Active for Life after Cancer: Association of Physical Activity with Cancer Patients’ Interpersonal Competence, Quality of Life, and Survival Beliefs
Abstract
1. Introduction
2. Literature Interview and Hypothesis Development
2.1. Physical Activity, Interpersonal Competence, and Quality of Life
2.2. The Mediating Effect
3. Methods
3.1. Participants and Procedures
- Participation by filling out the questionnaire was voluntary;
- If they felt uncomfortable during the questionnaire filling process, they could refuse to continue at any time;
- The questionnaire was anonymous;
- The data analysis would be used only for academic research;
- Personal information and data would not be shared with anyone.
3.2. Measures
3.2.1. Physical Activity
3.2.2. Interpersonal Competence
3.2.3. Quality of Life
3.2.4. Survival Beliefs
3.3. Data Analysis
4. Results
4.1. Measurement Model
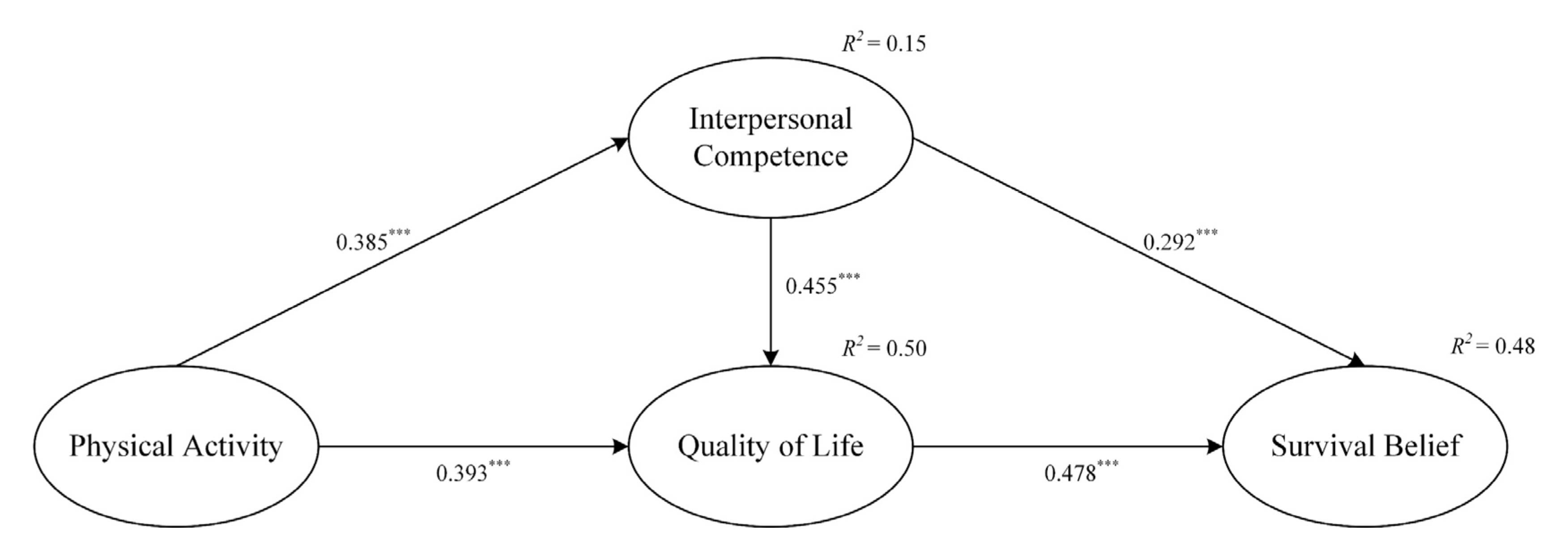
4.2. Structural Path Model
5. Discussion
5.1. Contributions
5.2. Implications
5.3. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sener, S.F.; Grey, N. The global burden of cancer. J. Surg. Oncol. 2005, 92, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Whitford, H.S.; Olver, I.N. The multidimensionality of spiritual wellbeing: Peace, meaning, and faith and their association with quality of life and coping in oncology. Psycho-Oncology 2012, 21, 602–610. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, J.-M.; Kim, S.-W.; Shin, I.-S.; Bae, K.-Y.; Shim, H.-J.; Hwang, J.-E.; Bae, W.-K.; Cho, S.-H.; Chung, I.-J.; et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psycho-Oncology 2013, 22, 2206–2213. [Google Scholar] [CrossRef]
- Morrison, E.J.; Novotny, P.J.; Sloan, J.A.; Yang, P.; Patten, C.A.; Ruddy, K.J.; Clark, M.M. Emotional problems, quality of life, and symptom burden in patients with lung cancer. Clin. Lung Cancer 2017, 18, 497–503. [Google Scholar] [CrossRef]
- Otto, A.K.; Szczesny, E.C.; Soriano, E.C.; Laurenceau, J.P.; Siegel, S.D. Effects of a randomized gratitude intervention on death-related fear of recurrence in breast cancer survivors. Health Psychol. 2016, 35, 1320–1328. [Google Scholar] [CrossRef]
- Forbes, L.J.L.; Simon, A.E.; Warburton, F.; Boniface, D.; Brain, K.E.; Dessaix, A.; Donnelly, C.; Haynes, K.; Hvidberg, L.; Lagerlund, M.; et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): Do they contribute to differences in cancer survival? Brit. J. Cancer 2013, 108, 292–300. [Google Scholar] [CrossRef]
- Biere, S.; Henegouwen, M.I.V.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.M.; et al. Minimally invasive vs. open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of radiosurgery alone vs. radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. J. Am. Med. Assoc. 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef]
- Mann, E.; Smith, M.J.; Hellier, J.; Balabanovic, J.A.; Hamed, H.; Grunfeld, E.A.; Hunter, M.S. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): A randomised controlled trial. Lancet Oncol. 2012, 13, 309–318. [Google Scholar] [CrossRef]
- Bradt, J.; Dileo, C.; Myers-Coffman, K.; Biondo, J. Music interventions for improving psychological and physical outcomes in people with cancer. Cochrane Database Syst. Rev. 2021, 10, CD006911. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public. Health Rep. 1985, 100, 126–131. [Google Scholar]
- Harridge, S.D.R.; Lazarus, N.R. Physical Activity, Aging, and Physiological Function. Physiology 2017, 32, 152–161. [Google Scholar] [CrossRef]
- Penedo, F.J.; Dahn, J.R. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatr. 2005, 18, 189–193. [Google Scholar] [CrossRef]
- Guicciardi, M.; Carta, M.; Pau, M.; Cocco, E. The relationships between physical activity, self-efficacy, and quality oflife in people with multiple sclerosis. Behav. Sci. 2019, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.B.; Bentele, C.N.; Grossman, H.B.; Le, Y.; Jang, H.; Steger, M.F. You, me, and meaning: An integrative review of connections between relationships and meaning in life. J. Psychol. Afr. 2014, 24, 44–50. [Google Scholar] [CrossRef]
- Chang, C.M.; Chou, Y.H.; Hsieh, H.H.; Huange, C.K. The Effect of Participation Motivations on Interpersonal Relationships and Learning Achievement of Female College Students in Sports Club: Moderating Role of Club Involvement. Int. J. Environ. Res. Public. Health 2020, 17, 6514. [Google Scholar] [CrossRef]
- Huang, J.; Du, C.; Liu, J.; Tan, G. Meta-Analysis on Intervention Effects of Physical Activities on Children and Adolescents with Autism. Int. J. Environ. Res. Public. Health 2020, 17, 1950. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Lu, T.; Tao, B.; Gao, Y.; Yan, J. The Relationship between Physical Activity and College Students’ Mobile Phone Addiction: The Chain-Based Mediating Role of Psychological Capital and Social Adaptation. Int. J. Environ. Res. Public Health 2022, 19, 9286. [Google Scholar] [CrossRef]
- Group, W. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Mori, M.; Gao, R.; Nail, L.M.; King, M.E. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med. Sci. Sports Exerc. 2001, 33, 718–723. [Google Scholar] [CrossRef]
- Segal, R.; Evans, W.; Johnson, D.; Smith, J.; Colletta, S.; Gayton, J.; Woodard, S.; Wells, G.; Reid, R. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J. Clin. Oncol. 2001, 19, 657–665. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Courneya, K.S. Exercise in cancer survivors: An overview of research. Med. Sci. Sports Exerc. 2003, 35, 1846–1852. [Google Scholar] [CrossRef]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep. Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Haskell, W.L.; Taylor, C.B.; Kraemer, H.C.; DeBusk, R.F. Group- vs. home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA 1991, 266, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Vilalta, A.; Valls, J.; Porta, J.; Viñas, J. Evaluation of spiritual needs of patients with advanced cancer in a palliative care unit. J. Palliat. Med. 2014, 17, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, M.; Khoshknab, M.F.; Maddah, S.S.; Ahmadi, F. Iranian cancer patients’ perception of spirituality: A qualitative content analysis study. BMC Nurs. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Demiralay, C.; Huber, C.G. Influence of religious aspects and personal beliefs on psychological behavior: Focus on anxiety disorders. Psychol. Res. Behav. Manag. 2014, 7, 93–101. [Google Scholar] [CrossRef]
- Torabi, F.; Rassouli, M.; Nourian, M.; Borumandnia, N.; Shirinabadi Farahani, A.; Nikseresht, F. The Effect of Spiritual Care on Adolescents Coping With Cancer. Holist. Nurs. Pract. 2018, 32, 149–159. [Google Scholar] [CrossRef]
- Hampton, D.M.; Hollis, D.E.; Lloyd, D.A.; Taylor, J.; McMillan, S.C. Spiritual needs of persons with advanced cancer. Am. J. Hosp. Palliat. Care 2007, 24, 42–48. [Google Scholar] [CrossRef]
- Zeighamy, H.; Sadeghi, N. Spiritual/Religious Needs of Adolescents with Cancer. Religions 2016, 7, 91. [Google Scholar] [CrossRef]
- Cella, D.F. Quality of life: Concepts and definition. J. Pain. Symptom. Manag. 1994, 9, 186–192. [Google Scholar] [CrossRef]
- Hurny, C.; Bernhard, J. Lessons learned from measuring health-related quality of life in oncology. J. Clin. Oncol. 1994, 12, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ho, K.-Y.; Lam, K.-K.-W.; Lam, W.-Y.-Y.; Cheng, E.-H.-L.; Ching, S.-S.-Y.; Wong, F.-K.-Y. A Descriptive and Phenomenological Exploration of the Spiritual Needs of Chinese Children Hospitalized with Cancer. Int. J. Environ. Res. Public. Health 2022, 19, 13217. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.S.; Lee, J.W.; Phillips, L.R.; Zhang, X.E.; Jaceldo, K.B. An adaptation of Brislin’s translation model for cross-cultural research. Nurs. Res. 2001, 50, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, C.L.; Winterstein, A.G. Validity and reliability of measurement instruments used in research. Am. J. Health Syst. Pharm. 2008, 65, 2276–2284. [Google Scholar] [CrossRef]
- Fornell, C.; Larcker, D.F. Evaluating structuralequation models with unobservable variables and measurement error. J. Marketing Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Andersen, L.G.; Groenvold, M.; Jorgensen, T.; Aadahl, M. Construct validity of a revised Physical Activity Scale and testing by cognitive interviewing. Scand. J. Public. Health 2010, 38, 707–714. [Google Scholar] [CrossRef]
- Buhrmester, D.; Furman, W.; Wittenberg, M.T.; Reis, H.T. Five domains of interpersonal competence in peer relationships. J. Pers. Soc. Psychol. 1988, 55, 991–1008. [Google Scholar] [CrossRef]
- Group, T.W. Development of the world health organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Linehan, M.M.; Goodstein, J.L.; Nielsen, S.L.; Chiles, J.A. Reasons for staying alive when you are thinking of killing yourself: The Reasons for Living Inventory. J. Consult. Clin. Psych. 1983, 51, 276–286. [Google Scholar] [CrossRef]
- Hair, J.F.; Sarstedt, M.; Ringle, C.M.; Mena, J.A. An assessment of the use of partial least squares structural equation modeling in marketing research. J. Acad. Market Sci. 2011, 40, 414–433. [Google Scholar] [CrossRef]
- Anderson, J.C.; Gerbing, D.W. Structural equation modeling in practice: A review and recommended two-step approach. Psychol. Bull. 1988, 103, 411–423. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis: A Global Perspective; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Bollen, K.A.; Stine, R. Direct and indirect effects: Classical and bootstrap estimates of variability. Sociol. Methodol. 1990, 20, 115–140. [Google Scholar] [CrossRef]
- Podsakoff, P.M.; Organ, D.W. Self-reports in organizational research: Problems and prospects. J. Manag. 1986, 12, 531–544. [Google Scholar] [CrossRef]
- Podsakoff, P.M.; MacKenzie, S.B.; Podsakoff, N.P. Sources of Method Bias in Social Science Research and Recommendations on How to Control It. Annu. Rev. Psychol. 2011, 63, 539–569. [Google Scholar] [CrossRef]
- Mossholder, K.W.; Bennett, N.; Kemery, E.R.; Wesolowski, M.A. Relationships between Bases of Power and Work Reactions: The Mediational Role of Procedural Justice. J. Manag. 1998, 24, 533–552. [Google Scholar] [CrossRef]
- Patsou, E.D.; Alexias, G.T.; Anagnostopoulos, F.G.; Karamouzis, M.V. Physical activity and sociodemographic variables related to global health, quality of life, and psychological factors in breast cancer survivors. Psychol. Res. Behav. Manag. 2018, 11, 371–381. [Google Scholar] [CrossRef]
- Nowak, P.F.; Bożek, A.; Blukacz, M. Physical Activity, Sedentary Behavior, and Quality of Life among University Students. Biomed. Res. Int. 2019, 2019, 9791281. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Lin, Y.; Liu, Y.; Xu, Z.; Yang, J. Keep Moving to Retain the Healthy Self: The Influence of Physical Exercise in Health Anxiety among Chinese Menopausal Women. Behav. Sci. 2023, 13, 140. [Google Scholar] [CrossRef]
- Scully, D.; Kremer, J.; Meade, M.M.; Graham, R.; Dudgeon, K. Physical exercise and psychological well being: A critical review. Brit J. Sport. Med. 1998, 32, 111–120. [Google Scholar] [CrossRef]
- Fossati, C.; Torre, G.; Vasta, S.; Giombini, A.; Quaranta, F.; Papalia, R.; Pigozzi, F. Physical Exercise and Mental Health: The Routes of a Reciprocal Relation. Int. J. Environ. Res. Public. Health 2021, 18, 12364. [Google Scholar] [CrossRef]
- Wu, R.; Jing, L.; Liu, Y.; Wang, H.; Yang, J. Effects of physical activity on regulatory emotional self-efficacy, resilience, and emotional intelligence of nurses during the COVID-19 pandemic. Front. Psychol. 2022, 13, 1059786. [Google Scholar] [CrossRef] [PubMed]
- Peplau, H.E. Peplau’s theory of interpersonal relations. Nurs. Sci. Quart. 1997, 10, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Buchman-Schmitt, J.M.; Stanley, I.H.; Hom, M.A.; Tucker, R.P.; Hagan, C.R.; Rogers, M.L.; Podlogar, M.C.; Chiurliza, B.; Ringer, F.B.; et al. The interpersonal theory of suicide: A systematic review and meta-analysis of a decade of cross-national research. Psychol. Bull. 2017, 143, 1313–1345. [Google Scholar] [CrossRef] [PubMed]
- Given, B.A.; Given, C.W.; Kozachik, S. Family support in advanced cancer. CA Cancer J. Clin. 2001, 51, 213–231. [Google Scholar] [CrossRef]
- Luthans, F.; Luthans, K.W.; Luthans, B.C. Positive psychological capital: Beyond human and social capital. Bus. Horiz. 2004, 47, 45–50. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]

| Variables | N | % | |
|---|---|---|---|
| Gender | Male | 139 | 55.2 |
| Female | 113 | 44.8 | |
| Age (years) | Mean: 57 | ||
| Range: 23–78 | |||
| <60 | 130 | 51.6 | |
| ≥60 | 122 | 48.4 |
| Items | Loadings | Cα | AVE | CR |
|---|---|---|---|---|
| Physical Activity (PA) | 0.791 | 0.564 | 0.795 | |
| PA1 | 0.770 | |||
| PA2 | 0.756 | |||
| PA 3 | 0.726 | |||
| Interpersonal Competence (IC) | 0.799 | 0.526 | 0.814 | |
| IC1 | 0.791 | |||
| IC2 | 0.640 | |||
| IC3 | 0.801 | |||
| IC4 | 0.653 | |||
| Quality of Life (QoL) | 0.831 | 0.626 | 0.833 | |
| QoL1 | 0.780 | |||
| QoL2 | 0.847 | |||
| QoL3 | 0.742 | |||
| Survival Beliefs (SB) | 0.910 | 0.728 | 0.914 | |
| SB1 | 0.869 | |||
| SB2 | 0.859 | |||
| SB3 | 0.918 | |||
| SB4 | 0.759 |
| Construct | PA | IC | QoL | SB |
|---|---|---|---|---|
| PA | (0.751) | |||
| IC | 0.310 ** | (0.725) | ||
| QoL | 0.473 ** | 0.499 ** | (0.791) | |
| SB | 0.368 ** | 0.520 ** | 0.590 ** | (0.853) |
| Point Estimate | Product of Coefficients | Bootstrapping | ||||||
|---|---|---|---|---|---|---|---|---|
| Percentile 95% CI | Bias-Corrected 95% CI | Two-Tailed Significance | ||||||
| SE | Z | Lower | Upper | Lower | Upper | |||
| Indirect effects | ||||||||
| PA→ QoL | 0.175 | 0.055 | 3.182 | 0.079 | 0.289 | 0.081 | 0.293 | 0.001(**) |
| PA → SB | 0.384 | 0.066 | 5.818 | 0.259 | 0.520 | 0.259 | 0.518 | 0.000(***) |
| IC → SB | 0.218 | 0.088 | 2.477 | 0.083 | 0.425 | 0.081 | 0.416 | 0.013(*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jing, L.; Liu, Y.; Wang, H.; Yuan, T.; Yang, J. Active for Life after Cancer: Association of Physical Activity with Cancer Patients’ Interpersonal Competence, Quality of Life, and Survival Beliefs. Behav. Sci. 2023, 13, 449. https://doi.org/10.3390/bs13060449
Liu Y, Jing L, Liu Y, Wang H, Yuan T, Yang J. Active for Life after Cancer: Association of Physical Activity with Cancer Patients’ Interpersonal Competence, Quality of Life, and Survival Beliefs. Behavioral Sciences. 2023; 13(6):449. https://doi.org/10.3390/bs13060449
Chicago/Turabian StyleLiu, Ying, Longjun Jing, Yang Liu, Huilin Wang, Tinggang Yuan, and Jingyu Yang. 2023. "Active for Life after Cancer: Association of Physical Activity with Cancer Patients’ Interpersonal Competence, Quality of Life, and Survival Beliefs" Behavioral Sciences 13, no. 6: 449. https://doi.org/10.3390/bs13060449
APA StyleLiu, Y., Jing, L., Liu, Y., Wang, H., Yuan, T., & Yang, J. (2023). Active for Life after Cancer: Association of Physical Activity with Cancer Patients’ Interpersonal Competence, Quality of Life, and Survival Beliefs. Behavioral Sciences, 13(6), 449. https://doi.org/10.3390/bs13060449







