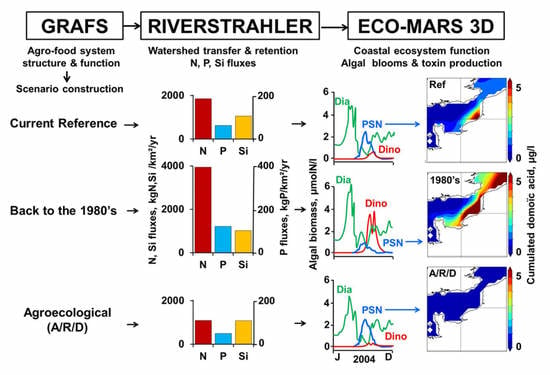
Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel
Abstract
Share and Cite
Garnier, J.; Riou, P.; Le Gendre, R.; Ramarson, A.; Billen, G.; Cugier, P.; Schapira, M.; Théry, S.; Thieu, V.; Ménesguen, A. Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel. Geosciences 2019, 9, 441. https://doi.org/10.3390/geosciences9100441
Garnier J, Riou P, Le Gendre R, Ramarson A, Billen G, Cugier P, Schapira M, Théry S, Thieu V, Ménesguen A. Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel. Geosciences. 2019; 9(10):441. https://doi.org/10.3390/geosciences9100441
Chicago/Turabian StyleGarnier, Josette, Philippe Riou, Romain Le Gendre, Antsiva Ramarson, Gilles Billen, Philippe Cugier, Mathilde Schapira, Sylvain Théry, Vincent Thieu, and Alain Ménesguen. 2019. "Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel" Geosciences 9, no. 10: 441. https://doi.org/10.3390/geosciences9100441
APA StyleGarnier, J., Riou, P., Le Gendre, R., Ramarson, A., Billen, G., Cugier, P., Schapira, M., Théry, S., Thieu, V., & Ménesguen, A. (2019). Managing the Agri-Food System of Watersheds to Combat Coastal Eutrophication: A Land-to-Sea Modelling Approach to the French Coastal English Channel. Geosciences, 9(10), 441. https://doi.org/10.3390/geosciences9100441