Ballasted Flocs Capture Pelagic Primary Production and Alter the Local Sediment Characteristics in the Coastal German Bight (North Sea)
Abstract
1. Introduction
Motivation for This Study
2. Materials and Methods
2.1. Working Area and Samples
2.2. Grain Size
2.3. Porosity
2.4. Permeability
2.5. Chlorins
2.6. Specific Respiration Rate
2.7. Data Availability
3. Results
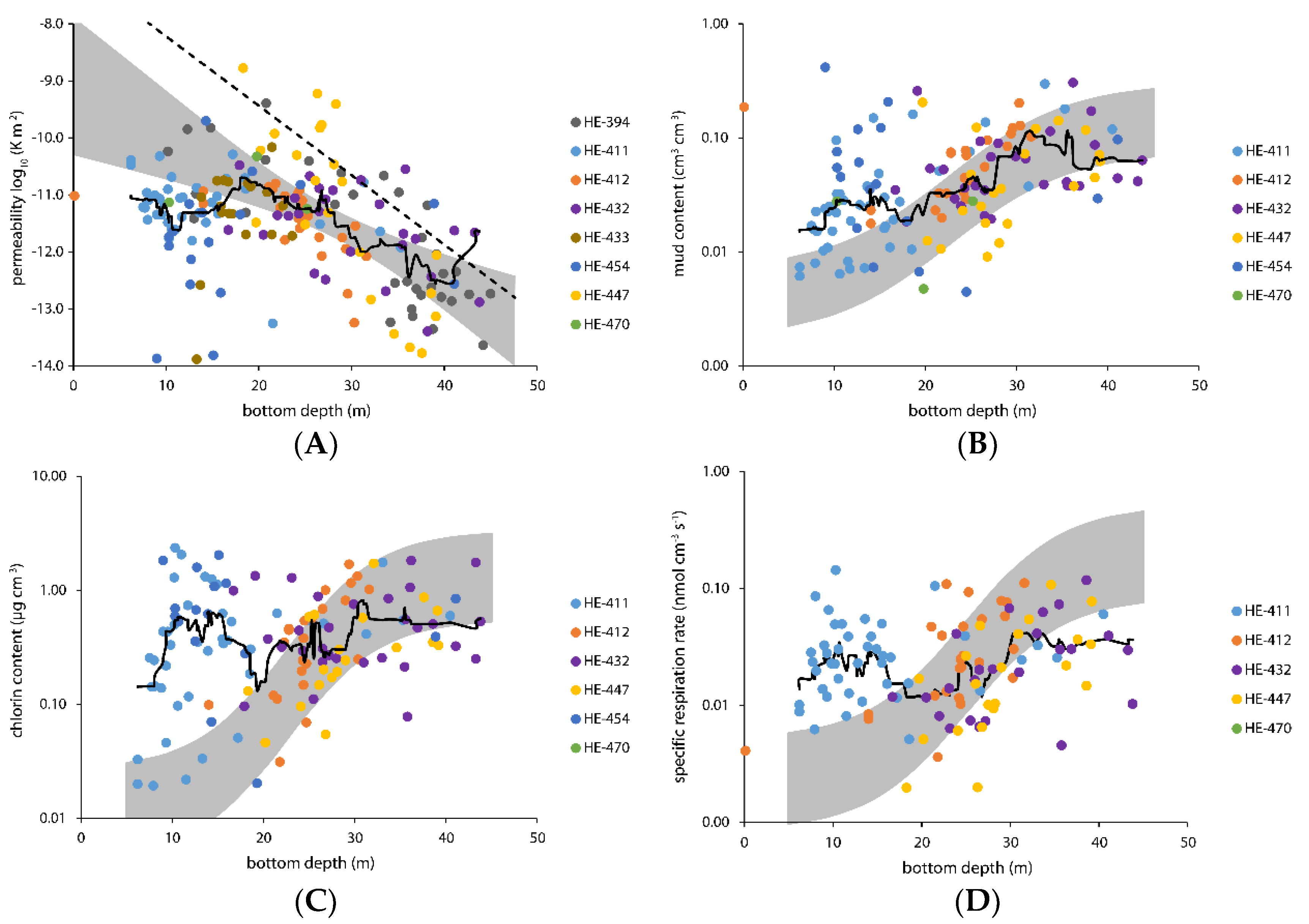
3.1. Grain Size Analysis and Sediment Description
3.2. Specific Respiration Rate and Pigment Content
4. Discussion
4.1. A Continuum of Sediment Types
4.2. Can We Detect a Benthic Effect of Ballasted Flocs?
4.3. After the Flocs Have Settled
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Notation and Abbreviations
| CO2 | oxygen concentration (µmol L−1) |
| Chl | chlorin content per sediment bulk volume (µg cm−3) |
| Di, Dmode | grain size (µm) |
| EPS | Extracellular, polymeric substance |
| k | permeability (m2) |
| MRE | median of relative error |
| mudL | volume fraction of mud content measured by laser-diffraction (cm3 cm−3) |
| P17, P83 | 17th and 83rd percentile of relative error distribution |
| p | probability of null-hypothesis |
| POM | particulate organic matter |
| φ | porosity |
| R | specific respiration rate per sediment bulk volume (nmol cm−3 s−2), temperature-normalized to 10 °C. |
| ρs | Spearman’s rank correlation coefficient |
| SPM | suspended particulate matter |
| t | time (s) |
| τ | bed shear stress (N m−2) |
References
- Alldredge, A.L.; Gotschalk, C. In situ settling behavior of marine snow. Limnol. Oceanogr. 1988, 33, 339–351. [Google Scholar] [CrossRef]
- Hamm, C.E. Interactive aggregation and sedimentation of diatoms and clay-sized lithogenic material. Limnol. Oceanogr. 2002, 47, 1790–1795. [Google Scholar] [CrossRef]
- Peterson, M.L.; Wakeham, S.G.; Lee, C.; Askea, M.A.; Miquel, J.C. Novel techniques for collection of sinking particles in the ocean and determining their settling rates. Limnol. Oceanogr. 2005, 3, 520–532. [Google Scholar] [CrossRef]
- Claessens, M.; Prast, M. Concentration of fixed plankton samples via settling: How long is long enough? J. Plankton Res. 2008, 30, 57–64. [Google Scholar] [CrossRef]
- Ptacnik, R.; Diehl, S.; Berger, S. Performance of sinking and nonsinking phytoplankton taxa in a gradient of mixing depths. Limnol. Oceanogr. 2003, 5, 1903–1912. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Fettweis, M.; Baeye, M.; Van der Zande, D.; Van den Eynde, D.; Lee, B.J. Seasonality of floc strength in the southern North Sea. J. Geophys. Res. Oceans 2014, 119, 1911–1926. [Google Scholar] [CrossRef]
- Eisma, D. Flocculation and de-flocculation of suspended matter in estuaries. J. Sea Res. 1986, 20, 183–199. [Google Scholar] [CrossRef]
- De La Rocha, C.; Passow, U. Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res. II 2007, 54, 639–658. [Google Scholar] [CrossRef]
- Maerz, J.; Hofmeister, R.; van der Lee, E.M.; Gräwe, U.; Riethmüller, R.; Wirtz, K.W. Maximum sinking velocities of suspended particulate matter in a coastal transition zone. Biogeosciences 2016, 13, 4863–4876. [Google Scholar] [CrossRef]
- Malarkey, J.; Baas, J.H.; Hope, J.A.; Aspden, R.J.; Parsons, D.R.; Peakall, J.; Paterson, D.M.; Schindler, R.J.; Ye, L.; Lichtman, I.D.; et al. The pervasive role of biological cohesion in bedform development. Nat. Commun. 2015, 6, 6257. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.R.; Schindler, R.J.; Hope, J.A.; Malarkey, J.; Baas, J.H.; Peakall, J.; Manning, A.J.; Ye, L.; Simmons, S.; Paterson, D.M.; et al. The role of biophysical cohesion on subaqueous bed form size. Geophys. Res. Lett. 2016, 43, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Baas, J.H.; Davies, A.G.; Malarkey, J. Bedform development in mixed sand–mud: The contrasting role of cohesive forces in flow and bed. Geomorphology 2013, 182, 19–32. [Google Scholar] [CrossRef]
- Soulsby, R. Dynamics of Marine Sands; Thomas Telford: London, UK, 1997; ISBN 978-0-7277-2584-4. [Google Scholar]
- Van Rijn, L.C. Principles of Sediment Transport in Rivers, Estuaries and Coastal Areas; Aqua Publications: Amsterdam, The Netherlands, 1993; ISBN 90-800356-2-9. [Google Scholar]
- CoastMap Data Portal. Available online: www.hzg.de/institutes_platforms/coastmap/index.php.en (accessed on 27 June 2019).
- Schartau, M.; Riethmüller, R.; Flöser, G.; van Beusekom, J.E.E.; Krasemann, H.; Hofmeister, R.; Wirtz, K. On the separation between inorganic and organic fractions of suspended matter in a marine coastal environment. Prog. Oceanogr. 2019, 171, 231–250. [Google Scholar] [CrossRef]
- Hass, H.C.; Kuhn, G.; Monien, P.; Brumsack, H.-J.; Forwick, M. Climate fluctuations during the past two millennia as recorded in sediments from Maxwell Bay, South Shetland Islands, West Antarctica. In Fjordic Depositional Systems and Archives; Howe, J., Austin, W.E.N., Paetzel, M., Forwick, M., Eds.; Geological Society of London: London, UK, 2010; pp. 243–260. [Google Scholar]
- Neumann, A.; Möbius, J.; Hass, H.C.; Puls, W.; Friedrich, J. Empirical model to estimate permeability of surface sediments in the German Bight (North Sea). J. Sea Res. 2016, 127, 36–45. [Google Scholar] [CrossRef]
- Bear, J. Dynamics of Fluids in Porous Media; American Elsevier Publishing Company: New York, NY, USA, 1972. [Google Scholar]
- Glover, P.W.; Walker, E. Grain-size to effective pore-size transformation derived from electrokinetic theory. Geophysics 2009, 74, E17–E29. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- de Beer, D.; Wenzhöfer, F.; Ferdelman, T.G.; Boehme, S.E.; Huettel, M.; van Beusekom, J.E.E.; Böttcher, M.E.; Musat, N.; Dubilier, N. Transport and mineralization rates in North Sea sandy intertidal sediments, Sylt-Rømø Basin, Wadden Sea. Limnol. Oceanogr. 2005, 50, 113–127. [Google Scholar] [CrossRef]
- Thamdrup, B.; Hansen, J.W.; Jørgensen, B.B. Temperature dependence of aerobic respiration in a coastal sediment. FEMS Microbiol. Ecol. 1998, 25, 189–200. [Google Scholar] [CrossRef]
- Hancke, K.; Glud, R.N. Temperature effects on respiration and photosynthesis in three diatom-dominated benthic communities. Aquat. Microb. Ecol. 2004, 37, 265–281. [Google Scholar] [CrossRef]
- Provoost, P.; Braeckman, U.; Van Gansbeke, D.; Moodley, L.; Soetaert, K.; Middelburg, J.J.; Vanaverbeke, J. Modelling benthic oxygen consumption and benthic-pelagic coupling at a shallow station in the southern North Sea. Estuar. Coast. Shelf Sci. 2013, 120, 1–11. [Google Scholar] [CrossRef]

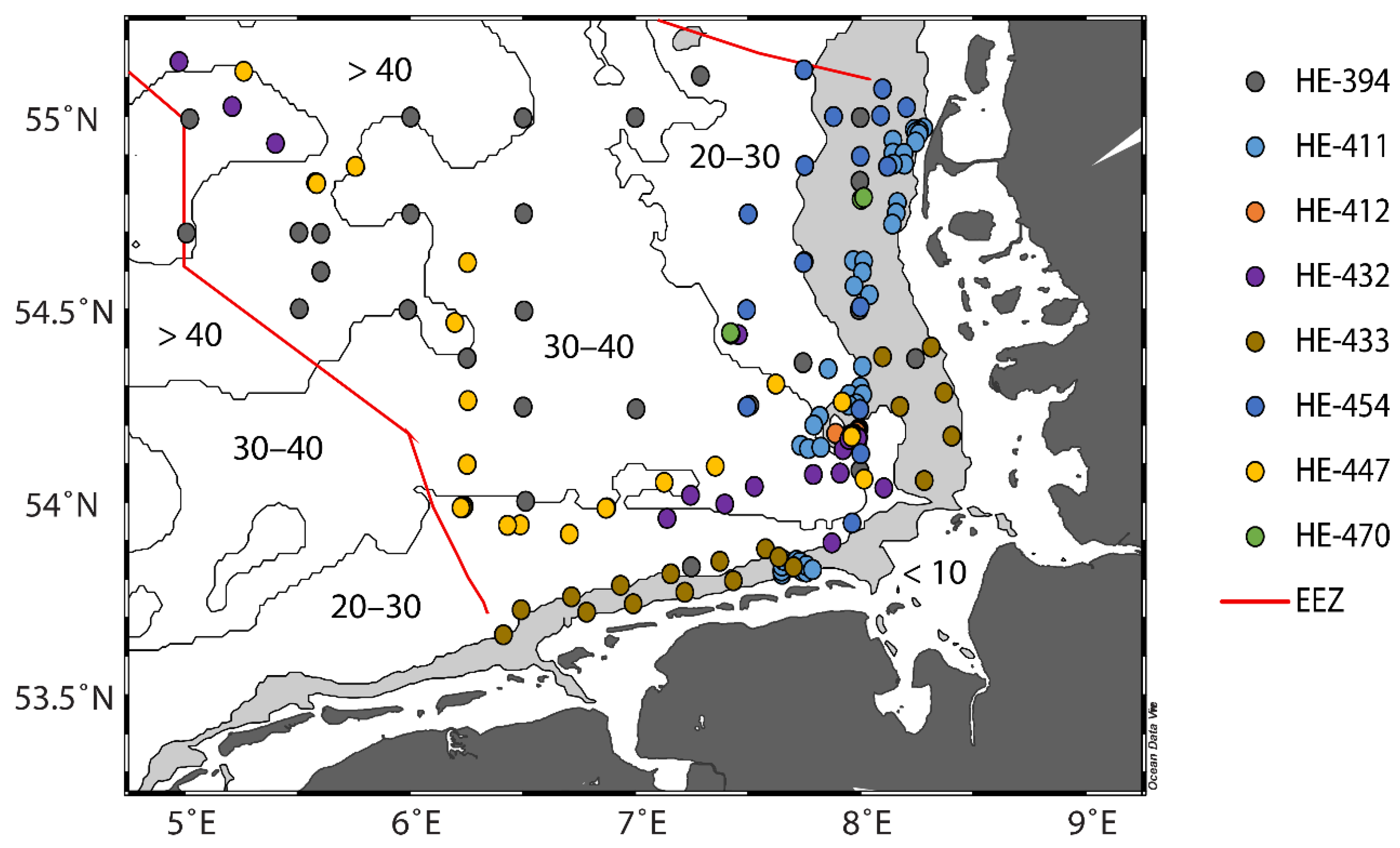




| Cruise | Date | Samples |
|---|---|---|
| HE-394 | 3/2013 | perm |
| HE-411 | 10/2013 | gr-ld, perm, poro, chl, resp |
| HE-412 | 10/2013 | gr-ld, perm, poro, chl, resp |
| HE-432 | 9/2014 | gr-ld, perm, poro, chl, resp |
| HE-433 | 10/2014 | perm |
| HE-447 | 6/2015 | gr-ld, perm, poro, chl, resp |
| HE-454 | 11/2015 | gr-ld, perm, poro, chl |
| HE-470 | 8/2016 | gr-ld, perm, poro, chl, resp |
| Equation | Formula | P17 | MRE | P83 |
|---|---|---|---|---|
| 1 | 0.06 | 0.01 | 0.11 | |
| 2 | 0.74 | 0.02 | 1.61 | |
| 3 | 0.44 | −0.08 | 0.92 | |
| 4 | 0.61 | 0.07 | 2.38 | |
| 5 | 0.06 | 0.02 | 0.10 | |
| 6 | 0.64 | −0.06 | 1.07 | |
| 7 | 0.56 | 0.02 | 1.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, A.; Hass, H.C.; Möbius, J.; Naderipour, C. Ballasted Flocs Capture Pelagic Primary Production and Alter the Local Sediment Characteristics in the Coastal German Bight (North Sea). Geosciences 2019, 9, 344. https://doi.org/10.3390/geosciences9080344
Neumann A, Hass HC, Möbius J, Naderipour C. Ballasted Flocs Capture Pelagic Primary Production and Alter the Local Sediment Characteristics in the Coastal German Bight (North Sea). Geosciences. 2019; 9(8):344. https://doi.org/10.3390/geosciences9080344
Chicago/Turabian StyleNeumann, Andreas, H. Christian Hass, Jürgen Möbius, and Céline Naderipour. 2019. "Ballasted Flocs Capture Pelagic Primary Production and Alter the Local Sediment Characteristics in the Coastal German Bight (North Sea)" Geosciences 9, no. 8: 344. https://doi.org/10.3390/geosciences9080344
APA StyleNeumann, A., Hass, H. C., Möbius, J., & Naderipour, C. (2019). Ballasted Flocs Capture Pelagic Primary Production and Alter the Local Sediment Characteristics in the Coastal German Bight (North Sea). Geosciences, 9(8), 344. https://doi.org/10.3390/geosciences9080344





