The Influence of Classroom Size and Window View on Young Children’s Executive Functions and Physiological Responses, Based on VR Technology
Abstract
:1. Introduction
1.1. The Impact of Visual Exposure to Nature on Humans
1.2. Impact of Room Size on Humans
1.3. Research Questions and Hypotheses
- RQ1.
- Does classroom size (large vs. small) influence young children’s performance in EF tasks or their physiological responses (i.e., cortisol and HRV)?
- RQ1.1.
- Does classroom size (large vs. small) influence young children’s performance in EF tasks?
- RQ1.2.
- Does classroom size (large vs. small) influence young children’s physiological responses (i.e., cortisol and HRV)?
- RQ2.
- Does window view (nature vs. built) influence young children’s performance in EF tasks or their physiological responses (i.e., cortisol and HRV)?
- RQ2.1.
- Does window view (nature vs. built) influence young children’s performance in EF tasks?
- RQ2.2.
- Does window view (nature vs. built) influence young children’s physiological responses (i.e., cortisol and HRV)?
2. Materials and Methods
2.1. Participants
2.2. Procedures
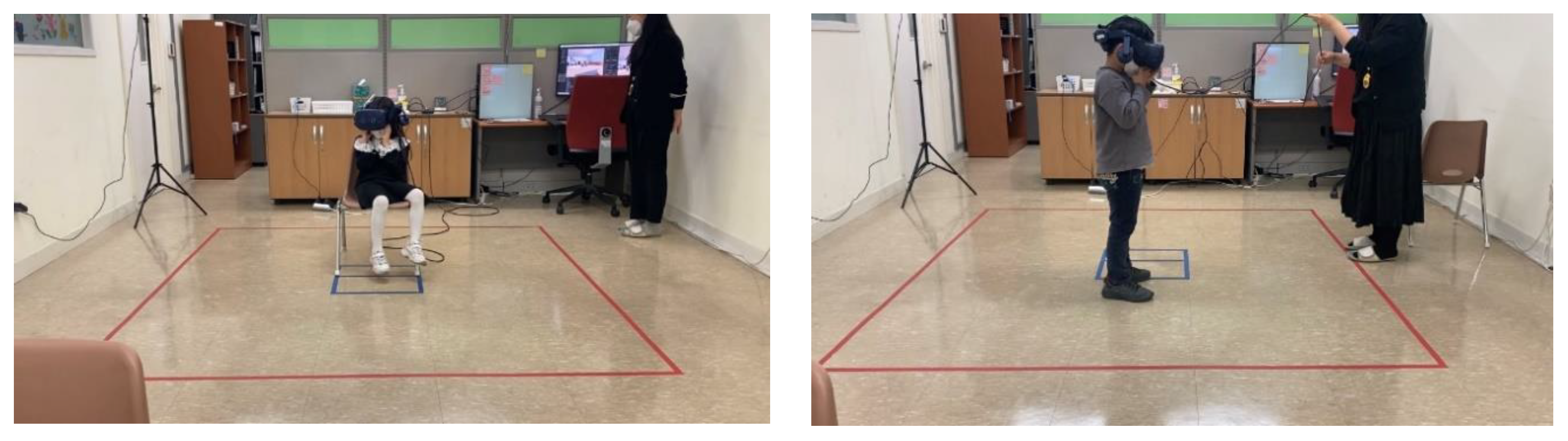
2.2.1. VR Development and Equipment
2.2.2. VR Classroom Design Conditions
2.2.3. Experimental Procedures
2.3. Measures
2.4. Analyses
3. Results
3.1. Descriptive Statistics and Correlational Analysis
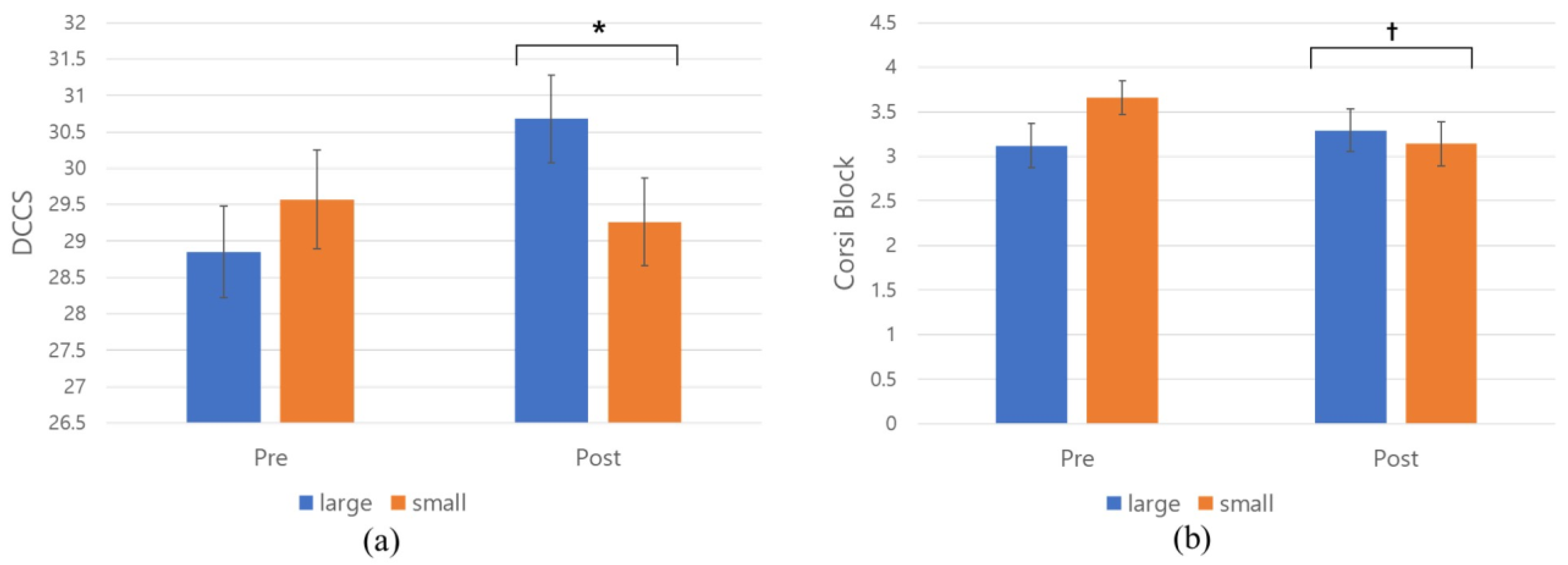
3.2. RQ 1.1: Classroom Size (Large vs. Small) and Children’s Performance on EF Tasks
3.3. RQ 1.2: Classroom Size (Large vs. Small) and Children’s Cortisol and HRV
3.4. RQ 2.1: Window View (Nature vs. Built) and Children’s Performance on EF Tasks
3.5. RQ 2.2: Window View (Nature vs. Built) and Children’s Cortisol and HRV
4. Discussion
4.1. The Impact of Classroom Size and Window View on Young Children’s EFs
4.2. Physiological Responses in Relation to VR Conditions and Children’s EFs
4.3. Limitations and Future Studies
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berti, S.; Cigala, A.; Sharmahd, N. Early childhood education and care physical environment and child development: State of the art and reflections on future orientations and methodologies. Educ. Psychol. Rev. 2019, 31, 991–1021. [Google Scholar] [CrossRef]
- OECD. Encouraging Quality in Early Childhood Education and Care (ECEC). In Starting Strong III: A Quality Toolbox for Early Childhood Education and Care; OECD, Ed.; OECD Co.: Paris, France, 2013; pp. 23–42. [Google Scholar]
- Brunsek, A.; Perlman, M.; Falenchuk, O.; McMullen, E.; Fletcher, B.; Shah, P.S. The relationship between the Early Childhood Environment Rating Scale and its revised form and child outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178512. [Google Scholar] [CrossRef] [PubMed]
- Mashburn, A.J. Quality of social and physical environments in preschools and children’s development of academic, language, and literacy skills. Appl. Dev. Sci. 2008, 12, 113–127. [Google Scholar] [CrossRef]
- Maxwell, L.E. Multiple effects of home and day care crowding. Environ. Behav. 1996, 28, 494–511. [Google Scholar] [CrossRef]
- Al-Ayash, A.; Kane, R.T.; Smith, D.; Green-Armytage, P. The influence of color on student emotion, heart rate, and performance in learning environments. Color Res. Appl. 2016, 41, 196–205. [Google Scholar] [CrossRef]
- Banaei, M.; Hatami, J.; Yazdanfar, A.; Gramann, K. Walking through architectural spaces: The impact of interior forms on human brain dynamics. Front. Hum. Neurosci. 2017, 11, 477. [Google Scholar] [CrossRef]
- Benfield, J.A.; Rainbolt, G.N.; Bell, P.A.; Donovan, G.H. Classrooms with nature views: Evidence of differing student perceptions and behaviors. Environ. Behav. 2015, 47, 140–157. [Google Scholar] [CrossRef]
- Li, D.; Sullivan, W.C. Impact of views to school landscapes on recovery from stress and mental fatigue. Landsc. Urban Plan. 2016, 148, 149–158. [Google Scholar] [CrossRef]
- Palanica, A.; Lyons, A.; Cooper, M.; Lee, A.; Fossat, Y. A comparison of nature and urban environments on creative thinking across different levels of reality. J. Environ. Psychol. 2019, 63, 44–51. [Google Scholar] [CrossRef]
- Tuszyńska-Bogucka, W.; Kwiatkowski, B.; Chmielewska, M.; Dzieńkowski, M.; Kocki, W.; Pełka, J.; Przesmycka, N.; Bogucki, J.; Galkowski, D. The effects of interior design on wellness—Eye tracking analysis in determining emotional experience of architectural space. A survey on a group of volunteers from the Lublin Region, Eastern Poland. Ann. Agric. Environ. Med. 2020, 27, 113–122. [Google Scholar] [CrossRef]
- Abbas, M.Y.; Othman, M.; Rahman, P.Z.M.A. Pre-School Children’s Play Behaviour Influenced by Classroom’s Spatial Definitions? Asian J. Environ. Stud. 2016, 1, 49–65. [Google Scholar] [CrossRef]
- Read, M.A.; Sugawara, A.I.; Brandt, J.A. Impact of space and color in the physical environment on preschool children’s cooperative behavior. Environ. Behav. 1999, 31, 413–428. [Google Scholar] [CrossRef]
- Van Liempd, I.H.; Oudgenoeg–Paz, O.; Leseman, P.P. Do spatial characteristics influence behavior and development in early childhood education and care? J. Environ. Psychol. 2019, 67, 101385. [Google Scholar] [CrossRef]
- Aries, M.B.; Veitch, J.A.; Newsham, G.R. Windows, view, and office characteristics predict physical and psycho-logical discomfort. J. Environ. Psychol. 2010, 30, 533–541. [Google Scholar] [CrossRef]
- Bratman, G.N.; Hamilton, J.P.; Daily, G.C. The impacts of nature experience on human cognitive function and mental health. Ann. N. Y. Acad. Sci. 2012, 1249, 118–136. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Dravigne, A.; Waliczek, T.M.; Lineberger, R.D.; Zajicek, J.M. The effect of live plants and window views of green spaces on employee perceptions of job satisfaction. Hort. Sci. 2008, 43, 183–187. [Google Scholar] [CrossRef]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Matsuoka, R.H. Student performance and high school landscapes: Examining the links. Landsc. Urban Plan. 2010, 97, 273–282. [Google Scholar] [CrossRef]
- Studente, S.; Seppala, N.; Sadowska, N. Facilitating creative thinking in the classroom: Investigating the effects of plants and the colour green on visual and verbal creativity. Think. Ski. Creat. 2016, 19, 1–8. [Google Scholar] [CrossRef]
- Tennessen, C.M.; Cimprich, B. Views to nature: Effects on attention. J. Environ. Psychol. 1995, 15, 77–85. [Google Scholar] [CrossRef]
- Daly, J.; Burchett, M.; Torpy, F. Plants in the Classroom Can Improve Student Performance; National Interior Plantscape Association: Kippa-Ring, QLD, Australia, 2010; pp. 1–9. [Google Scholar]
- Lindemann-Matthies, P.; Benkowitz, D.; Hellinger, F. Associations between the naturalness of window and interior classroom views, subjective well-being of primary school children and their performance in an attention and concentration test. Landsc. Urban Plan. 2021, 214, 104146. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; Wesselius, J.E.; Maas, J.; Tanja-Dijkstra, K. Green walls for a restorative classroom environment: A controlled evaluation study. Environ. Behav. 2016, 49, 791–813. [Google Scholar] [CrossRef]
- Zhou, Z.; Ergan, S. Where do we look? An eye-tracking study of architectural features in building design. In Proceedings of the 35th CIB W78 2018 Conference: IT in Design, Construction, and Management, Chicago, IL, USA, 1–3 October 2018. [Google Scholar]
- Cruz-Garza, J.G.; Darfler, M.; Rounds, J.D.; Gao, E.; Kalantari, S. EEG-based investigation of the impact of room size and window placement on cognitive performance. J. Build. Eng. 2022, 53, 104540. [Google Scholar] [CrossRef]
- Fowler, W.J.; Walberg, H.J. School size, characteristics, and outcomes. Educ. Evaluation Policy Anal. 1991, 13, 189. [Google Scholar] [CrossRef]
- Ehrenberg, R.G.; Brewer, D.J.; Gamoran, A.; Willms, J.D. Class size and student achievement. Psychol. Sci. Public Interest 2001, 2, 1–30. [Google Scholar] [CrossRef]
- Maxwell, L.E. Home and school density effects on elementary school children: The role of spatial density. Environ. Behav. 2003, 35, 566–578. [Google Scholar] [CrossRef]
- Burgess, J.W.; Fordyce, W.K. Effects of preschool environments on nonverbal social behavior: Toddlers’ interpersonal distances to teachers and classmates change with environmental density, classroom design, and parent–child interactions. J. Child Psychol. Psychiatry 1989, 30, 261–276. [Google Scholar] [CrossRef]
- Loo, C.M. The effects of spatial density on the social behavior of children. J. Appl. Soc. Psychol. 1972, 2, 372–381. [Google Scholar] [CrossRef]
- Smith, P.K.; Connolly, K.J. The Ecology of Preschool Behavior; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Kantrowitz, E.J.; Evans, G.W. The relation between the ratio of children per activity area and off-task behavior and type of play in day care centers. Environ. Behav. 2004, 36, 541–557. [Google Scholar] [CrossRef]
- Evans, G.W. The built environment and mental health. J. Urban Health 2003, 80, 536–555. [Google Scholar] [CrossRef]
- Bailey, J.O.; Bailenson, J.N.; Obradović, J.; Aguiar, N.R. Virtual reality’s effect on children’s inhibitory control, social compliance, and sharing. J. Appl. Dev. Psychol. 2019, 64, 101052. [Google Scholar] [CrossRef]
- Segovia, K.Y.; Bailenson, J.N. Virtually true: Children’s acquisition of false memories in virtual reality. Media Psychol. 2009, 12, 371–393. [Google Scholar] [CrossRef]
- Bull, R.; Scerif, G. Developmental neuropsychology callosal contribution to procedural learning in children. Dev. Neuropsychol. 2010, 19, 37–41. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Sundstrom, E. Crowding as a sequential process: Review of research on the effects of population density on humans. In Human Response to Crowding; Baum, A., Epstein, Y.M., Eds.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1978; pp. 31–116. [Google Scholar]
- Saegert, S. High density environments: Their personal and social consequences. Hum. Response Crowd. 1978, 257–281. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Mccraty, R.; Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Magnon, V.; Vallet, G.T.; Benson, A.; Mermillod, M.; Chausse, P.; Lacroix, A.; Bouillon-Minois, J.-B.; Dutheil, F. Does heart rate variability predict better executive functioning? A systematic review and meta-analysis. Cortex 2022, 155, 218–236. [Google Scholar] [CrossRef]
- Putnam, S.P.; Rothbart, M.K. Development of short and very short forms of the Children’s Behavior Questionnaire. J. Personal. Assess. 2006, 87, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M. Developmentally sensitive measures of executive function in preschool. Dev. Neuropsychol. 2005, 28, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Stevenson, H.W. Cross-linguistic differences in digit span of preschool children. J. Exp. Child Psychol. 1988, 46, 150–158. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; van Zandvoort, M.J.E.; Postma, A.; Kappelle, L.J.; de Haan, E.H.F. The Corsi block-tapping task: Standardization and normative data. Appl. Neuropsychol. 2000, 7, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat. Protoc. 2006, 1, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lewis, C. Korean preschoolers’ executive function skills: A comparison with US children. Early Child. Res. Q. 2018, 42, 144–152. [Google Scholar]
- Cowan, M.J. Measurement of heart rate variability. West. J. Nurs. Res. 1995, 17, 32–48. [Google Scholar] [CrossRef]
- Hernández-Vicente, A.; Hernando, D.; Santos-Lozano, A.; Rodríguez-Romo, G.; Vicente-Rodríguez, G.; Pueyo, E.; Bailón, R.; Garatachea, N. Heart rate variability and exceptional longevity. Front. Physiol. 2020, 11, 566399. [Google Scholar] [CrossRef]
- Davis, E.P.; Bruce, J.; Gunnar, M.R. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Dev. Psychobiol. 2001, 40, 43–56. [Google Scholar] [CrossRef]
- Moore, D.S.; McCabe, G.P.; Craig, B.A. Introduction to the Practice of Statistics, 9th ed.; Freeman & Company, W&H: New York, NY, USA, 2017. [Google Scholar]
- McCormick, C.; Lewis, E.; Somley, B.; Kahan, T. Individual differences in cortisol levels and performance on a test of executive function in men and women. Physiol. Behav. 2007, 91, 87–94. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Fredrickson, B.L. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. Am. Psychol. 2001, 56, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, B.L.; Branigan, C. Positive emotions broaden the scope of attention and thought-action repertoires. Cogn. Emot. 2005, 19, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Gasper, K.; Clore, G.L. Attending to the big picture: Mood and global versus local processing of visual information. Psychol. Sci. 2002, 13, 34–40. [Google Scholar] [CrossRef]
- Hartikainen, K.M. Emotion-attention interaction in the right hemisphere. Brain Sci. 2021, 11, 1006. [Google Scholar] [CrossRef]
- Kalia, V.; Vishwanath, K.; Knauft, K.; Von Der Vellen, B.; Luebbe, A.; Williams, A. Acute stress attenuates cognitive flexibility in males only: An fNIRS examination. Front. Psychol. 2018, 9, 2084. [Google Scholar] [CrossRef]
- Moran, T.P. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol. Bull. 2016, 142, 831–864. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. In The Psychology of Learning and Motivation: Advances in Research and Theory; Bower, G.H., Ed.; Academic Press: New York, NY, USA, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Papagno, C.; Comi, A.; Riva, M.; Bizzi, A.; Vernice, M.; Casarotti, A.; Fava, E.; Bello, L. Mapping the brain network of the phonological loop. Hum. Brain Mapp. 2017, 38, 3011–3024. [Google Scholar] [CrossRef]
- Scherf, K.S.; Sweeney, J.A.; Luna, B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006, 18, 1045–1058. [Google Scholar] [CrossRef]
- Shackman, A.J.; Sarinopoulos, I.; Maxwell, J.S.; Pizzagalli, D.A.; Lavric, A.; Davidson, R.J. Anxiety selectively disrupts visuospatial working memory. Emotion 2006, 6, 40–61. [Google Scholar] [CrossRef]
- Vytal, K.E.; Cornwell, B.R.; Letkiewicz, A.M.; Arkin, N.E.; Grillon, C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front. Hum. Neurosci. 2013, 7, 93. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; Yonelinas, A.P. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016, 68, 651–668. [Google Scholar] [CrossRef]
- Becker, L.; Rohleder, N. Time course of the physiological stress response to an acute stressor and its associations with the primacy and recency effect of the serial position curve. PLoS ONE 2019, 14, e0213883. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Herman, J.P. The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocr. 2015, 27, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Sapolsky, L.; Romero, M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green spaces and cognitive development in primary schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W.; Saegert, S.; Harris, R. Residential density and psychological health among children in low-income families. Environ. Behav. 2001, 33, 165–180. [Google Scholar] [CrossRef]
- Xia, G.; Henry, P.; Li, M.; Queiroz, F.; Westland, S.; Yu, L. A Comparative study of colour effects on cognitive per-formance in real-world and VR environments. Brain Sci. 2021, 12, 31. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Surg | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||
| 2. NE | −0.31 *** | − | − | − | − | − | − | − | − | − | − | − | − | − | ||||
| 3. EC | 0.04 | −0.15 | − | − | − | − | − | − | − | − | − | − | − | − | ||||
| 4. Pre-DS | −0.09 | −0.03 | 0.10 | − | − | − | − | − | − | − | − | − | − | − | ||||
| 5. Post-DS | −0.11 | 0.02 | −0.02 | 0.53 *** | − | − | − | − | − | − | − | − | − | − | ||||
| 6. Pre-CB | −0.11 | 0.06 | −0.10 | 0.08 | 0.11 | − | − | − | − | − | − | − | − | − | ||||
| 7. Post-CB | −0.28 | 0.05 | 0.07 | 0.12 | 0.18 * | 0.49 *** | − | − | − | − | − | − | − | − | ||||
| 8. Pre-DCCS | 0.07 | −0.08 | −0.02 | 0.09 | 0.03 | 0.19 * | 0.18 * | − | − | − | − | − | − | − | ||||
| 9. Post-DCCS | 0.08 | −0.12 | 0.04 | 0.06 | 0.00 | 0.17 * | 0.08 | 0.43 *** | − | − | − | − | − | − | ||||
| 10. Pre-Corti | 0.07 | 0.13 | 0.01 | 0.15 | 0.11 | 0.02 | 0.01 | 0.11 | 0.00 | − | − | − | − | |||||
| 11. Post-Corti | −0.04 | 0.05 | 0.05 | 0.14 | 0.07 | −0.01 | −0.01 | 0.10 | 0.02 | 0.76 *** | − | − | − | − | ||||
| 12. SDNN | −0.04 | −0.07 | 0.05 | 0.07 | 0.11 | −0.05 | 0.03 | 0.00 | −0.14 | 0.18 * | 0.04 | |||||||
| 13. NN50 | 0.03 | −0.13 | 0.07 | 0.09 | 0.07 | −0.14 | −0.08 | −0.13 | −0.09 | 0.06 | −0.00 | 0.70 *** | ||||||
| 14. pNN50 | 0.04 | −0.01 | 0.11 | 0.06 | 0.03 | −0.08 | −0.03 | −0.13 | −0.05 | 0.13 | 0.04 | 0.73 *** | 0.82 *** | |||||
| 15. RMSSD | −0.03 | −0.04 | 0.05 | 0.05 | 0.09 | −0.04 | 0.04 | 0.01 | −0.13 | 0.19 | 0.04 | 0.99 *** | 0.65 *** | 0.73 *** | ||||
| 16. FE | 0.03 | −0.08 | −0.04 | 0.09 | −0.03 | 0.04 | 0.01 | 0.04 | 0.05 | 0.07 | 0.10 | 0.14 | 0.09 | 0.06 | 0.12 | − | − | − |
| 17. ME | 0.06 | 0.04 | −0.08 | 0.16 | 0.13 | −0.01 | 0.02 | −0.02 | 0.01 | 0.11 | 0.08 | 0.00 | −0.04 | −0.02 | 0.00 | 0.42 *** | − | − |
| 18. Income | −0.05 | −0.11 | 0.01 | 0.17 * | 0.16+ | −0.01 | 0.11 | −0.01 | −0.00 | 0.25 ** | 0.14 | 0.05 | 0.01 | 0.02 | 0.04 | 0.26 ** | 0.29 *** | − |
| 19. Child sex | −0.00 | 0.00 | 0.03 *** | 0.00 | 0.01 | −0.02 | 0.12 | −0.06 | 0.11 | 0.11 | 0.03 | 0.12 | 0.08 | 0.21 * | 0.13 | 0.04 | 0.09 | 0.20 * |
| N | M | SD | Min | Max | |
|---|---|---|---|---|---|
| Temperament | |||||
| Surgency | 141 | 4.15 | 0.75 | 2.33 | 6.42 |
| Negative emotionality | 141 | 3.86 | 0.67 | 2.00 | 5.67 |
| Effortful control | 141 | 5.54 | 0.66 | 3.25 | 6.92 |
| Executive Functions | |||||
| Digit span (pre) | 141 | 3.50 | 0.95 | 0 | 6 |
| Digit span (post) | 141 | 3.67 | 0.97 | 2 | 7 |
| Corsi block (pre) | 141 | 3.18 | 1.32 | 0 | 6 |
| Corsi block (post) | 141 | 3.06 | 1.43 | 0 | 7 |
| DCCS (pre) | 141 | 28.68 | 3.92 | 15.00 | 36.00 |
| DCCS (post) | 141 | 29.74 | 3.66 | 20.00 | 36.00 |
| Room Size | Window View | ||||
|---|---|---|---|---|---|
| Large (n = 34) | Small (n = 35) | Nature (n = 34) | Built (n = 38) | ||
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Digit Span | Pre | 3.67 (0.68) | 3.57 (0.88) | 3.50 (1.05) | 3.26 (1.08) |
| Post | 3.79 (0.97) | 3.65 (0.93) | 3.76 (1.13) | 3.50 (0.86) | |
| Corsi block | Pre | 3.12 (1.45) | 3.66 (1.13) | 3.18 (1.35) | 2.79 (1.21) |
| Post | 3.29 (1.40) | 3.14 (1.42) | 3.14 (1.37) | 2.71 (1.50) | |
| DCCS | Pre | 28.85 (3.70) | 29.57 (4.05) | 28.26 (4.26) | 28.08 (3.67) |
| Post | 30.68 (3.51) | 29.26 (3.57) | 29.76 (3.54) | 29.32 (3.97) | |
| Cortisol | Pre | 7.19 (3.55) | 6.47 (2.77) | 6.49 (2.83) | 5.83 (2.48) |
| Post | 6.21 (2.45) | 5.77 (2.01) | 5.81 (2.32) | 5.55 (3.01) | |
| HRV | SDNN (ms) | 127.78 | 95.21 | 98.63 | 99.75 |
| NN50 (ms) | 39.88 | 31.55 | 34.17 | 30.62 | |
| pNN50 (%) | 0.19 | 0.16 | 0.17 | 0.16 | |
| RMSSD (ms) | 164.62 | 121.09 | 124.40 | 120.73 | |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | ηp2 |
|---|---|---|---|---|---|---|
| Dependent variable: Post-digit span | ||||||
| Model | 9.46 | 2 | 4.73 | 5.97 | 0.00 ** | 0.15 |
| Classroom size | 0.13 | 1 | 0.13 | 0.17 | 0.68 | 0.00 |
| Pre-digit span | 9.13 | 1 | 9.14 | 11.53 | 0.00 ** | 0.15 |
| Residual | 52.31 | 66 | 0.79 | |||
| Total | 61.77 | 68 | 0.91 | |||
| Dependent variable: Post-Corsi block | ||||||
| Model | 33.25 | 2 | 16.63 | 9.92 | 0.00 *** | 0.23 |
| Classroom size | 4.55 | 1 | 4.55 | 2.71 | 0.10 † | 0.04 |
| Pre-Corsi block | 32.28 | 1 | 32.28 | 19.25 | 0.00 *** | 0.23 |
| Residual | 110.66 | 66 | 1.68 | |||
| Total | 143.91 | 68 | 2.12 | |||
| Dependent variable: Post-DCCS | ||||||
| Model | 157.49 | 2 | 78.74 | 7.26 | 0.00 ** | 0.18 |
| Classroom size | 47.66 | 1 | 47.66 | 4.40 | 0.04 * | 0.06 |
| Pre-DCCS | 122.74 | 1 | 122.74 | 11.32 | 0.00 ** | 0.15 |
| Residual | 715.38 | 66 | 10.84 | |||
| Total | 872.87 | 68 | 12.84 | |||
| Dependent variable: Post-cortisol | ||||||
| Model | 220.32 | 2 | 110.16 | 61.21 | 0.00 ** | 0.65 |
| Classroom size | 0.014 | 1 | 0.01 | 0.01 | 0.93 | 0.00 |
| Pre-cortisol | 217.08 | 1 | 217.08 | 120.62 | 0.00 *** | 0.65 |
| Residual | 118.78 | 66 | 1.80 | |||
| Total | 339.10 | 68 | 4.99 | |||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | ηp2 |
|---|---|---|---|---|---|---|
| Dependent variable: Post-digit span | ||||||
| Model | 28.92 | 2 | 14.46 | 23.77 | 0.00 *** | 0.41 |
| Window view | 0.28 | 1 | 0.28 | 0.46 | 0.50 | 0.01 |
| Pre-digit span | 27.66 | 1 | 27.66 | 45.48 | 0.00 *** | 0.40 |
| Residual | 41.96 | 69 | 0.61 | |||
| Total | 70.88 | 71 | 1.00 | |||
| Dependent variable: Post-Corsi block | ||||||
| Model | 40.97 | 2 | 20.49 | 13.03 | 0.00 *** | 0.27 |
| Window view | 0.82 | 1 | 0.82 | 0.52 | 0.47 | 0.01 |
| Pre-Corsi block | 37.57 | 1 | 37.57 | 23.89 | 0.00 *** | 0.26 |
| Residual | 108.51 | 69 | 1.57 | |||
| Total | 149.50 | 71 | 2.11 | |||
| Dependent variable: Post-DCCS | ||||||
| Model | 245.47 | 2 | 122.73 | 11.22 | 0.00 *** | 0.25 |
| Window view | 2.35 | 1 | 2.35 | 0.21 | 0.64 | 0.00 |
| Pre-DCCS | 241.85 | 1 | 241.85 | 22.12 | 0.00 *** | 0.24 |
| Residual | 754.48 | 69 | 10.93 | |||
| Total | 999.94 | 71 | 14.08 | |||
| Dependent variable: Post-cortisol | ||||||
| Model | 284.73 | 2 | 142.36 | 43.09 | 0.00 *** | 0.66 |
| Window view | 1.04 | 1 | 1.04 | 0.32 | 0.58 | 0.08 |
| Pre-cortisol | 283.52 | 1 | 283.52 | 85.81 | 0.00 *** | 0.66 |
| Residual | 227.98 | 69 | 3.30 | |||
| Total | 512.71 | 71 | 7.22 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, K. The Influence of Classroom Size and Window View on Young Children’s Executive Functions and Physiological Responses, Based on VR Technology. Behav. Sci. 2023, 13, 936. https://doi.org/10.3390/bs13110936
Cha K. The Influence of Classroom Size and Window View on Young Children’s Executive Functions and Physiological Responses, Based on VR Technology. Behavioral Sciences. 2023; 13(11):936. https://doi.org/10.3390/bs13110936
Chicago/Turabian StyleCha, Kijoo. 2023. "The Influence of Classroom Size and Window View on Young Children’s Executive Functions and Physiological Responses, Based on VR Technology" Behavioral Sciences 13, no. 11: 936. https://doi.org/10.3390/bs13110936
APA StyleCha, K. (2023). The Influence of Classroom Size and Window View on Young Children’s Executive Functions and Physiological Responses, Based on VR Technology. Behavioral Sciences, 13(11), 936. https://doi.org/10.3390/bs13110936




