Eye Movements in Response to Pain-Related Feelings in the Presence of Low and High Cognitive Loads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
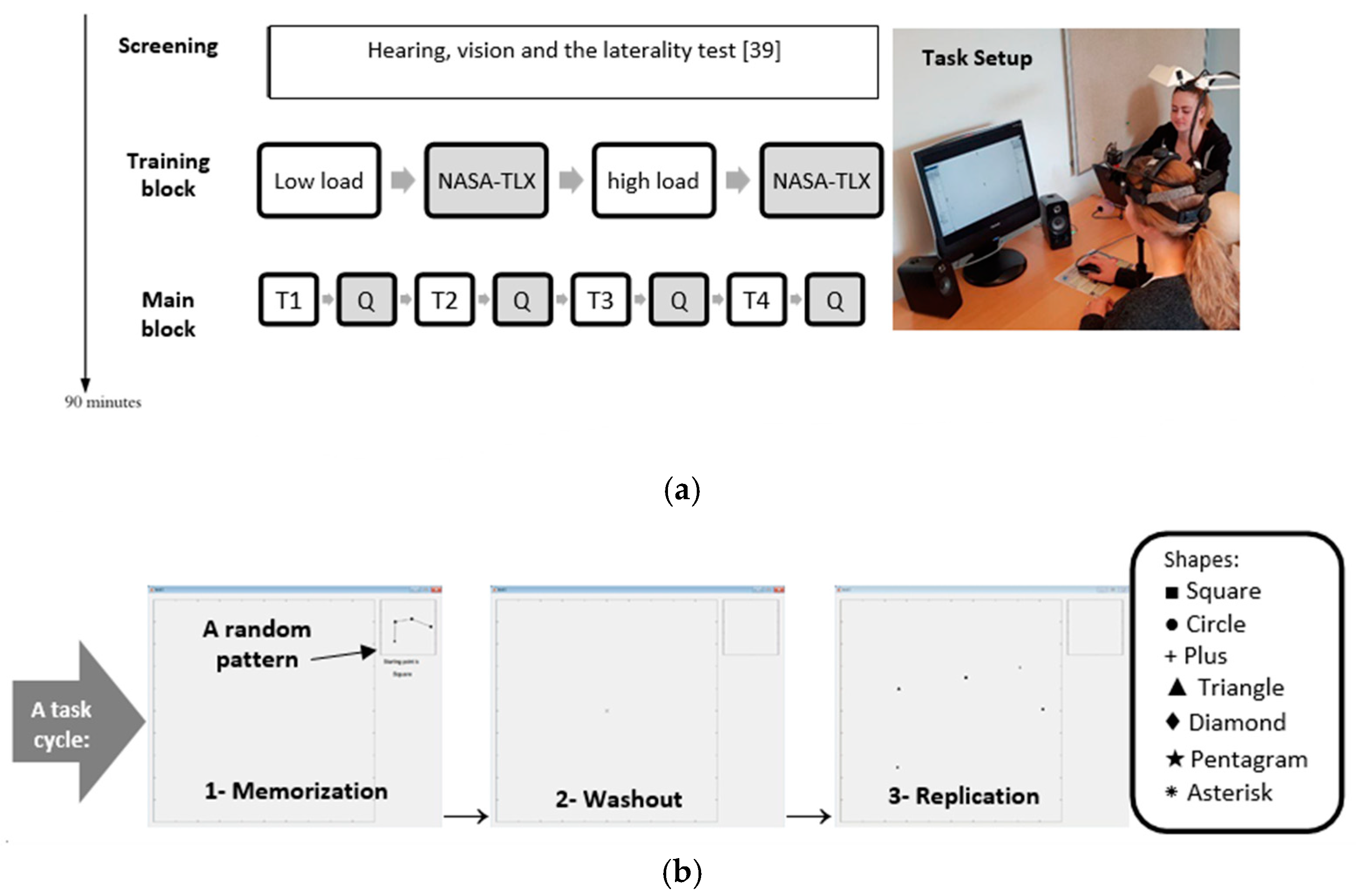
2.2. Experimental Procedure
2.3. Auditory Stimuli
2.4. Experimental Task
3. Data Collection and Analysis
Statistical Analysis
4. Results
4.1. Saccades
4.2. Fixations
4.3. Blinks
4.4. Pupillary Responses
4.5. Overall Performance
4.6. Subjective Ratings
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, F.; Zhu, X.; Luo, Y.; Cheng, J. Working memory load modulates the neural response to other’s pain: Evidence from an ERP study. Neurosci. Lett. 2017, 644, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lyons, I.M.; Beilock, S.L. When math hurts: Math anxiety predicts pain network activation in anticipation of doing math. PLoS ONE 2012, 7, e48076. [Google Scholar] [CrossRef] [PubMed]
- Merskey, H.; Bogduk, N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd ed.; International Association for the Study of Pain: Seattle, WA, USA, 1994; ISBN 0-931092-05-1. [Google Scholar]
- Erk, S.; Abler, B.; Walter, H. Cognitive modulation of emotion anticipation. Eur. J. Neurosci. 2006, 24, 1227–1236. [Google Scholar] [CrossRef]
- Porro, C.A.; Baraldi, P.; Pagnoni, G.; Serafini, M.; Facchin, P.; Maieron, M.; Nichelli, P. Does anticipation of pain affect cortical nociceptive systems? J. Neurosci. 2002, 22, 3206–3214. [Google Scholar] [CrossRef]
- Schmidt, K.; Gamer, M.; Forkmann, K.; Bingel, U. Pain affects visual orientation: An eye-tracking study. J. Pain 2018, 19, 135–145. [Google Scholar] [CrossRef]
- Jackson, T.; Yang, Z.; Su, L. Pain-related gaze biases and later functioning among adults with chronic pain: A longitudinal eye-tracking study. Pain 2019, 160, 2221–2228. [Google Scholar] [CrossRef]
- Duchowski, A. Eye Tracking Methodology Theory and Practice, 3rd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 9781846286087. [Google Scholar]
- Oliva, M.; Anikin, A. Pupil dilation reflects the time course of emotion recognition in human vocalizations. Sci. Rep. 2018, 8, 4871. [Google Scholar] [CrossRef] [Green Version]
- Marandi, R.Z.; Madeleine, P.; Omland, Ø.; Vuillerme, N.; Samani, A. Eye movement characteristics reflected fatigue development in both young and elderly individuals. Sci. Rep. 2018, 8, 13148. [Google Scholar] [CrossRef]
- Marandi, R.Z.; Madeleine, P.; Omland, Ø.; Vuillerme, N.; Samani, A. An oculometrics-based biofeedback system to impede fatigue development during computer work: A proof-of-concept study. PLoS ONE 2019, 14, e0213704. [Google Scholar] [CrossRef] [Green Version]
- Marandi, R.Z.; Gazerani, P. Aging and eye tracking: In the quest for objective biomarkers. Future Neurol. 2019, 14, FNL33. [Google Scholar] [CrossRef]
- Han, S.; Soleiman, M.; Soden, M.; Zweifel, L.; Palmiter, R.D. Elucidating an affective pain circuit that creates a threat memory. Cell 2015, 162, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Fairhurst, M.; Fairhurst, K.; Berna, C.; Tracey, I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS ONE 2012, 7, e48711. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Mishima, R.; Yamamura, K.; Mostafeezur, R.; Zakir, H.M.D.; Kurose, M.; Yamada, Y. Difference in physiological responses to sound stimulation in subjects with and without fear of dental treatments. Odontology 2008, 96, 44–49. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.; Kim, S.; Chetana, M.; Jo, D.; Ruley, H.E.; Lin, S.Y.; Rabah, D.; Kinet, J.P.; Shin, H.S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 2010, 13, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Mee, S.; Bunney, B.G.; Reist, C.; Potkin, S.G.; Bunney, W.E. Psychological pain: A review of evidence. J. Psychiatr. Res. 2006, 40, 680–690. [Google Scholar] [CrossRef]
- Papini, M.R.; Fuchs, P.N.; Torres, C. Behavioral neuroscience of psychological pain. Neurosci. Biobehav. Rev. 2015, 48, 53–69. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 2009, 87, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.R.; Farmer, M.A.; Baliki, M.N.; Apkarian, A.V. Chronic pain: The role of learning and brain plasticity. Restor. Neurol. Neurosci. 2014, 32, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Golwala, P. Phantom headache: Pain-memory-emotion hypothesis for chronic daily headache? J. Headache Pain 2011, 12, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Sandkühler, J.; Lee, J. How to erase memory traces of pain and fear. Trends Neurosci. 2013, 36, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Reist, C.; Mee, S.; Fujimoto, K.; Rajani, V.; Bunney, W.E.; Bunney, B.G. Assessment of psychological pain in suicidal veterans. PLoS ONE 2017, 12, e0177974. [Google Scholar] [CrossRef]
- Yang, G.; Baad-Hansen, L.; Wang, K.; Xie, Q.F.; Svensson, P. Effect of negative emotions evoked by light, noise and taste on trigeminal thermal sensitivity. J. Headache Pain 2014, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Seminowicz, D.A.; Mikulis, D.J.; Davis, K.D. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain 2004, 112, 48–58. [Google Scholar] [CrossRef]
- Van Ryckeghem, D.M.L.; Crombez, G.; Eccleston, C.; Legrain, V.; Van Damme, S. Keeping pain out of your mind: The role of attentional set in pain. Eur. J. Pain 2013, 17, 402–411. [Google Scholar] [CrossRef]
- Roa Romero, Y.; Straube, T.; Nitsch, A.; Miltner, W.H.R.; Weiss, T. Interaction between stimulus intensity and perceptual load in the attentional control of pain. Pain 2013, 154, 135–140. [Google Scholar] [CrossRef]
- Lavie, N.; Beck, D.M.; Konstantinou, N. Blinded by the load: Attention, awareness and the role of perceptual load. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130205. [Google Scholar] [CrossRef] [Green Version]
- Dick, B.D.; Connolly, J.F.; McGrath, P.J.; Finley, G.A.; Stroink, G.; Houlihan, M.E.; Clark, A.J. The disruptive effect of chronic pain on mismatch negativity. Clin. Neurophysiol. 2003, 114, 1497–1506. [Google Scholar] [CrossRef]
- Kewman, D.G.; Vaishampayan, N.; Zald, D.; Han, B. Cognitive impairment in musculoskeletal pain patients. Int. J. Psychiatry Med. 1991, 21, 253–262. [Google Scholar] [CrossRef]
- Marandi, R.Z.; Madeleine, P.; Omland, Ø.; Vuillerme, N.; Samani, A. Reliability of oculometrics during a mentally demanding task in young and old adults. IEEE Access 2018, 6, 17500–17517. [Google Scholar] [CrossRef]
- Rosch, J.L.; Vogel-Walcutt, J.J. A review of eye-tracking applications as tools for training. Cogn. Technol. Work 2013, 15, 313–327. [Google Scholar] [CrossRef]
- Fashler, S.R.; Katz, J. Keeping an eye on pain: Investigating visual attention biases in individuals with chronic pain using eye-tracking methodology. J. Pain Res. 2016, 9, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attridge, N.; Noonan, D.; Eccleston, C.; Keogh, E. The disruptive effects of pain on n-back task performance in a large general population sample. Pain 2015, 156, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Jameson, E.; Trevena, J.; Swain, N. Electronic gaming as pain distraction. Pain Res. Manag. 2011, 16, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malloy, K.M.; Milling, L.S. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Sharek, D. A Useable, Online NASA-TLX Tool. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2011, 55, 1375–1379. [Google Scholar] [CrossRef]
- Melzack, R. The short-form McGill Pain Questionnaire (sf-MPQ). Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- McMahon, S.B.; Koltzenburg, M.; Tracey, I.; Turk, D.C. Wall & Melzack’s Textbook of Pain: Expert Consult-Online and Print; Elsevier Health Sciences: Philadelphia, PA, USA, 2013; ISBN 0443027153. [Google Scholar]
- Marandi, R.Z. WAME1.0, a Standardized Computer Task to Induce Cognitive Load, and Develop Fatigue. 2019. Available online: https://osf.io/2fc7s/ (accessed on 1 May 2020).
- Laring, J.; Forsman, M.; Kadefors, R.; Örtengren, R. MTM-based ergonomic workload analysis. Int. J. Ind. Ergon. 2002, 30, 135–148. [Google Scholar] [CrossRef]
- Salvucci, D.D.; Goldberg, J.H. Identifying Fixations and Saccades in Eye-Tracking Protocols. Proc. Eye Track. Res. Appl. Symp. 2000, 71–78. [Google Scholar] [CrossRef]
- Chen, S.; Epps, J. Using task-induced pupil diameter and blink rate to infer cognitive load. Hum. Comput. Interact. 2014, 29, 390–413. [Google Scholar] [CrossRef]
- Hopstaken, J.F.; van der Linden, D.; Bakker, A.B.; Kompier, M.A. The window of my eyes: Task disengagement and mental fatigue covary with pupil dynamics. Biol. Psychol. 2015, 110, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Knapen, T.; De Gee, J.W.; Brascamp, J.; Nuiten, S.; Hoppenbrouwers, S.; Theeuwes, J. Cognitive and ocular factors jointly determine pupil responses under equiluminance. PLoS ONE 2016, 11, e0155574. [Google Scholar] [CrossRef] [Green Version]
- Pedrotti, M.; Mirzaei, M.A.; Tedesco, A.; Chardonnet, J.R.; Mérienne, F.; Benedetto, S.; Baccino, T. Automatic stress classification with pupil diameter analysis. Int. J. Hum. Comput. Interact. 2014, 30, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Ly-Liu, D.; Reinoso-Barbero, F. Immediate postoperative pain can also be predicted by pupillary pain index in children. Br. J. Anaesth. 2015, 114, 345–346. [Google Scholar] [CrossRef] [Green Version]
- Brokjær, A.; Olesen, A.E.; Christrup, L.L.; Dahan, A.; Drewes, A.M. The effects of morphine and methylnaltrexone on gastrointestinal pain in healthy male participants. Neurogastroenterol. Motil. 2015, 27, 693–704. [Google Scholar] [CrossRef]
- Connelly, M.A.; Brown, J.T.; Kearns, G.L.; Anderson, R.A.; St Peter, S.D.; Neville, K.A. Pupillometry: A non-invasive technique for pain assessment in paediatric patients. Arch. Dis. Child. 2014, 99, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Eisenach, J.C.; Curry, R.; Aschenbrenner, C.A.; Coghill, R.C.; Houle, T.T. Pupil responses and pain ratings to heat stimuli: Reliability and effects of expectations and a conditioning pain stimulus. J. Neurosci. Methods 2017, 279, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Ellermeier, W.; Westphal, W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain 1995, 61, 435–439. [Google Scholar] [CrossRef]
- Geuter, S.; Gamer, M.; Onat, S.; Büchel, C. Parametric trial-by-trial prediction of pain by easily available physiological measures. Pain 2014, 155, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Kyle, B.N.; McNeil, D.W. Autonomic arousal and experimentally induced pain: A critical review of the literature. Pain Res. Manag. 2014, 19, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Chapman, C.R.; Kim, B.; Nakajima, I.; Shimizu, O.; Oi, Y. Pupil dilation response to noxious stimulation: Effect of varying nitrous oxide concentration. Clin. Neurophysiol. 2007, 118, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Priebe, J.A.; Messingschlager, M.; Lautenbacher, S. Gaze behaviour when monitoring pain faces: An eye-tracking study. Eur. J. Pain 2015, 19, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.K.; Wang, J.Y.; Luo, F. Experimental pain induces attentional bias that is modified by enhanced motivation: An eye tracking study. Eur. J. Pain 2016, 20, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Duncan, G.H.; Hofbauer, R.K.; Ha, B.; Chen, J.-I.; Carrier, B. Pain perception: Is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. USA 1999, 96, 7705–7709. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Hu, L.; Shen, L.; Yang, Z.; Chen, H.; Huang, X.; Jackson, T. Emotional primes modulate the responses to others’ pain: An ERP study. Exp. Brain Res. 2012, 220, 277–286. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Macdonald, R.A.R.; Knussen, C.; Serpell, M.G. A survey investigation of the effects of music listening on chronic pain. Psychol. Music 2007, 35, 37–57. [Google Scholar] [CrossRef]
- Gardner, W.J.; Licklider, J.C.R. Auditory analgesia in dental operations. J. Am. Dent. Assoc. 1959, 59, 1144–1149. [Google Scholar] [CrossRef]
- Hermann, C.; Blanchard, E.B. Biofeedback in the treatment of headache and other childhood pain. Appl. Psychophysiol. Biofeedback 2002, 27, 143–162. [Google Scholar] [CrossRef]

| Control Sounds | Pain-Related Sounds |
|---|---|
| Barred owl | Dental scaler |
| Heart beats | Dental turbine |
| Vivid human voice in crowd | Horn |
| Babbling brook water streaming | Sawing machine |
| Waterfall | Medical device alarm |
| Air | Civil defense siren |
| Variable | L | LP | H | HP |
|---|---|---|---|---|
| Oculometrics | ||||
| SPV (°/s) | 119 ± 9 | 116 ± 10 | 113 ± 6 | 114 ± 9 |
| SF (Hz) | 1.35 ± 0.21 | 1.43 ± 0.19 | 1.26 ± 0.18 | 1.25 ± 0.22 |
| FD (s) | 0.47 ± 0.10 | 0.48 ± 0.10 | 0.51 ± 0.10 | 0.52 ± 0.10 |
| FF (Hz) | 1.8 ± 0.3 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 |
| PDR (mm) | 0.31 ± 0.11 | 0.35 ± 0.13 | 0.38 ± 0.13 | 0.39 ± 0.14 |
| BD (s) | 0.19 ± 0.03 | 0.18 ± 0.03 | 0.18 ± 0.03 | 0.18 ± 0.03 |
| BF (Hz) | 0.20 ± 0.18 | 0.19 ± 0.15 | 0.19 ± 0.16 | 0.18 ± 0.15 |
| Performance | ||||
| OP | 0.42 ± 0.10 | 0.43 ± 0.11 | 0.27 ± 0.10 | 0.29 ± 0.14 |
| Main Study | Validation Study | |||||
|---|---|---|---|---|---|---|
| L | LP | H | HP | PR | C | |
| Pain scores | 0.6 ± 0.6 | 0.9 ± 1.1 | 1.1 ± 0.7 | 0.8 ± 1.0 | 3.5 ± 0.1 | 1.8 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargari Marandi, R.; Fjelsted, C.A.; Hrustanovic, I.; Dan Olesen, R.; Gazerani, P. Eye Movements in Response to Pain-Related Feelings in the Presence of Low and High Cognitive Loads. Behav. Sci. 2020, 10, 92. https://doi.org/10.3390/bs10050092
Zargari Marandi R, Fjelsted CA, Hrustanovic I, Dan Olesen R, Gazerani P. Eye Movements in Response to Pain-Related Feelings in the Presence of Low and High Cognitive Loads. Behavioral Sciences. 2020; 10(5):92. https://doi.org/10.3390/bs10050092
Chicago/Turabian StyleZargari Marandi, Ramtin, Camilla Ann Fjelsted, Iris Hrustanovic, Rikke Dan Olesen, and Parisa Gazerani. 2020. "Eye Movements in Response to Pain-Related Feelings in the Presence of Low and High Cognitive Loads" Behavioral Sciences 10, no. 5: 92. https://doi.org/10.3390/bs10050092





