1. Introduction
Sleep disturbances are commonly reported in children with developmental disorders. Poor sleep habits may trigger impairments in cognitive and behavioural functioning [
1,
2], including lower academic performance [
3], reduced attentional capacities (e.g., [
4]), and poor executive functioning [
5]. A suboptimal quality of sleep has also been associated with adverse and challenging anti-social behaviours [
6], such as aggression, tantrums, non-compliance, and impulsivity [
2,
7,
8]. Sleep disturbances have been found to be predictors of a reduced quality of life, related to stress, depression, and overall family functioning [
9,
10]. In childhood, a reduced sleep duration has been associated with developmental delays in language acquisition and consolidation [
11,
12]. Sleep disturbances have also been reported in infants with neurodevelopmental disabilities such as Williams Syndrome [
13], which raises the importance of early identification and intervention during development, as these could mitigate against the cognitive and behavioural consequences of poor sleep.
Williams Syndrome (WS) is a rare neurodevelopmental disorder affecting approximately 1 in 20,000 individuals in the United Kingdom. WS is equally prevalent in male and female populations, and is not specific to ethnicity [
14]. The disorder is caused by a micro-deletion of approximately 26–28 genes from the long arm of chromosome 7 at the point q11.23 [
14]. Sleep concerns have been reported in infants with WS [
15]. In early childhood, children with WS have been found to display long sleep latencies, reduced sleep efficiency and increased night wakings [
16,
17,
18,
19], frequent bed wetting and sleep anxiety [
16,
17], and movements during sleep [
20]. Moreover, a few studies have reported an atypical sleep architecture, comprised of reduced rapid eye movement (REM) and amplified slow-wave sleep (SWS) stages [
21,
22]. Additionally, associations between elevated bedtime cortisol, a sleep-related hormone, and sleep onset have been reported in children with WS [
23].
Cortisol has an established 24-h circadian rhythm with peak levels in the morning, particularly up to around thirty minutes after waking, before its concentration decreases throughout the day, with the lowest level being observed before night-time [
24,
25]. It has been argued that there is a bi-directional relationship between sleep deprivation and the secretion of cortisol, where poorer sleep is often associated with higher cortisol levels [
24,
26,
27]. However, several recent studies have shown a rather different pattern. For instance, [
28] observed a flattened curve in individuals with sickle-cell disease, which was associated with deficits in neurocognitive profiles that were moderated by sleep quality [
28]. In another study, mothers of autistic children who exhibited a lack of diurnal variation suffered from sleep disturbances and high depression scores [
9].
The present study aimed to examine the relationships between sleep patterns, anxiety, and cortisol levels in adolescents with WS compared to TD healthy adolescents. We hypothesised that (1) adolescents with WS would show a different cortisol profile than the age-matched TD controls, and (2) the cortisol profiles would be related to sleep parameters and anxiety scores.
4. Discussion
Adolescence is considered a sensitive period, during which some of the largest sleep disruptions occur (e.g., [
31,
32]). This “perfect storm” has been related to pubertal changes, alterations in the circadian rhythm (including later bed- and wake-times), and changes in social functioning [
33]. The current study supported the notion that problematic sleep is a general characteristic of adolescence, as most participants (82%, n = 11 TD; n = 10 WS) were categorised as having sleep disturbances. However, unlike TD adolescents, who restricted their sleep duration, the range of sleep problems was varied and persistent across developmental stages in individuals with WS. Actigraphy data showed that adolescents with WS spent a longer time in bed and experienced more sleep, albeit marginally, compared to their TD peers. The National Sleep Foundation recommendations suggest that adolescents should optimally receive 8–10 h of sleep per night [
34]. However, the actual mean duration of sleep was 7:36 for the WS group and 7:05 for the TD group.
The sleep profiles obtained from the CSHQ and actigraphy suggested that both groups experienced disturbed sleep. Similar to previous studies, the actigraphy data and the parental report (CSHQ) indicated discordance (see [
17,
35]). The findings and effect sizes of the CSHQ and actigraphy data suggested that it is suitable for the two measures to be used in conjunction with each other in young populations. Used together, the instruments can provide more holistic information on different aspects of sleep health profiles, which could be informative for healthcare professionals in the sleep management of patients.
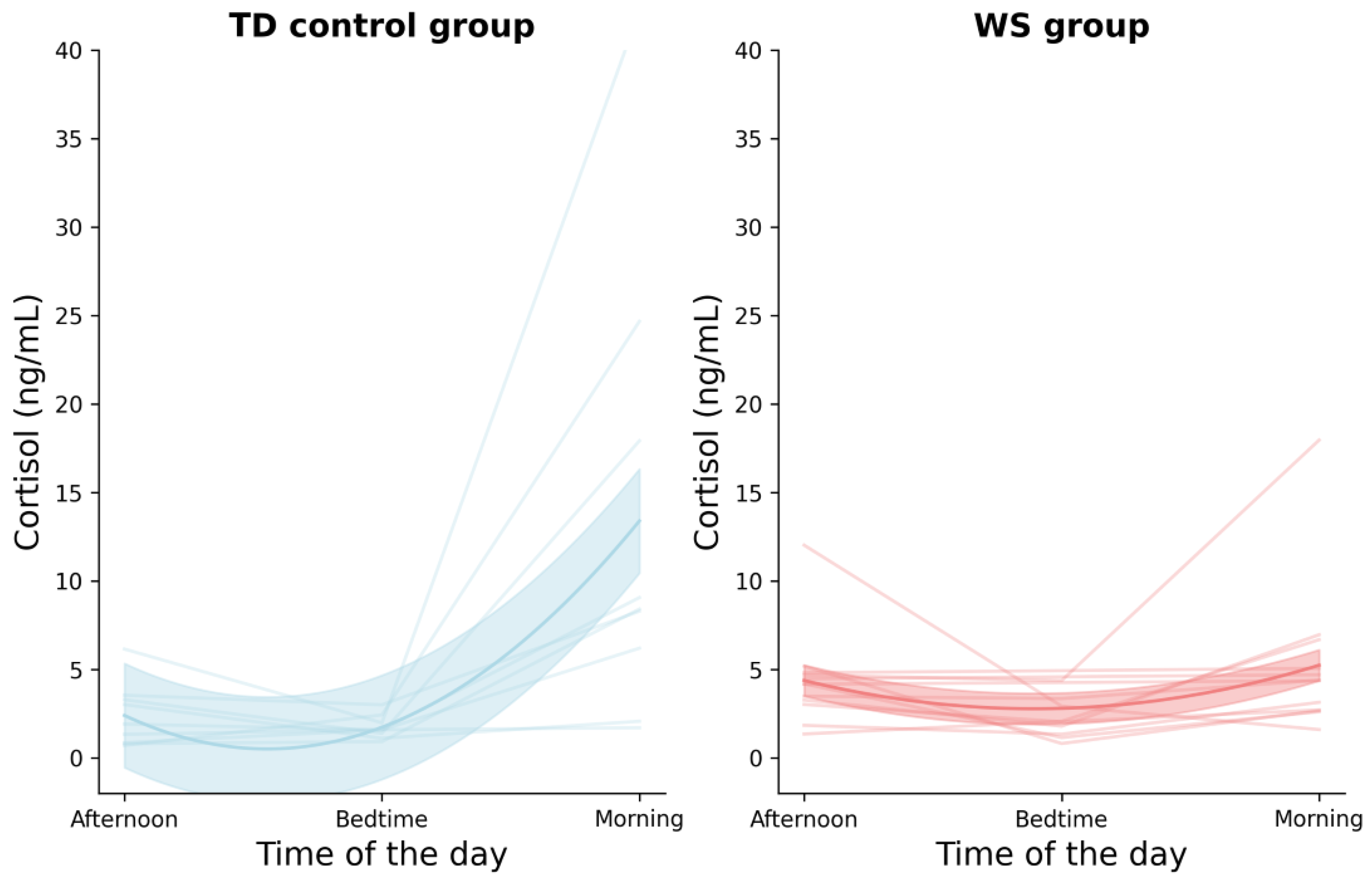
Previous work has indicated that sleep-related problems in adolescence are closely associated with elevated anxiety levels [
36], and this relationship was explored in the context of cortisol in the current study. Compared to the TD group, the WS group showed a lack of diurnal cortisol variation, with similar concentrations observed across the three time points. This pattern was in line with recent studies (e.g., [
28]), which similarly revealed that individuals with sickle-cell disease showed a flattened diurnal cortisol curve. It is important to note that the literature currently lacks a sufficient understanding of the optimal cortisol secretion within this developmental period. To date, researchers are still not cognizant of how the different medical conditions in individuals with WS might impact cortisol secretion and interact with the sleep/wake cycle.
Significant results were reported in the WS group relative to the total anxiety score, including the subscales of general anxiety, separation anxiety, and panic and aggravation. These findings suggested that adolescents with WS experienced higher total anxiety compared to their TD peers, which could explain the larger effect sizes in the sleep anxiety dimension of the CSHQ. This result concurred with the established literature, according to which elevated general anxiety is considered a persistent behavioural phenotype in WS [
37,
38,
39,
40], as is separation anxiety [
41] and panic-related anxiety [
42,
43,
44]. Non-significant findings were reported in the following anxiety subscales: physical injury, obsessive–compulsive disorder, and social anxiety. Arguably, this could be due to the pro-social behaviour exhibited by adolescents with WS. Further, the effect sizes indicated trends in terms of variance, which suggested that a larger sample size could have potentially yielded a statistically significant result. Though the prevalence of anxiety in individuals with WS exceeded that in TD individuals, its persistence over time is relatively stable compared to TD adolescents (who have been noted to experience remission within the first 12 months of diagnosis [
40,
45]). Chronic anxiety in individuals with WS might be associated with the lack of variation in cortisol secretion, though caution needs to be exercised here, as this finding ought to be examined further.
The findings presented here were based on a cross-sectional design with a small sample size of participants at the adolescent stage of life only. In the continued search for the possible mechanisms involved in the interaction between sleep, anxiety, and cortisol, future studies should employ either a longitudinal approach to examine individuals with WS across the lifespan, or adopt a larger collaborative international study that could buttress the sample size. Finally, future research could further examine the interplay and associations between cortisol, actigraphy, sleep disturbances, and anxiety in WS populations.