The Reward-Related Shift of Emotional Contagion from the Observer’s Perspective Correlates to Their Intimacy with the Expresser
Abstract
:1. Introduction
1.1. The Social Function of Emotional Contagion
1.2. Reward Context and Social Functions of Emotional Contagion
1.3. Overview of the Present Research
2. Materials and Methods
2.1. Participants
2.2. Stimuli
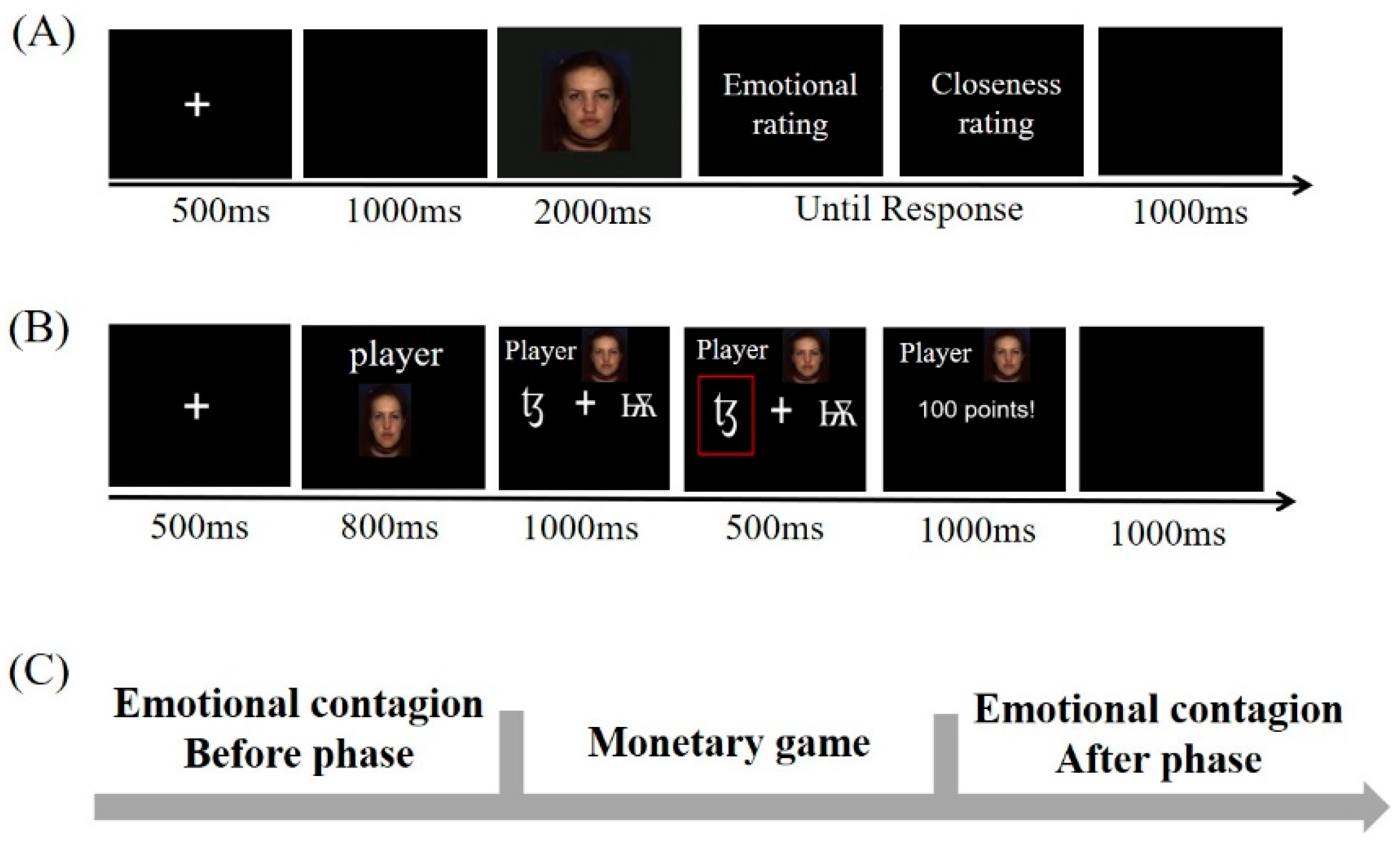
2.3. Procedure
2.4. Data Acquisition and Analysis
2.5. Statistical Analysis
3. Results
3.1. The Effect of Reward Outcome on the Closeness
3.2. The Effect of Reward Outcome on the Emotional Contagion
3.2.1. The Manipulation Checks of Emotional Contagion Presence
3.2.2. The Effect of Reward Outcome on the Emotional Contagion
3.3. Control Analysis
3.4. The Effect of Reward Outcome on Brain Activities
3.5. Neural Activity Changes Linked to Closeness and Emotional Contagion Changes
3.6. The Effect of Reward Outcome on the Social Functions of Emotional Contagion
3.7. Mediation Analysis

4. Discussion
4.1. The Social Function of Emotional Contagion
4.2. The Effect of Reward Outcome on the Changes in Closeness, Emotional Contagion, and Brain Activity
4.3. The Effect of Reward Context on the Social Functioning of Emotional Contagion
4.4. Limitations and Future Outlooks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumeister, R.F.; Leary, M.R. The Need to Belong—Desire for Interpersonal Attachments as a Fundamental Human-Motivation. Psychol. Bull. 1995, 117, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Hess, U.; Fischer, A. Emotional mimicry as social regulator: Theoretical considerations. Cogn. Emot. 2022, 36, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Saporta, N.; Marton-Alper, I.Z.; Gvirts, H.Z. Herding Brains: A Core Neural Mechanism for Social Alignment. Trends Cogn. Sci. 2019, 23, 174–186. [Google Scholar] [CrossRef]
- Hatfield, E.; Rapson, R.L.; Le, Y.C. Emotional contagion and empathy. In The Social Neuroscience of Empathy; Decety, J., Ickes, W., Eds.; MIT Press: Cambridge, UK, 2009; pp. 19–30. [Google Scholar]
- Sims, T.B.; van Reekum, C.M.; Johnstone, T.; Chakrabarti, B. How reward modulates mimicry: EMG evidence of greater facial mimicry of more rewarding happy faces. Psychophysiology 2012, 49, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Belkin, L.Y. Emotional Contagion in the Electronic Communication Context: Conceptualizing the Dynamics and Implications of Electronic Emotional Encounters in Organizations. J. Organ. Cult. Commun. Confl. 2009, 13, 105. [Google Scholar]
- Dezecache, G.; Jacob, P.; Grèzes, J. Emotional contagion: Its scope and limits. Trends Cogn. Sci. 2015, 19, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Sonnby-Borgström, M.; Hess, U.; Fischer, A.H. Emotional mimicry: Underlying mechanisms and individual differences. In Emotional Mimicry in Social Context; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Barsade, S.G. The Ripple Effect: Emotional Contagion and its Influence on Group Behavior. Adm. Sci. Q. 2002, 47, 644–675. [Google Scholar] [CrossRef]
- Chartrand, T.L.; Bargh, J.A. The chameleon effect: The perception-behavior link and social interaction. J. Personal. Soc. Psychol. 1999, 76, 893–910. [Google Scholar] [CrossRef] [PubMed]
- van Kleef, G.A.; Côté, S. The Social Effects of Emotions. Annu. Rev. Psychol. 2021, 73, 629–658. [Google Scholar] [CrossRef]
- Stel, M.; Rispens, S.; Leliveld, M.C.; Lokhorst, A.M. The consequences of mimicry for prosocials and proselfs: Effects of social value orientation on the mimicry–liking link. Eur. J. Soc. Psychol. 2011, 41, 269–274. [Google Scholar] [CrossRef]
- Kühn, S.; Muller, B.C.; van der Leij, A.; Dijksterhuis, A.; Brass, M.; van Baaren, R.B. Neural correlates of emotional synchrony. Soc. Cogn. Affect. Neurosci. 2011, 6, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Mauersberger, H.; Hess, U. When smiling back helps and scowling back hurts: Individual differences in emotional mimicry are associated with self-reported interaction quality during conflict interactions. Motiv. Emot. 2019, 43, 471–482. [Google Scholar] [CrossRef]
- Salazar Kämpf, M.; Liebermann, H.; Kerschreiter, R.; Krause, S.; Nestler, S.; Schmukle, S.C. Disentangling the Sources of Mimicry: Social Relations Analyses of the Link Between Mimicry and Liking. Psychol. Sci. 2018, 29, 131–138. [Google Scholar] [CrossRef]
- Hess, U.; Fischer, A.H. Emotional Mimicry as Social Regulation. Personal. Soc. Psychol. Rev. 2013, 17, 142–157. [Google Scholar] [CrossRef]
- Stel, M.; Van Baaren, R.B.; Vonk, R. Effects of mimicking: Acting prosocially by being emotionally moved. Eur. J. Soc. Psychol. 2008, 38, 965–976. [Google Scholar] [CrossRef]
- Stel, M.; van den Bos, K.; Sim, S.; Rispens, S. Mimicry and just world beliefs: Mimicking makes men view the world as more personally just. Br. J. Soc. Psychol. 2013, 52, 397–411. [Google Scholar] [CrossRef]
- Hale, J.; Hamilton, A.F. Cognitive mechanisms for responding to mimicry from others. Neurosci. Biobehav. Rev. 2016, 63, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Hess, U. Who to whom and why: The social nature of emotional mimicry. Psychophysiology 2021, 58, e13675. [Google Scholar] [CrossRef]
- Dalton, A.N.; Chartrand, T.L.; Finkel, E.J. The schema-driven chameleon: How mimicry affects executive and self-regulatory resources. J. Pers. Soc. Psychol. 2010, 98, 605–617. [Google Scholar] [CrossRef]
- Sims, T.B.; Neufeld, J.; Johnstone, T.; Chakrabarti, B. Autistic traits modulate frontostriatal connectivity during processing of rewarding faces. Soc. Cogn. Affect. Neurosci. 2014, 9, 2010–2016. [Google Scholar] [CrossRef]
- Korb, S.; Goldman, R.; Davidson, R.J.; Niedenthal, P.M. Increased Medial Prefrontal Cortex and Decreased Zygomaticus Activation in Response to Disliked Smiles Suggest Top-Down Inhibition of Facial Mimicry. Front. Psychol. 2019, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Forbes, P.A.G.; Korb, S.; Radloff, A.; Lamm, C. The effects of self-relevance vs. reward value on facial mimicry. Acta Psychol. 2021, 212, 103193. [Google Scholar] [CrossRef]
- Wang, Y.; Hamilton, A. Social top-down response modulation (STORM): A model of the control of mimicry in social interaction. Front. Hum. Neurosci. 2012, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Trilla, I.; Drimalla, H.; Bajbouj, M.; Dziobek, I. The Influence of Reward on Facial Mimicry: No Evidence for a Significant Effect of Oxytocin. Front. Behav. Neurosci. 2020, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Kozakevich Arbel, E.; Shamay-Tsoory, S.G.; Hertz, U. Adaptive Empathy: Empathic Response Selection as a Dynamic, Feedback-Based Learning Process. Front. Psychiatry 2021, 12, 706474. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, J.P.; Brinberg, M.; Ram, N.; Hollenstein, T. Emotion socialization as a dynamic process across emotion contexts. Dev. Psychol. 2020, 56, 553–565. [Google Scholar] [CrossRef]
- Hein, G.; Engelmann, J.B.; Vollberg, M.C.; Tobler, P.N. How learning shapes the empathic brain. Proc. Natl. Acad. Sci. USA 2016, 113, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Kühn, S.; Müller, B.C.; van Baaren, R.B.; Wietzker, A.; Dijksterhuis, A.; Brass, M. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Soc. Neurosci. 2010, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Vrtička, P.; Vuilleumier, P. Neuroscience of human social interactions and adult attachment style. Front. Hum. Neurosci. 2012, 6, 212. [Google Scholar] [CrossRef]
- Cui, F.; Ma, N.; Luo, Y.J. Moral judgment modulates neural responses to the perception of other’s pain: An ERP study. Sci. Rep. 2016, 6, 20851. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Meng, J.; Cui, F. Scarcity mindset reduces empathic responses to others’ pain: The behavioral and neural evidence. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad012. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Peng, W.; Li, Z.; Shen, L. The interaction between pain and attractiveness perception in others. Sci. Rep. 2020, 10, 5528. [Google Scholar] [CrossRef]
- Peng, W.; Meng, J.; Lou, Y.; Li, X.; Lei, Y.; Yan, D. Reduced empathic pain processing in patients with somatoform pain disorder: Evidence from behavioral and neurophysiological measures. Int. J. Psychophysiol. 2019, 139, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bruder, M.; Fischer, A.H.; Manstead, A.S.R. Social appraisal as a cause of collective emotions. In Collective Emotions; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Wróbel, M. I can see that you’re happy but you’re not my friend. J. Soc. Pers. Relatsh. 2018, 35, 1301–1318. [Google Scholar] [CrossRef]
- Wróbel, M.; Królewiak, K. Do We Feel the Same Way If We Think the Same Way? Shared Attitudes and the Social Induction of Affect. Basic Appl. Soc. Psychol. 2017, 39, 19–37. [Google Scholar] [CrossRef]
- Bourgeois, A.; Chelazzi, L.; Vuilleumier, P. How motivation and reward learning modulate selective attention. Prog. Brain Res. 2016, 229, 325–342. [Google Scholar]
- Swart, J.C.; Froböse, M.I.; Cook, J.L.; Geurts, D.E.; Frank, M.J.; Cools, R.; den Ouden, H.E. Catecholaminergic challenge uncovers distinct Pavlovian and instrumental mechanisms of motivated (in)action. Elife 2017, 6, e22169. [Google Scholar] [CrossRef]
- Kret, M.E. Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Front. Psychol. 2015, 6, 711. [Google Scholar] [CrossRef]
- de Vignemont, F.; Singer, T. The empathic brain: How, when and why? Trends Cogn. Sci. 2006, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Heyes, C. Empathy is not in our genes. Neurosci. Biobehav. Rev. 2018, 95, 499–507. [Google Scholar] [CrossRef]
- Yin, L.; Chen, X.; Sun, Y.; Worm, T.; Reale, M. A high-resolution 3D dynamic facial expression database. In Proceedings of the 2008 8th IEEE International Conference on Automatic Face & Gesture Recognition, Amsterdam, The Netherlands, 17–19 September 2008. [Google Scholar]
- Sarkheil, P.; Goebel, R.; Schneider, F.; Mathiak, K. Emotion unfolded by motion: A role for parietal lobe in decoding dynamic facial expressions. Soc. Cogn. Affect. Neurosci. 2013, 8, 950–957. [Google Scholar] [CrossRef]
- Schacher, M.; Winkler, R.; Grunwald, T.; Kraemer, G.; Kurthen, M.; Reed, V.; Jokeit, H. Mesial temporal lobe epilepsy impairs advanced social cognition. Epilepsia 2006, 47, 2141–2146. [Google Scholar] [CrossRef]
- Sollfrank, T.; Kohnen, O.; Hilfiker, P.; Kegel, L.C.; Jokeit, H.; Brugger, P.; Loertscher, M.L.; Rey, A.; Mersch, D.; Sternagel, J.; et al. The Effects of Dynamic and Static Emotional Facial Expressions of Humans and Their Avatars on the EEG: An ERP and ERD/ERS Study. Front. Neurosci. 2021, 15, 651044. [Google Scholar] [CrossRef]
- Lockwood, P.L.; Apps, M.A.; Valton, V.; Viding, E.; Roiser, J.P. Neurocomputational mechanisms of prosocial learning and links to empathy. Proc. Natl. Acad. Sci. USA 2016, 113, 9763–9768. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Eta-Squared and Partial Eta-Squared in Fixed Factor Anova Designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Wager, T.D.; Davidson, M.L.; Hughes, B.L.; Lindquist, M.A.; Ochsner, K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008, 59, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Hu, P. Matching Your Face or Appraising the Situation: Two Paths to Emotional Contagion. Front. Psychol. 2017, 8, 2278. [Google Scholar] [CrossRef] [PubMed]
- Olszanowski, M.; Wróbel, M.; Hess, U. Mimicking and sharing emotions: A re-examination of the link between facial mimicry and emotional contagion. Cogn. Emot. 2020, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hess, U.; Blairy, S. Facial mimicry and emotional contagion to dynamic emotional facial expressions and their influence on decoding accuracy. Int. J. Psychophysiol. 2001, 40, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, L.-O.; Dimberg, U. Facial Expressions Are Contagious. J. Psychophysiol. 1995, 9, 203–211. [Google Scholar]
- Fan, Y.; Han, S. Temporal dynamic of neural mechanisms involved in empathy for pain: An event-related brain potential study. Neuropsychologia 2008, 46, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Jackson, T.; Chen, H.; Hu, L.; Yang, Z.; Su, Y.; Huang, X. Pain perception in the self and observation of others: An ERP investigation. Neuroimage 2013, 72, 164–173. [Google Scholar] [CrossRef]
- Rauchbauer, B.; Majdandžić, J.; Hummer, A.; Windischberger, C.; Lamm, C. Distinct neural processes are engaged in the modulation of mimicry by social group-membership and emotional expressions. Cortex 2015, 70, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, W.; Liu, M.; Ou, Y.; Xu, E.; Hu, P. Light makeup decreases receivers’ negative emotional experience. Sci. Rep. 2021, 11, 23802. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Iannone, N.E.; McCarty, M.K. Emotional contagion of anger is automatic: An evolutionary explanation. Br. J. Soc. Psychol. 2016, 55, 182–191. [Google Scholar] [CrossRef]
- Ibanez, A.; Melloni, M.; Huepe, D.; Helgiu, E.; Rivera-Rei, A.; Canales-Johnson, A.; Baker, P.; Moya, A. What event-related potentials (ERPs) bring to social neuroscience? Soc. Neurosci. 2012, 7, 632–649. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Yeung, N.; van den Wildenberg, W.; Ridderinkhof, K.R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 2003, 3, 17–26. [Google Scholar] [CrossRef]
- Gable, S.L. Approach and avoidance social motives and goals. J. Pers. 2006, 74, 175–222. [Google Scholar] [CrossRef] [PubMed]
- Laméris, D.W.; van Berlo, E.; Sterck, E.H.M.; Bionda, T.; Kret, M.E. Low relationship quality predicts scratch contagion during tense situations in orangutans (Pongo pygmaeus). Am. J. Primatol. 2020, 82, e23138. [Google Scholar] [CrossRef] [PubMed]
- Kret, M.E.; Akyüz, R. Mimicry eases prediction and thereby smoothens social interactions. Cogn. Emot. 2022, 36, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Keysers, C.; Knapska, E.; Moita, M.A.; Gazzola, V. Emotional contagion and prosocial behavior in rodents. Trends Cogn. Sci. 2022, 26, 688–706. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, W.; Zhang, L.; Wei, Y.; Hu, P. The Reward-Related Shift of Emotional Contagion from the Observer’s Perspective Correlates to Their Intimacy with the Expresser. Behav. Sci. 2023, 13, 934. https://doi.org/10.3390/bs13110934
Chen Y, Chen W, Zhang L, Wei Y, Hu P. The Reward-Related Shift of Emotional Contagion from the Observer’s Perspective Correlates to Their Intimacy with the Expresser. Behavioral Sciences. 2023; 13(11):934. https://doi.org/10.3390/bs13110934
Chicago/Turabian StyleChen, Ying, Wenfeng Chen, Ling Zhang, Yanqiu Wei, and Ping Hu. 2023. "The Reward-Related Shift of Emotional Contagion from the Observer’s Perspective Correlates to Their Intimacy with the Expresser" Behavioral Sciences 13, no. 11: 934. https://doi.org/10.3390/bs13110934
APA StyleChen, Y., Chen, W., Zhang, L., Wei, Y., & Hu, P. (2023). The Reward-Related Shift of Emotional Contagion from the Observer’s Perspective Correlates to Their Intimacy with the Expresser. Behavioral Sciences, 13(11), 934. https://doi.org/10.3390/bs13110934





