1. Introduction
Stressful life events can trigger a series of processes within the central nervous system that result in the fight-or-flight-response and cortisol secretion [
1]. The increase in cortisol secretion is known to suppress the immune system, alter the catabolic state, and increase energy [
2]. The number of hormones secreted during a stress response depends on the severity of the stressful experience; however, there is a consistent correlation between the activation of the catecholamines, cortisol, and stress [
1]. Research has shown that stress experienced long before learning can impair memory formation, whereas stress experienced shortly before or after the new information is presented can enhance memory performance [
3].
Electroencephalography (EEG) is a noninvasive method for measuring electrical activity in the cortex of the brain. Because of this capacity, the EEG has become a powerful tool to predict and assess comfort levels, epilepsy, and depression [
4,
5,
6]. Additionally, previous studies have shown alterations in the frequency of EEG activity during stress [
7,
8,
9]. In the week before examinations, medical students were found to have increases in the frequency of their beta activity when compared to their baseline EEGs [
9]. Another study found that when students took an exam while under pressure, there were decreases in frontal theta and alpha activity [
8]. Hewig et al. [
7] found that increases in cortisol after waking were correlated with increased alpha activity in the parietal lobe 6 weeks before the students’ exam period started and increased alpha activity in the left parietal lobe at the end of their exam period.
Cortisol is a steroid hormone, more specifically, a glucocorticoid, produced by the zona fasciculate of the adrenal gland [
2]. Individual differences can be found in all tasks; some individuals may produce high levels or low levels of cortisol, and others may not respond to stressors at all [
10]. Cortisol is the main glucocorticoid hormone, secreted by the adrenal glands in response to stress [
11]. Administration of cortisol has been found to increase right frontal alpha activity [
12] and increase the correlation between delta and beta wave activity [
13].
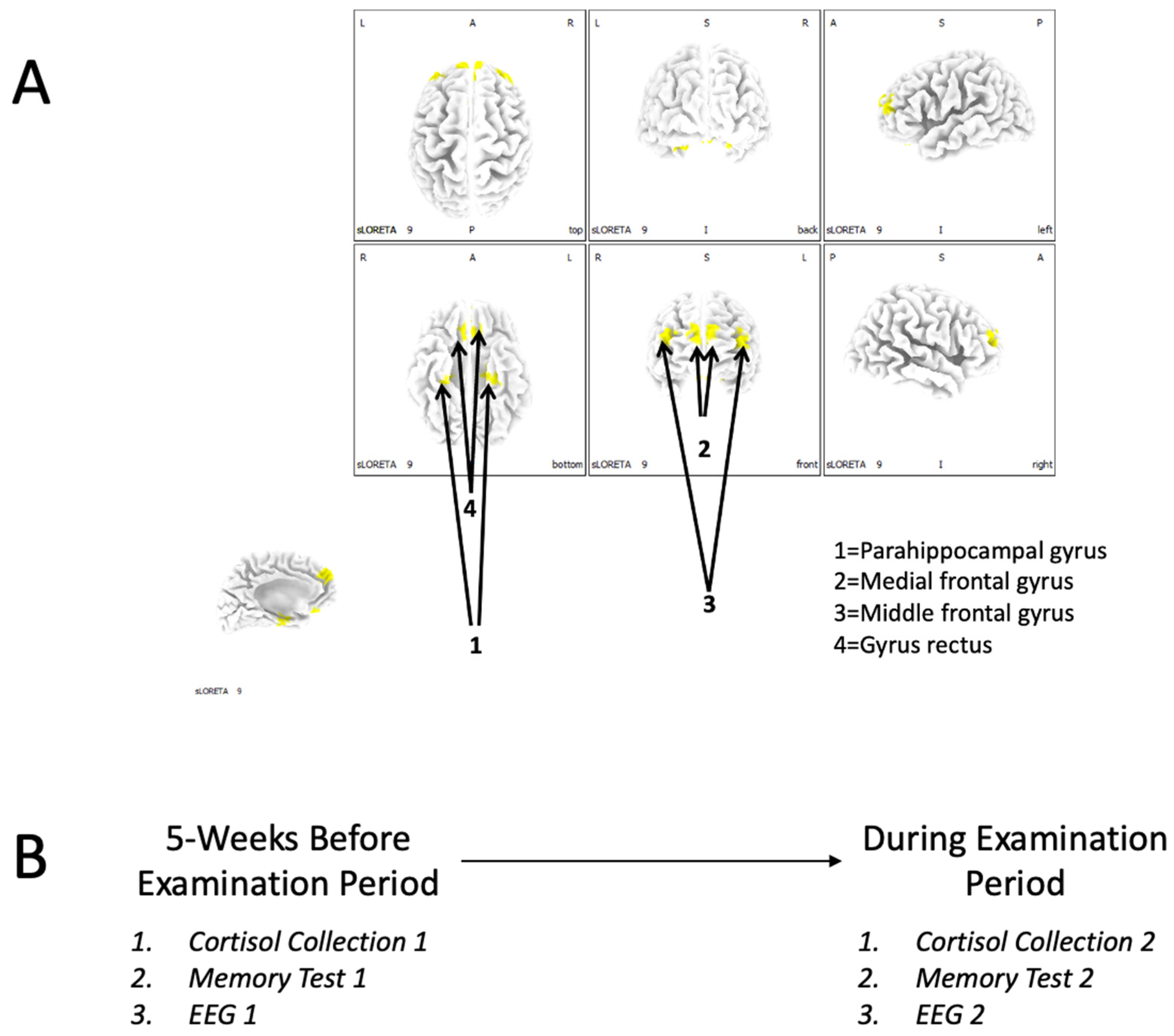
The aim of this study was to investigate whether an increase in cortisol levels was associated with impaired working-memory retrieval and recognition in students. This within-subject experiment measured the concentration of cortisol and electroencephalographic profiles at the beginning of the semester and again during an examination period. Our regions of interest (ROIs) were the parahippocampal gyrus, the medial frontal gyrus, and the middle frontal gyrus.
3. Results
On average, participants scored 75.7% on the memory task during the first trial (nonexam period) and 63.1% during the second trial (exam period). For those who completed both trials (N = 18), a paired-samples t-test indicated that there was a significant (t = 2.96, p < 0.05) decrease in the performance of the task when participants completed the test closer to their examination.
In order to further identify the structures that may be involved in this decreased performance, a measure of examination proximity was computed by calculating the difference in the number of days between the EEG measurement and the student’s examination. Data were then recoded into a variable (exam time) consisting of two groups: already completed examination (
N = 3) or to-complete examination (
N = 11). Separate repeated-measures general linear model analyses were completed for each of the 1 Hz frequency bins, using one between-levels (exam time) and three within-level interactions (region, hemisphere, and trial) and using the current source densities of the ROIs. The results of the analysis indicated a significant region by trial interaction (
F = 3.6,
p < 0.05, partial eta2 = 0.23) only within the 19–20 Hz band. Post hoc paired t-tests revealed that the source of the interaction was an increase in the 19–20 Hz current source density (uA/mm2) within the bilateral medial frontal gyri around the time of the examination period (
Figure 2).
We then used this 19–20 Hz band as a proxy to conduct ancillary exploratory analyses, given that this was the only bin to demonstrate any statistically significant effects. To explore the effects within the 19–20 Hz band, we calculated a measure of the difference in performance in the memory task by taking the absolute difference between each participant’s scores during trial 1 and trial 2. We also calculated the difference (trial 2–trial 1) in the current source density for each of the four regions within the 19–20 Hz band; these differences were completed on the averaged left/right hemispheric activity, hence bilaterally representing the activity within each respective region. This score thus represents the magnitude of the difference in performance between the two trials. Nonparametric Spearman rank correlations were then completed between the magnitude of the participant’s performance difference between the two trials and the difference scores of brain activity between the two trials. This indicated that the magnitude of the difference in memory performance was significantly (Rho = −0.54,
p < 0.05) and negatively correlated, with a 19–20 Hz current source density within the middle frontal gyrus (
Figure 3).
To discern if any of the regions selected for in the analysis were sensitive to the proximity examination within the 19–20 Hz band, we completed a series of Spearman rank correlations between the “time-to-examination” and current source densities within the various regions extracted during the sLORETA analysis. For this analysis, negative scores indicate that the exam was written X days before, 0 indicates that the participant was to write the examination on the day of trial 2, and positive values indicate how many days remain until the midterm examination. The results of the analysis indicated that the only significant relationship to emerge was a positive correlation between time-to-examination and CSD within the right parahippocampal gyrus (
Figure 4B). The results of these analyses were confirmed using a repeated-measures GLM, with time as a between-levels interaction and region and hemisphere as within-level. The results indicated a significant three-way interaction (
F = 3.85,
p < 0.05, partial eta2 = 0.24) between testing day, hemisphere, and region. The results of the post hoc paired t-tests indicated that the right parahippocampal gyrus current source density was higher for individuals who had not yet completed their examinations (
Figure 4A).
To determine if cortisol levels were affected by temporal proximity to a midterm examination, a paired t-test was completed. The results of the analysis indicated that there was no significant difference (p > 0.05) in cortisol levels, which suggests that cortisol was not altered during the midterm examination period. To investigate the relationships between memory, cortisol levels, and neuroelectric activity, all data were consolidated into one database consisting of each repeated measure as its own case. Because of the exploratory nature of this component of the analysis, we controlled for outliers by log transforming the ROI data; we have found this approach to be useful in exploratory analyses in minimizing spurious linear correlations driven by extreme cases. After the amalgamation of all available memory and cortisol data, there was a total of 27 valid cases.
Serial nonparametric correlational analyses were completed between memory scores and activity within each of the various regions extracted during the ROI analysis. The most consistent and strongest relationships found were between memory and low-frequency activity inferred from the left parahippocampal gyrus.
Figure 5 displays a plot of the effect size for each bivariate correlation computed across each of the 45 frequency bins (0–45 Hz) for each region of interest. Because of the most prominent peaks within the 5–9 Hz band (
Figure 5), it was assumed that parahippocampal activity within this band shared a common source of variance. Hence, a band between 5 and 9 Hz was constructed for the left parahippocampal region by averaging the spectral densities into one variable.
Three multiple regressions were completed separately to obtain residual scores for memory, 5–9 Hz activity within the left parahippocampal region, and cortisol levels after controlling for the time that each measure was taken (long before exam = 1, shortly before exam = 2); this allowed us to examine the relationship between these three variables, irrespective of the participant’s proximity to exams. A series of nonparametric correlations were then completed (
Figure 6). The results indicated that (1) cortisol levels were negatively correlated with memory performance (Rho = −0.48), (2) 5–9 Hz parahippocampal activity was positively correlated with memory performance (Rho = 0.54,
p < 0.05), and (3) that 5–9 Hz parahippocampal activity was negatively correlated with cortisol levels. Stated alternatively, the results suggest that increasing cortisol levels are associated with lower performance on a memory task, which may or may not be dependent on 5–9 Hz parahippocampal activity in the left hemisphere.
4. Discussion
Optimized stress levels can increase an individual’s performance; however, extreme levels of stress can deteriorate it. The main goal of this research was to demonstrate a direct relationship between poor test scores, stress, and specific brain areas. Here, we demonstrated a relationship between memory performance, activity in the parahippocampal gyrus, and cortisol levels. Furthermore, we found structures, including the parahippocampal gyrus, the medial frontal gyrus, and the middle frontal gyrus, which were significantly influenced by the presence of an exam or the proximity of an exam.
Memory processing is a main function of the frontal lobe. In this experiment, a specific interest was found in the middle frontal gyrus—an area important for working memory [
17]. When the process fails, working memory can be impaired, including not only the storage of information but also the simultaneous retrieval and processing of information in a capacity-limited store or computational workspace [
18]. Those who had a greater difference in memory performance between the first and second trials displayed less brain activity in the middle frontal gyrus. This suggests that, when stressed, in order to perform as well as when one is relaxed, the brain, specifically the middle frontal gyrus, produces heighted activity to maintain those same results. Due to its role in working memory, these results are sensible.
Although there was no significant interaction between cortisol levels and proximity to midterm examination, a relationship between memory, cortisol levels, and neuroelectric activity was found. Data obtained showed a positive correlation between the parahippocampal gyrus and memory performance, demonstrating that when activity in the parahippocampal gyrus increased, a concurrent increase in memory scores was noted. Corroboratively, the literature indicates that it has many connections with brain regions that are known to be essential in memory processes (e.g., the hippocampus); thus, one of its main roles is memory formation [
19]. This being said, it is not necessarily important for recollecting past memories, but it is important for new learning [
20].
Predictably, negative correlations between cortisol levels and the parahippocampal gyrus activity, as well as between cortisol and memory performance, were observed. Stated another way, when cortisol levels were high, memory scores and parahippocampal gyrus activity were low. These results are consistent with the data found in the literature. Memory consolidation initially depends on the hippocampus, but the storage, retrieval, and more permanent memory development are distributed in regions of the neocortex, which is in part found in the parahippocampal gyrus [
20]. Thus, the parahippocampal gyrus has a very important role in memory retrieval, and cortisol is discharged by a stress response. When cortisol is released, it disrupts the structures and impairs their capability to retrieve the information [
10].
Furthermore, the parahippocampal gyrus, specifically the right hemisphere, had a higher current source density in individuals who had not yet written their exams compared to those who did. This brain region encompasses the limbic cortex, which is part of the limbic system [
21]. The limbic system plays a vital role in behaviour and is activated during a stress response [
21]. Our results solidify the information found in the literature since those who have not yet written their exams are expected to be more stressed and anxious than individuals who have already completed their examination period. Once a stressful situation is complete (in this case an exam), individuals seemed to be more relaxed and their parahippocampal gyrus activity was lower.
In summary, the results support the involvement of the parahippocampal gyrus in stress and confirms that all three regions of interest (the parahippocampal gyrus, the middle gyrus, and the medial gyrus) have roles in memory processing. All three regions are also correlated with memory performance and exam proximity. Lastly, a further outlook on the biological aspects demonstrated that the individuals were indeed stressed when memory performance was lower. These findings provide further support for the role of these brain regions. They have also given evidence that the stress response (the release of cortisol) does have a negative effect on memory performance. With this, we can conclude that examination stress can have a negative effect on examination results as it disrupts brain areas responsible for the retrieval of memory.