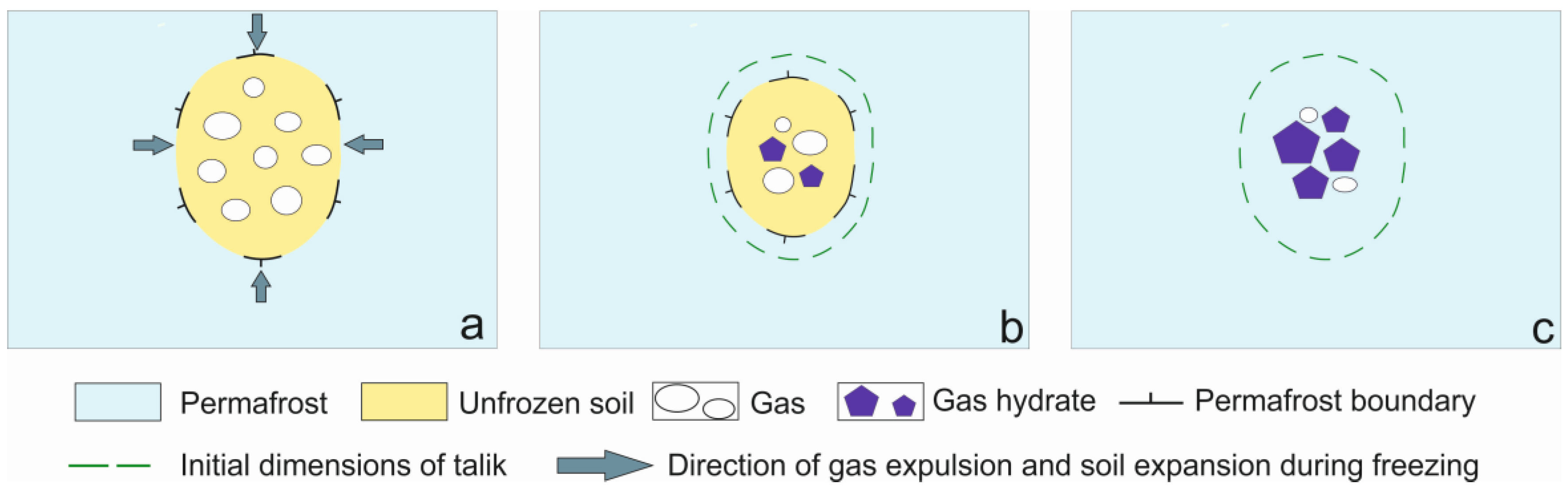
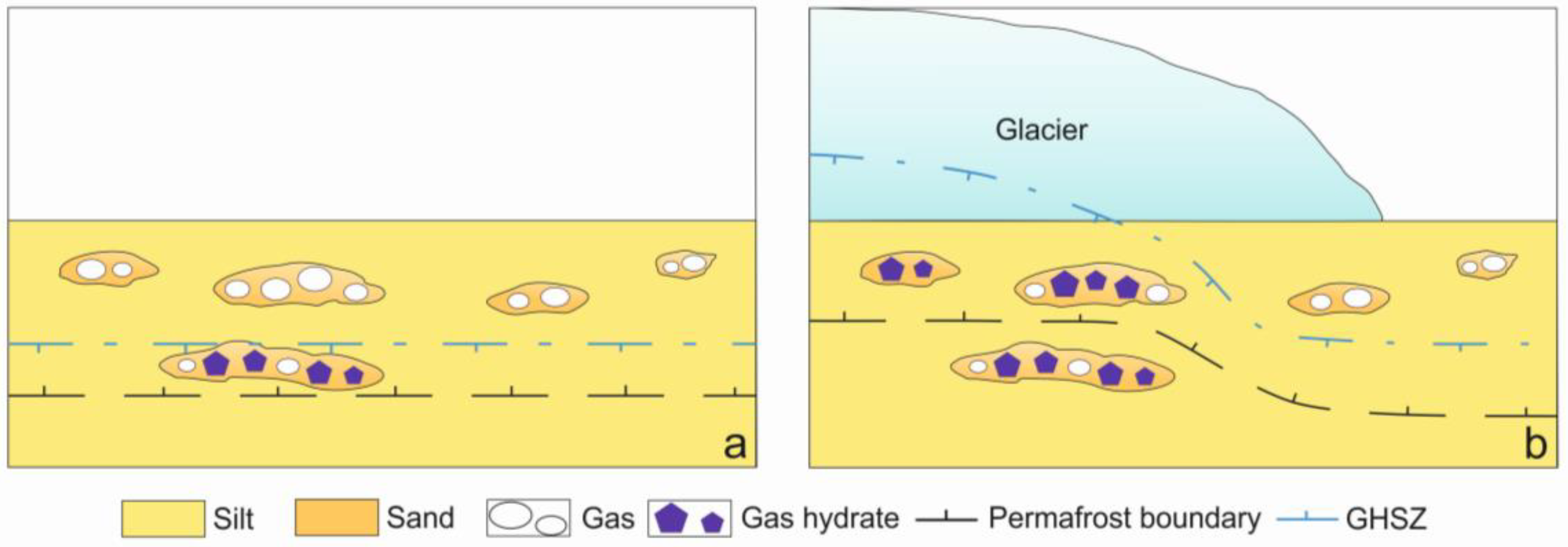
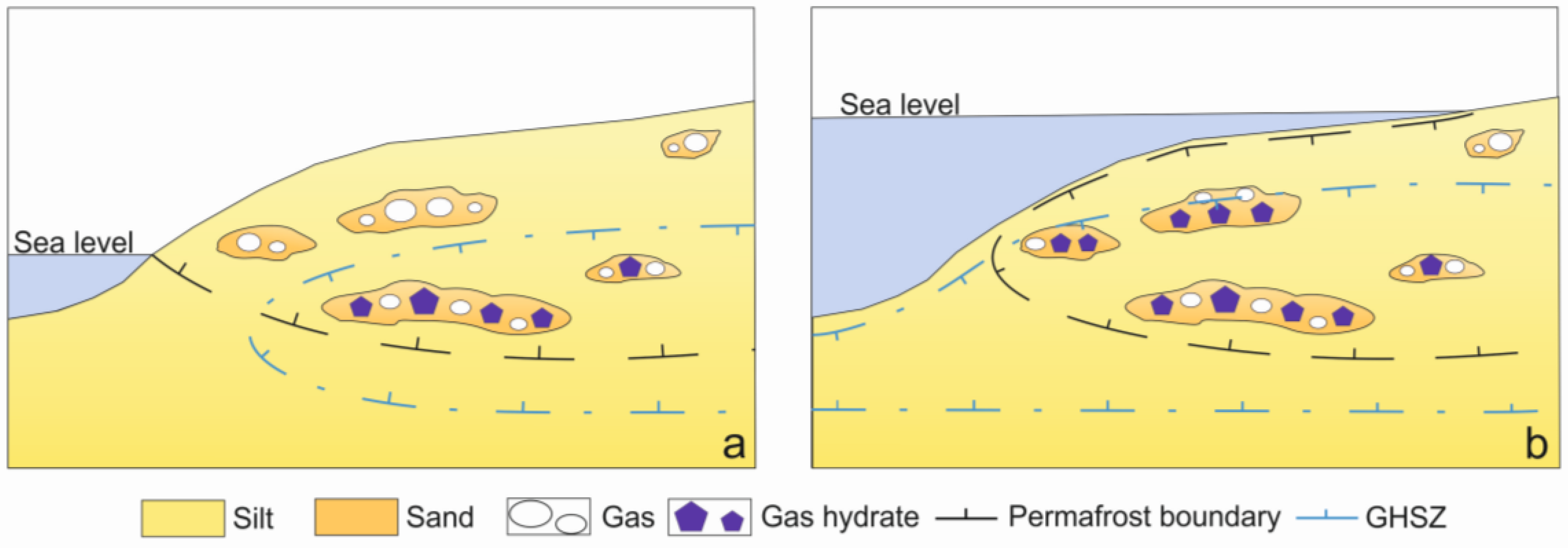
:1. Introduction
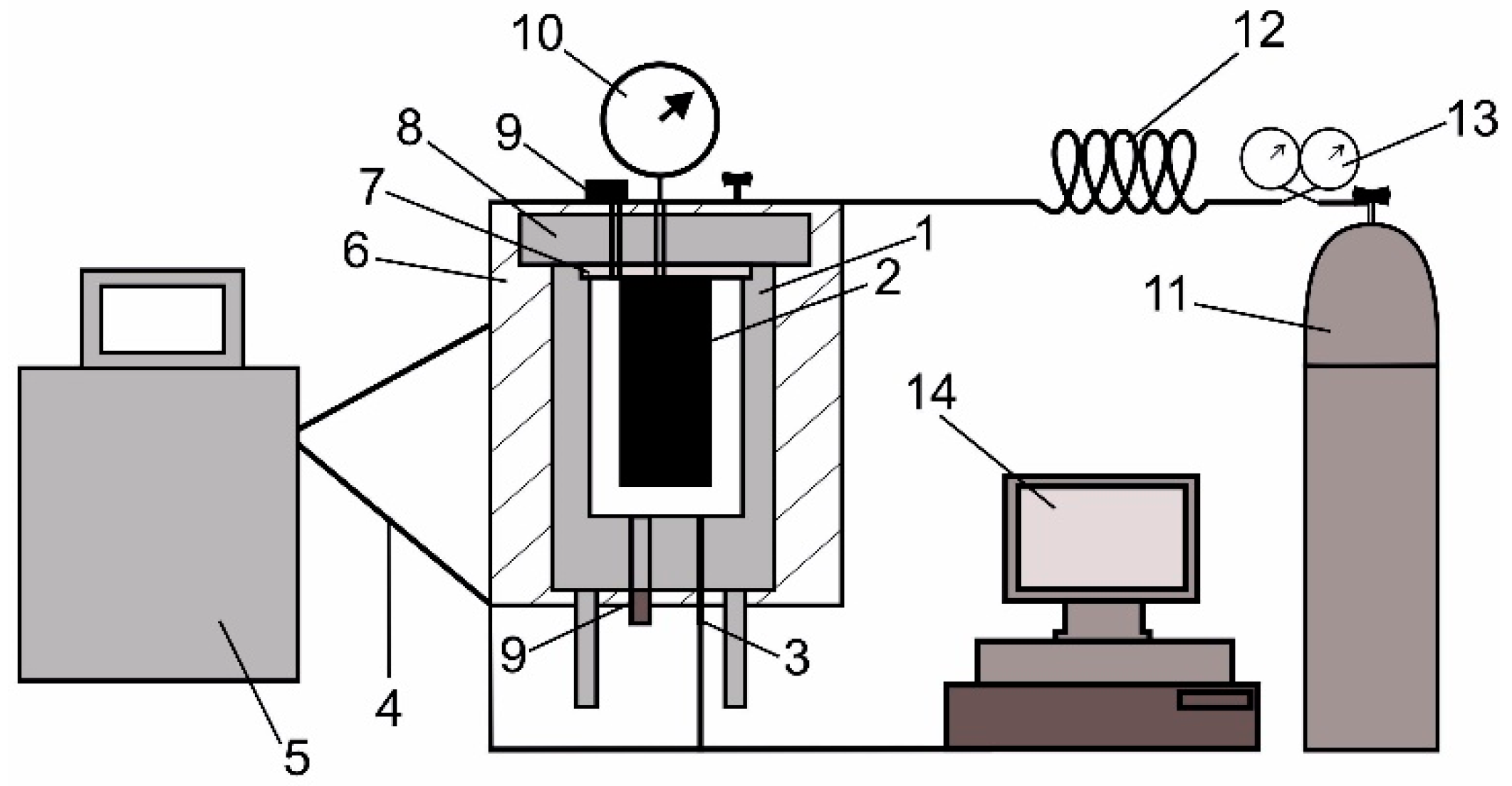
2. Materials and Methods
3. Results and Discussion
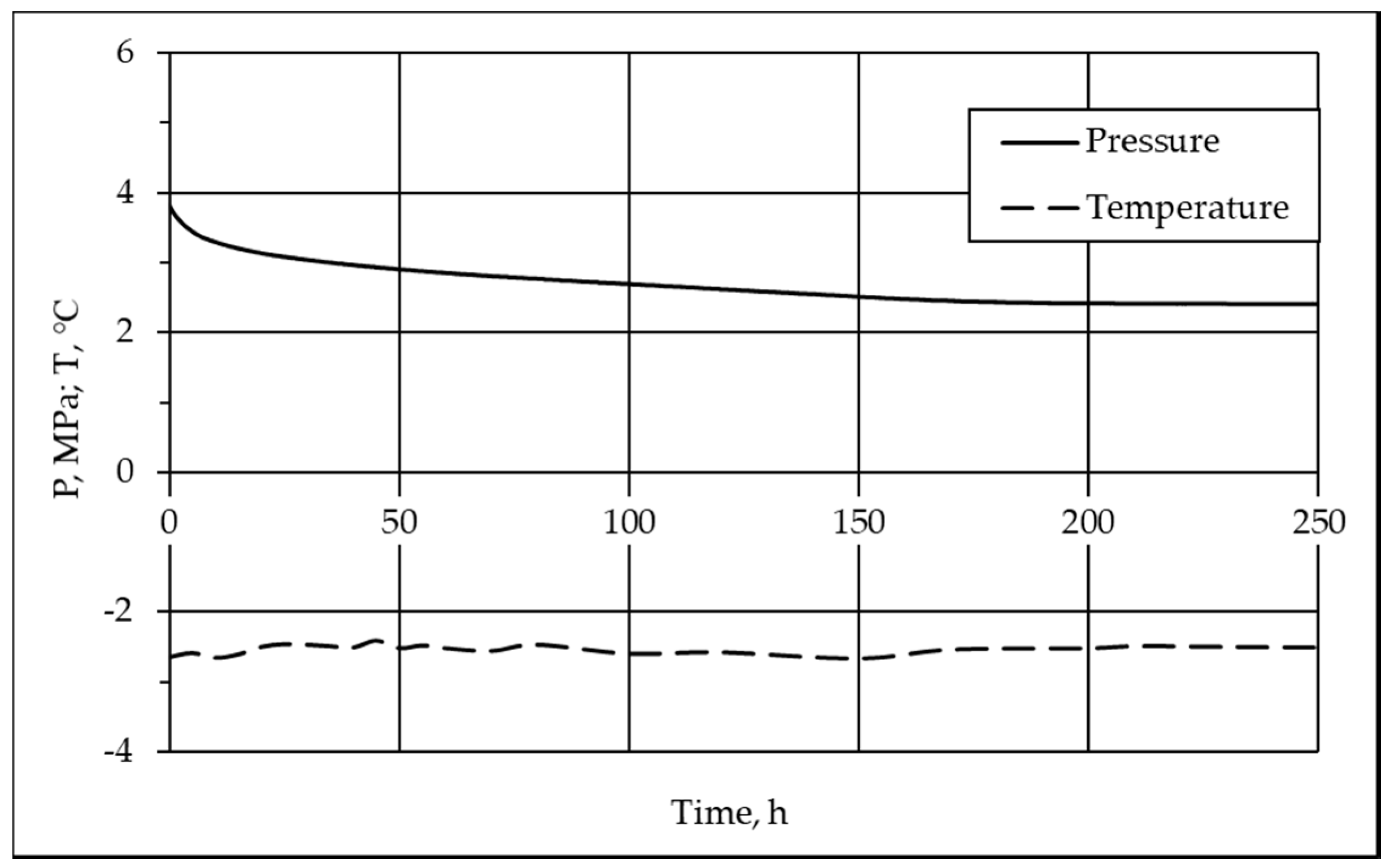
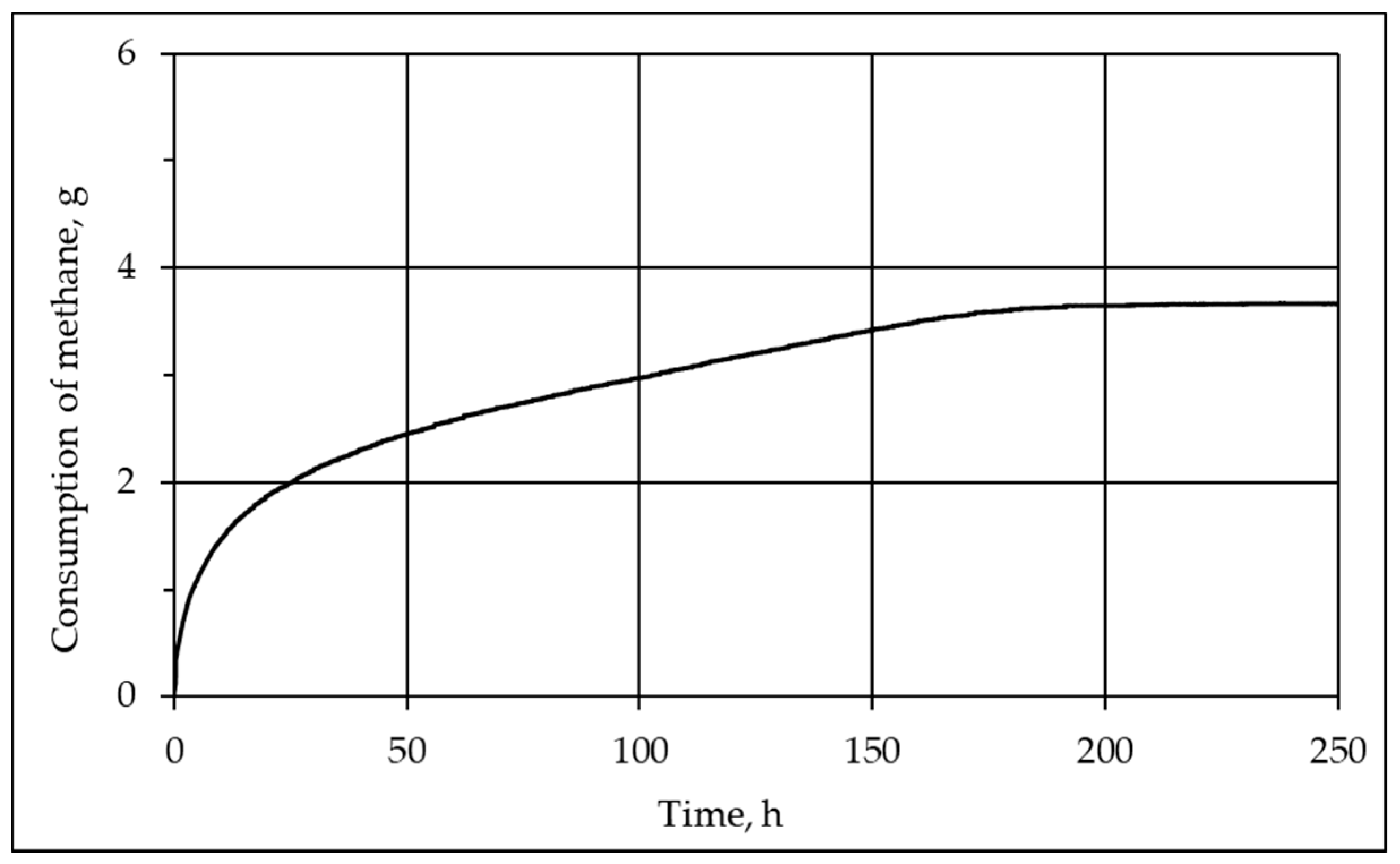
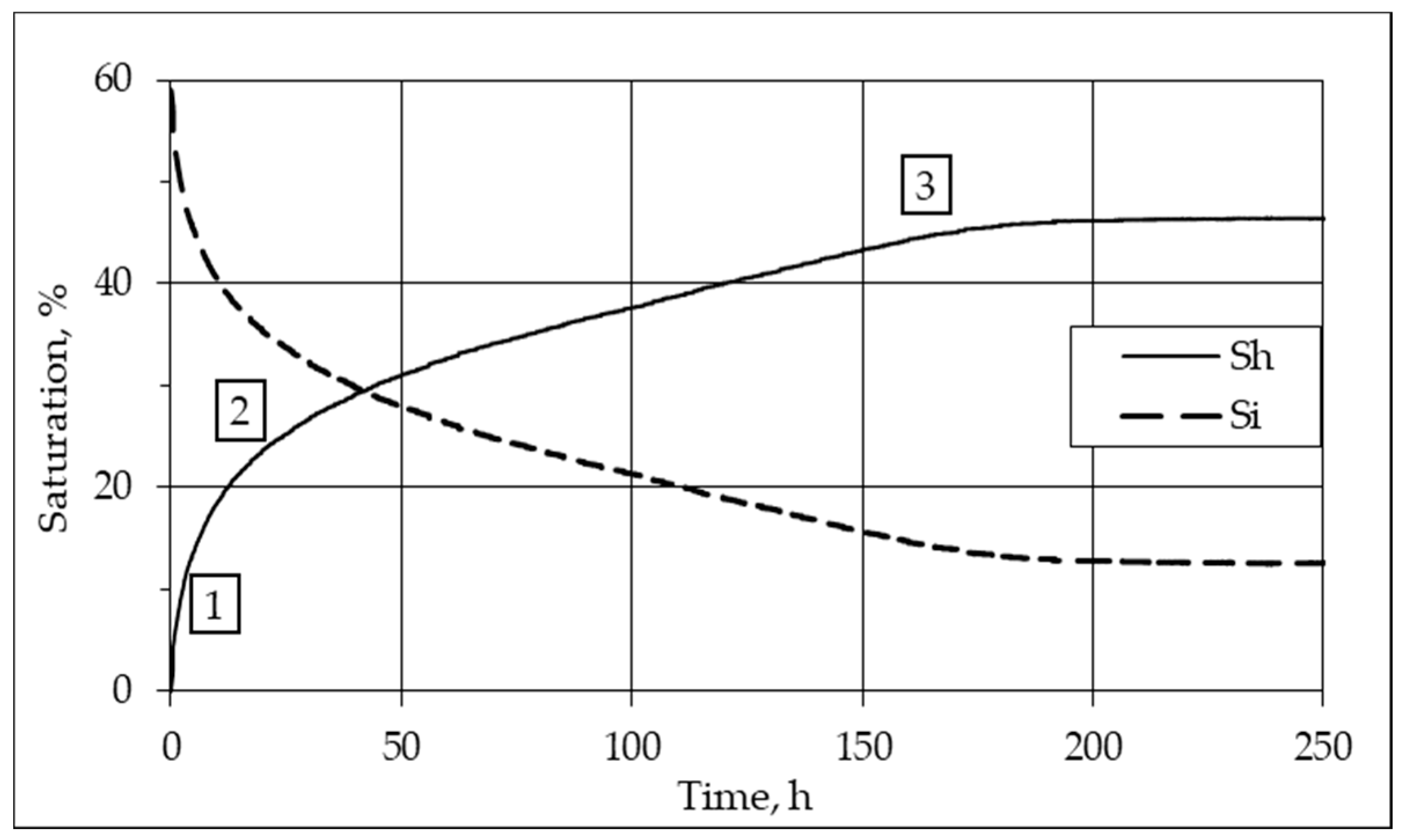
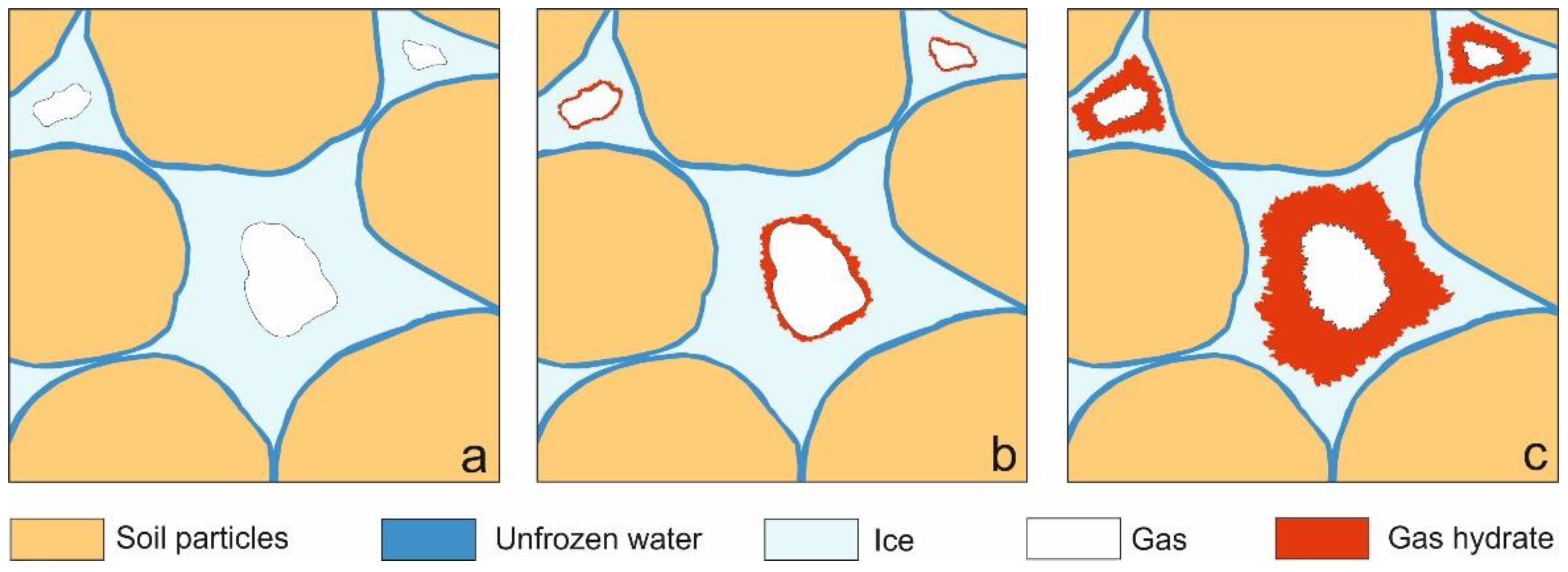
3.1. Mechanism and Kinetics of Pore Hydrate Formation at Negative Temperatures
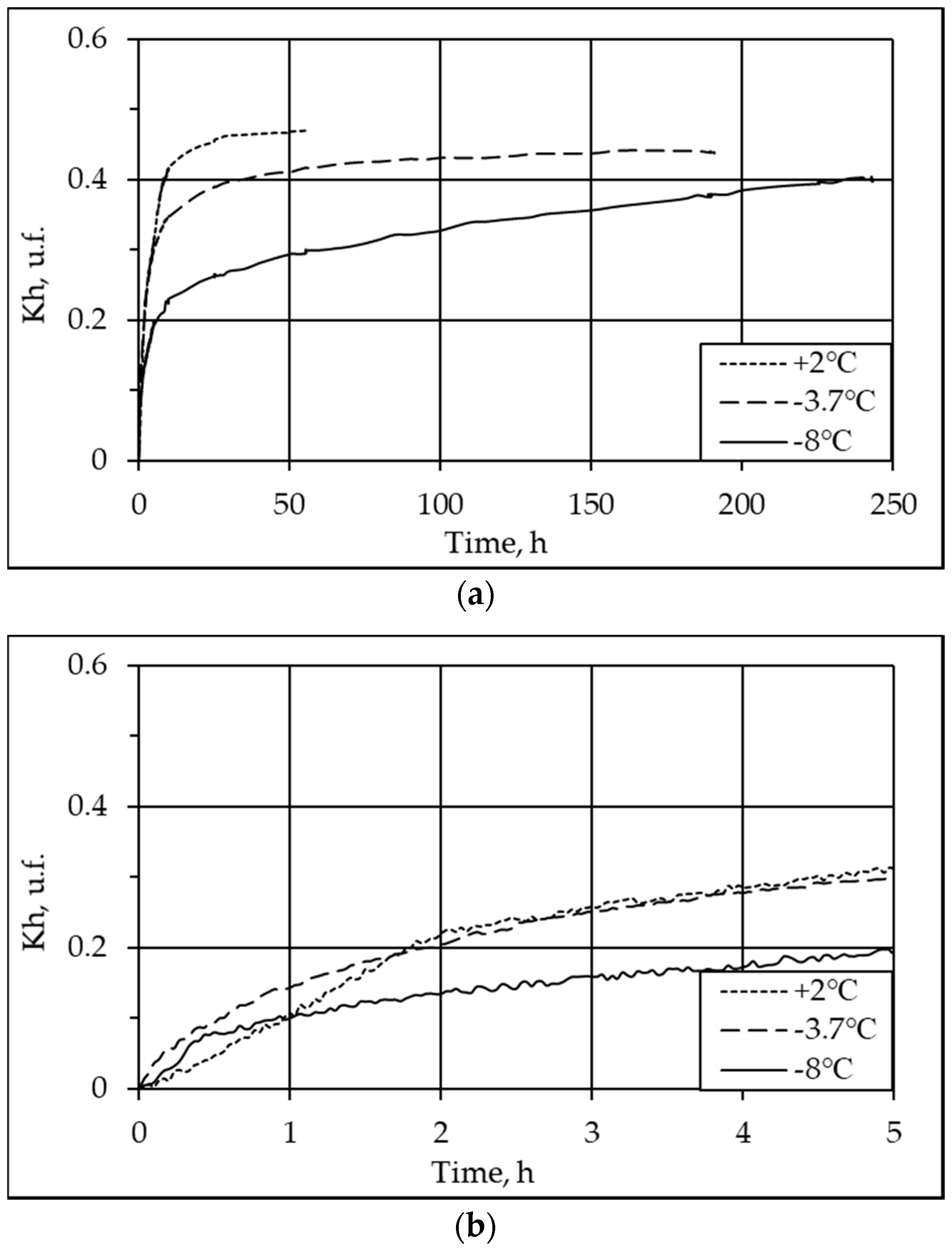
3.2. Effect of Temperature
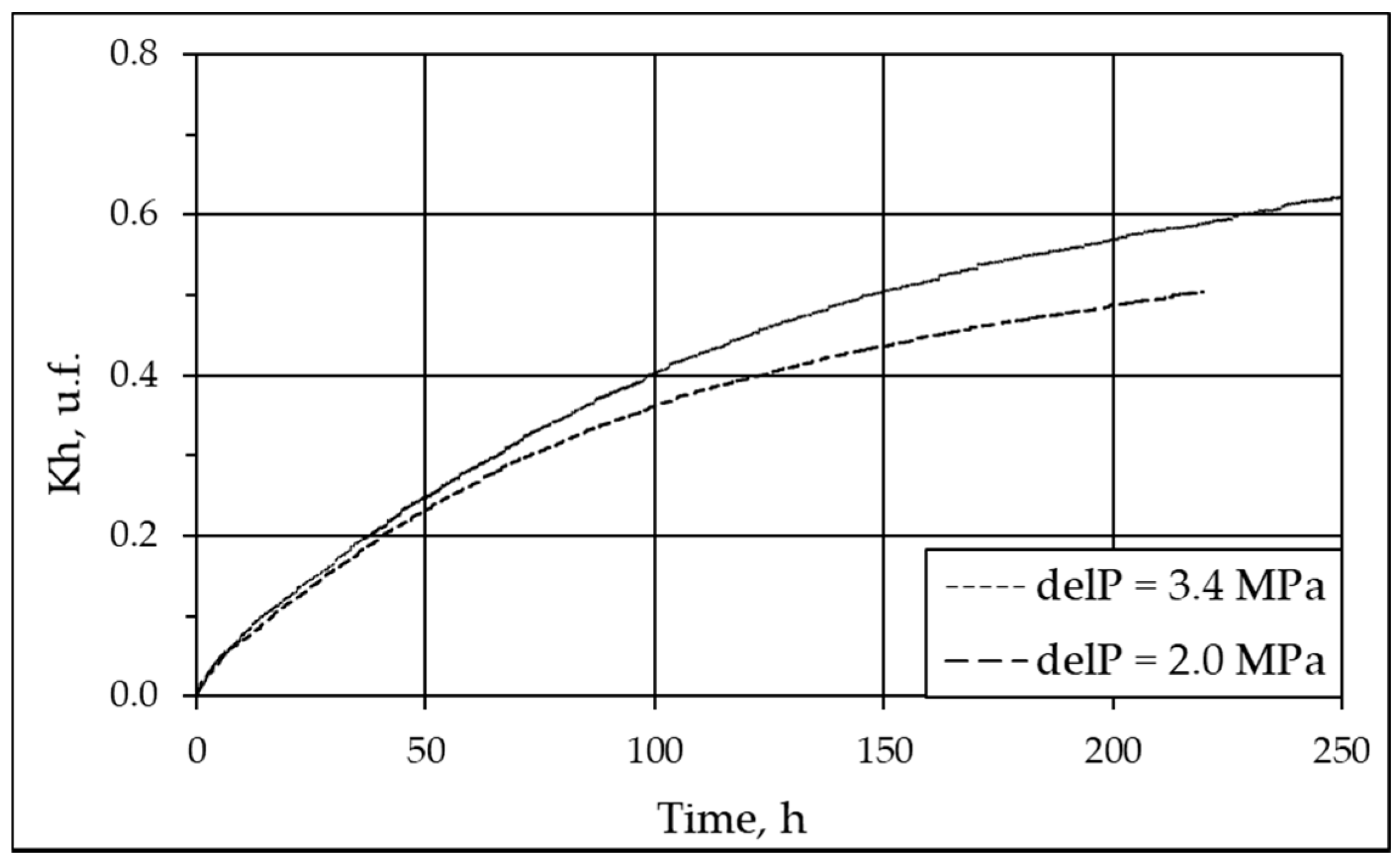
3.3. Effect of Gas Pressure
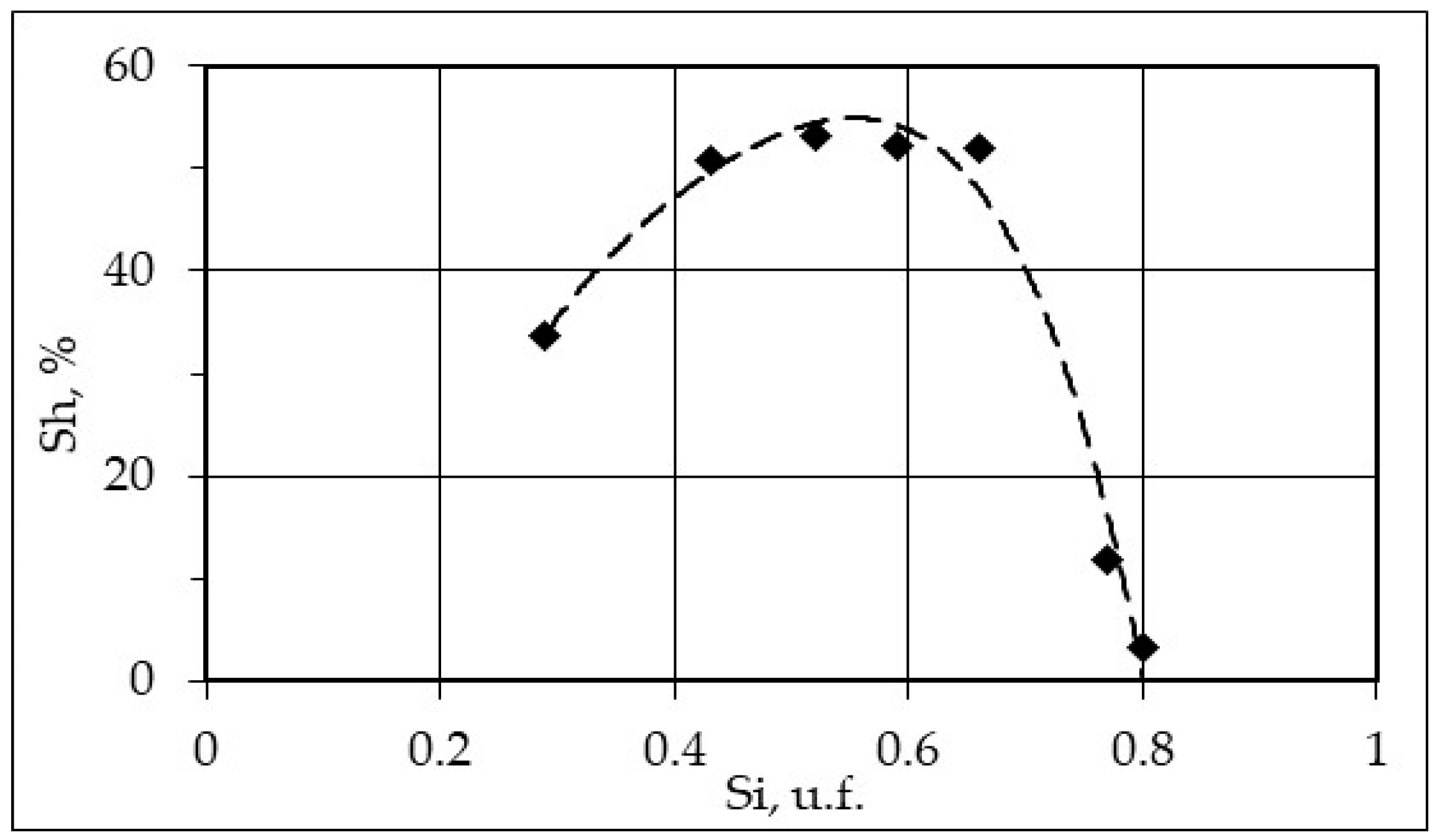
3.4. Effect of Ice Saturation
3.5. Effect of Soil Particle Size
3.6. Effect of Salinity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Makogon, Y.F. Hydrates of Natural Gases; NEDRA: Moscow, Russia, 1974; p. 208. ISBN 978-0878141654. (In Russian) [Google Scholar]
- Istomin, V.A.; Yakushev, V.S. Naturally Occurring Gas Hydrates; NEDRA: Moscow, Russia, 1992; p. 235. ISBN 5-247-02442-7. (In Russian) [Google Scholar]
- Sloan, E.D. Clathrate Hydrates of Natural Gases, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1998; p. 705. ISBN 0824799372. [Google Scholar]
- Max, M.D. Natural Gas Hydrate in Oceanic and Permafrost Environments; Kluwer Academic Publishers: Dordrecht, The Netherlands; London, UK, 2000; p. 414. ISBN 1384-6434. [Google Scholar] [CrossRef]
- Cherskiy, N.V.; Tsarev, V.P.; Nikitin, S.P. Conditions for Gas Accumulation in Gas Hydrate Deposits: Investigation and Prediction; Academy of Sciences of the USSR Publisher: Yakutsk, Russia, 1983; p. 156. (In Russian) [Google Scholar]
- Ginsburg, G.D.; Soloviev, V.A. Geological models of gas hydrate formation. Litologiya i Poleznye Iskopaemye 1990, 2, 76–87. (In Russian) [Google Scholar]
- Are, F.E. Problem of deep gas emission into the atmosphere. Kriosfera Zemli 1998, II, 42–50. (In Russian) [Google Scholar]
- Romanovskiy, N.N. Fundamentals of Cryogenesis in the Lithosphere; Moscow University Press: Moscow, Russia, 1993; p. 336. ISBN 5-211-02379-10. (In Russian) [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Gas and possible gas hydrates in the permafrost of Bovanenkovo gas field, Yamal Peninsula, West Siberia. Polarforschung 2000, 68, 215–219. [Google Scholar]
- Yakushev, V.S. One possible cause of gas bursts in permafrost. Geol. Neft. i Gaza 1989, 14, 45–46. (In Russian) [Google Scholar]
- Yakushev, V.S. Natural Gas and Gas Hydrates in Permafrost; VNIIGAZ: Moscow, Russia, 2009; p. 192. ISBN 978-5-89754-048-8. (In Russian) [Google Scholar]
- Rivkin, F.M.; Levantovskaya, N.P. Dynamics of sub-river channel taliks and the formation of gas hydrates. Kriosfera Zemli 2002, 6, 36–42. (In Russian) [Google Scholar]
- Kraev, G.; Schulze, E.-D.; Yurova, A.; Kholodov, A.; Chuvilin, E.; Rivkina, E. Cryogenic Displacement and Accumulation of Biogenic Methane in Frozen Soils. Atmosphere 2017, 8, 105. [Google Scholar] [CrossRef]
- Istomin, V.A.; Chuvilin, E.M.; Sergeeva, D.V.; Buhkanov, B.A.; Stanilovskaya, Y.V.; Green, E.; Badetz, C. Thermodynamic calculation of freezing temperature of gas-saturated pore water in talik zones. In Proceedings of the 5th European Conference Permafrost, Chamonix, France, 23 June–1 July 2018; pp. 480–481. [Google Scholar]
- Trofimuk, A.A.; Makogon, Y.F.; Yakushev, V.S. Influence of hydrate formation zones on the temperature regime of rocks in permafrost. Geologiya i Geofizika Sov. Geol. Geophys. 1986, 27, 3–10. (In Russian) [Google Scholar]
- Bily, C.; Dick, J.W.L. Natural gas hydrates in the Mackenzie Delta, Northwest Territories. Can. Pet. Geol. Bull. 1974, 340–352. [Google Scholar]
- Dallimore, S.R.; Uchida, T.; Collett, T.S. Scientific Results from JAPEX/JNOC/GSC Mallik 2L-38 Gas Hydrate Research Well, Mackenzie Delta, Northwest Territories, Canada. Geological Survey of Canada, Bulletin 544; US Government Printing Office: Washington, DC, USA, 1999; p. 403. ISBN 0-660-17777-3.
- Collet, T.S.; Dallimore, S.R. Permafrost-associated gas hydrate. In Natural Gas Hydrates in Oceanic and Permafrost Environments; Max, M.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; London, UK, 2000; p. 414. ISBN 978-94-011-4387-5. [Google Scholar]
- Lu, Z.; Zhu, Y.; Zhang, Y.; Wen, H.; Li, Y.; Liu, C. Gas hydrate occurrences in the Qilian Mountain permafrost, Qinghai Province, China. Cold Reg. Sci. Technol. 2011, 66, 93–104. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, J.; Li, J.; Lu, C.; Song, J. Gas hydrate formation and its accumulation potential in Mohe permafrost, China. Mar. Pet. Geol. 2012, 35, 166–175. [Google Scholar] [CrossRef]
- Dai, J.; Ni, Y.; Huang, S.; Peng, W.; Han, W.; Gong, D.; Wei, W. Genetic types of gas hydrates in China. Pet. Explor. Dev. 2017, 44, 837–848. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Omelchenko, R.Y. Messoyakha: Gas hydrate accumulation, its role and significance. Geol. i Polezn. Iskop. Mirovogo Okeana 2012, 3, 5–19. (In Russian) [Google Scholar]
- Makogon, Y.F. Appraisal of gas resources in the hydrate form. Gazov. Delo 1966, 1, 56. (In Russian) [Google Scholar]
- Zakirov, S.N.; Dubrovskiy, D.A.; Tolkach, V.M. Influence of Hydrates Dissociation Process on Messoyakha Deposit; VNIIEgazprom: Moscow, Russia, 1989; p. 84. (In Russian) [Google Scholar]
- Ginsburg, G.D.; Novozhilov, A.A. Hydrates in the Messoyakha gas field. Gazov. Prom. 1997, 2, 18–20. (In Russian) [Google Scholar]
- Yakushev, V.S.; Perlova, E.V.; Makhonina, N.A.; Chuvilin, E.M.; Kozlova, E.V. Gas hydrates in deposits on continents and islands. Ross. Khimicheskiy Zhurnal 2003, 3, 80–90. (In Russian) [Google Scholar]
- Dallimore, S.R.; Collett, T.S. Intrapermafrost gas hydrates from a deep core hole in the Mackenzie Delta, Northwest Territories, Canada. Geology 1995, 23, 527–530. [Google Scholar] [CrossRef]
- Cherskiy, N.V.; Tsarev, V.P. Prospects for Development of Gas Hydrate Deposits. In Improvement of Subsoil Use in the Northern and Eastern USSR, Part 1; Cherskiy, N.V., Ed.; Academy of Sciences of the USSR Publisher: Yakutsk, Russia, 1973; pp. 54–60. (In Russian) [Google Scholar]
- Ershov, E.D.; Lebedenko, Y.P.; Chuvilin, E.M.; Istomin, V.A.; Yakushev, V.S. Features of gas hydrates in permafrost. Dokl. Akad. Nauk. 1991, 321, 788–791. (In Russian) [Google Scholar]
- Dallimore, S.R.; Chuvilin, E.M.; Yakushev, V.S.; Grechischev, S.E.; Ponomarev, V.; Pavlov, A. Field and laboratory characterization of intrapermafrost gas hydrates, Mackenzie Delta, N.W.T., Canada. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 525–531. [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S. Structure and some properties of frozen hydrate-containing soils. In Proceedings of the International Symposium on Methane Hydrates Resourses in the Near Future, JNOC, Chiba City, Japan, 20–22 October 1998; pp. 239–246. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Carbon dioxide gas hydrates accumulation in freezing and frozen sediments. In Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008), Vancouver, BC, Canada, 6–20 July 2008; p. 5469. [Google Scholar]
- Chuvilin, E.M.; Grebenkin, S.I. Gas permeability variations in gas-filled soils upon hydrate formation and freezing: An experimental study. Kriosf. Zemli 2015, XIX, 67–74. [Google Scholar]
- Chuvilin, E.M.; Bukhanov, B.A. Effect of hydrate accumulation conditions on thermal conductivity of gas-saturated soils. Energy Fuels 2017, 31, 5246–5254. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Bukhanov, B.A.; Grebenkin, S.I.; Doroshin, V.V.; Iospa, A.V. Shear strength of frozen sand with dissociating pore methane hydrate: An experimental study. Cold Reg. Sci. Technol. 2018, 153, 101–105. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Experimental Study of Gas Hydrate Formation in Porous Media. In Advances in Cold-Region Thermal Engineering and Sciences; Hutter, K., Wang, Y., Beer, H., Eds.; Lecture Notes in Physics; Springer: Berlin/Heidelberg, Germany, 1999; Volume 533, pp. 431–440. ISBN 978-3-540-48410-3. [Google Scholar]
- Rivkina, E.; Gilichinsky, D.; McKay, C.; Dallimore, S. Methane Distribution in Permafrost: Evidence for an Interpore Pressure Methane Hydrate. In Permafrost Response on Economic Development, Environmental Security and Natural Potential; Paepe, R., Melnikov, V., Eds.; Kluwer Academic Publ.: Dordrecht, The Netherlands, 2001; pp. 487–496. ISBN 978-94-010-0684-2. [Google Scholar]
- Wright, J.F.; Chuvilin, E.M.; Dallimore, S.R.; Yakushev, V.S.; Nixon, F.M. Methane hydrate formation and dissociation in fine sands at temperature near 0 °C. In Proceedings of the 7th International Conference on Permafrost, Yellowknife, NT, Canada, 23–27 June 1998; pp. 1147–1153. [Google Scholar]
- Komai, T.; Sakamoto, Y.; Kawamura, T. Formation kinetics of CO2 gas hydrates in sandy sediments and change in permeability during crystal growth. In Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008), Vancouver, BC, Canada, 6–20 July 2008; p. 5342. [Google Scholar]
- Chuvilin, E.M.; Lupachik, M.V. Investigation of gas hydrate formation in frozen and thawing gas saturated sediments. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, UK, 17–21 July 2011. [Google Scholar]
- Chuvilin, E.M.; Kozlova, E.V. Experimental estimation of hydrate-bearing sediments stability. In Proceedings of the 5th International Conference on Gas Hydrate, Thermodynamic Aspects, Trondheim, Norway, 13–16 June 2005; Volume 3, pp. 1562–1567. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Experimental investigation of CO2 gas hydrate formation in porous media of frozen and freezing sediments. Kriosf. Zemli 2009, XIII, 70–79. (In Russian) [Google Scholar]
- Chuvilin, E.M.; Petrakova, S.Y.; Guryeva, O.M.; Istomin, V.A. Formation of Carbon Dioxide Gas Hydrates in Freezing Sediments and Decomposition Kinetics of the Hydrates Formed. In Proceedings of the 11th International Conference on the Physics and Chemistry of Ice, Bremerhaven, Germany, 23–28 July 2006; pp. 147–154. [Google Scholar]
- GOST 12536-2014. Soils. Methods of Laboratory Granulometric (Grain-Size) and Microaggregate Distribution; Standards Publishing: Moscow, Russia, 2014; p. 24. [Google Scholar]
- GOST 5180-2015. Soils. Laboratory Methods for Determination of Physical Characteristics; Standardinform: Moscow, Russia, 2015; p. 23. [Google Scholar]
- SNiP 2.02.04-88. Foundation Beds and Foundations in Permafrost Soils; CITP; Gosstroy (USSR): Moscow, Russia, 1990; p. 52.
- Trofimov, V.T.; Korolev, V.A.; Voznesensky, E.A.; Golodkovskaya, G.A.; Vasilchuk, Y.K.; Ziangirov, R.S. Soil Science; Publishing House of the Moscow State University and “Science”: Moscow, Russia, 2005; p. 1024. ISBN 5-211-04848-2. [Google Scholar]
- Yang, S.H.B.; Babu, P.; Chua, S.F.S.; Linga, P. Carbon dioxide hydrate kinetics in porous media with and without salts. Appl. Energy 2014, 162, 1131–1140. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.; Mu, C. Influence of temperature on methane hydrate formation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Ding, L.; Shi, B.; Huang, Q.; Gong, J. Study of hydrate formation in gas-emulsion multiphase flow systems. R. Soc. Chem. 2017, 7, 48127–48135. [Google Scholar] [CrossRef] [Green Version]
- Sychev, V.; Vassrman, L.; Zagoruchenko, V.; Kozlov, L.; Spiridonov, G.; Tsymarny, V. Thermodynamic Properties of Methane; Standards Publishing House: Moscow, Russia, 1979; p. 348. (In Russian) [Google Scholar]
- Staykova, D.K.; Hansen, T.; Salamatin, A.N.; Kuhs, W.F. Kinetic diffraction experiments on the formation of porous gas hydrates. In Proceedings of the 4th International Conference on Gas Hydrate, Yokohama Symposia, Yokohama, Japan, 19–23 May 2002; pp. 537–542. [Google Scholar]
- Staykova, D.K.; Kuhs, W.F.; Salamatin, A.N.; Hansen, T. Formation of porous gas hydrates from ice powders: Diffraction experiments and multistage model. Phys. Chem. B 2003, 107, 10299–10311. [Google Scholar] [CrossRef]
- Genov, G.; Kuhs, W.F.; Staykova, D.K.; Goreshnik, E.; Salamatin, A.N. Experimental studies on the formation of porous gas hydrates. Am. Miner. 2004, 89, 1228–1239. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Staykova, D.K.; Salamatin, A.N. Formation of methane hydrate from polydisperse ice powders. Phys. Chem. B 2006, 110, 13283–13295. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Yang, M.; Song, Y.; Zhang, L.; Li, Q.; Chen, Y. Experimental study on the methane hydrate formation from ice powders. In Proceedings of the 6th International Conference on Applied Energy (ICAE2014), Energy Procedia 61, Taipei, Taiwan, 30 May–2 June 2014; pp. 619–623. [Google Scholar] [CrossRef]
- Buldovicz, S.N.; Khilimonyuk, V.Z.; Bychkov, A.Y.; Ospennikov, E.N.; Vorobyev, S.A.; Gunar, A.Y.; Gorshkov, E.I.; Chuvilin, E.M.; Cherbunina, M.Y.; Kotov, P.I.; et al. Cryovolcanism on the Earth: Origin of a Spectacular Crater in the Yamal Peninsula (Russia). Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Hubberten, H.-W.; Gavrilov, A.V.; Eliseeva, A.A.; Tipenko, G.S.; Kholodov, A.L.; Romanovsky, V.E. Permafrost and gas hydrate stability zone evolution on the eastern part of the Eurasia Arctic sea shelf in the middle pleistocene–holocene. Kriosf. Zemli 2003, VII, 51–64. (In Russian) [Google Scholar]
- Istomin, V.A.; Chuvilin, E.M.; Sergeeva, D.V.; Bukhanov, B.A.; Stanilovskaya, Y.V.; Badetz, C. Influence of component composition and pressure of gas on ice and hydrate formation in gas-saturated pore solutions. Neftegazokhimiya 2018, 2, 33–42. [Google Scholar] [CrossRef]
- Shakhova, N.E.; Sergienko, V.I.; Semiletov, I.P. The contribution of the East Siberian shelf to the modern Methane Cycle. Her. Russ. Acad. Sci. 2009, 79, 237–246. [Google Scholar] [CrossRef]
- Shakhova, N.E.; Semiletov, I.P.; Leifer, I.; Salyuk, A.; Rekant, P.; Kosmach, D. Geochemical and geophysical evidence of methane release over the East Siberian Arctic Shelf. Geophys. Res. Oceans 2010, 115, C08007. [Google Scholar] [CrossRef]



| Sample | Origin, Age | Sampling Site | Mineralogy, % | Salinity, % | |
|---|---|---|---|---|---|
| Sand 1 | mJ3 | Lubertsy | Quartz | >90 | 0.01 |
| Sand 2 | gmQII2–4 | Kharasavey OGCF | Quartz | 93.7 | 0.07 |
| Kaolinite + chlorite | 3.5 | ||||
| Microcline | 2.3 | ||||
| Silt 1 | gmQII2–4 | Vorkuta | Microcline + albite | 45 | 0.08 |
| Quartz | 38 | ||||
| Illite | 9 | ||||
| Kaolinite + chlorite | 5 | ||||
| Montmorillonite | 3 | ||||
| Silt 2 | mQIII | Zapolarnoe OGCF | Quartz | 64 | 0.20 |
| XRA | 17 | ||||
| Microcline | 9 | ||||
| Albite | 5 | ||||
| Smectite + hydromica | 3 | ||||
| Silt 3 | gmQII2–4 | Bovanenkovo GCF | Quartz | 59.5 | 0.58 |
| Microcline | 13.6 | ||||
| Albite | 22 | ||||
| Kaolinite + chlorite | 2.4 | ||||
| Illite | 1.5 | ||||
| Sample | Particle size Distribution, % | Lithology * | ||
|---|---|---|---|---|
| 1–0.05 mm | 0.05–0.001 mm | <0.001 mm | ||
| Sand 1 | 94.8 | 3.1 | 2.1 | Fine sand |
| Sand 2 | 91.9 | 8.1 | 2 | Medium sand |
| Silt 1 | 41.8 | 53.7 | 4.5 | Heavy fine silt |
| Silt 2 | 88 | 4 | 8 | Heavy silt |
| Silt 3 | 59.9 | 35.1 | 5 | Heavy fine silt |
| Sample | Water Content | Density | Porosity | Ice Saturation |
|---|---|---|---|---|
| % | g/cm3 | % | % | |
| Silt 2 | 10 | 1.76 | 50 | 29 |
| 14 | 1.59 | 50 | 43 | |
| 18 | 1.55 | 50 | 52 | |
| 20 | 1.60 | 50 | 59 | |
| 23 | 1.56 | 53 | 66 | |
| 28 | 1.64 | 51 | 77 | |
| 27 | 1.68 | 50 | 80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Davletshina, D. Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling. Geosciences 2018, 8, 467. https://doi.org/10.3390/geosciences8120467
Chuvilin E, Davletshina D. Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling. Geosciences. 2018; 8(12):467. https://doi.org/10.3390/geosciences8120467
Chicago/Turabian StyleChuvilin, Evgeny, and Dinara Davletshina. 2018. "Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling" Geosciences 8, no. 12: 467. https://doi.org/10.3390/geosciences8120467
APA StyleChuvilin, E., & Davletshina, D. (2018). Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling. Geosciences, 8(12), 467. https://doi.org/10.3390/geosciences8120467




