Electroencephalogram-Based Subject Matching Learning (ESML): A Deep Learning Framework on Electroencephalogram-Based Biometrics and Task Identification
Abstract
:1. Introduction
- Generality—Biometric data should be generalizable to every normal individual.
- Uniqueness—Users with different identities should be distinguishable via their unique biometrics.
- Stability—It should not change over time (long-term).
- Accessibility—It should be easily accessible, easily quantifiable and its acquisition should not be harmful to the individual.
- Aliveness—EEG signals completely live with life and will disappear immediately if a subject dies.
- Stress-resistance (SR)—If a subject unwillingly accesses authentication systems under duress, this might incur a different pattern of EEG, which can potentially be detected.
- Anti-counterfeiting (AC)—Fingerprints can be found, especially when you leave them at many different systems. However, no one can obtain the brain signals of others.
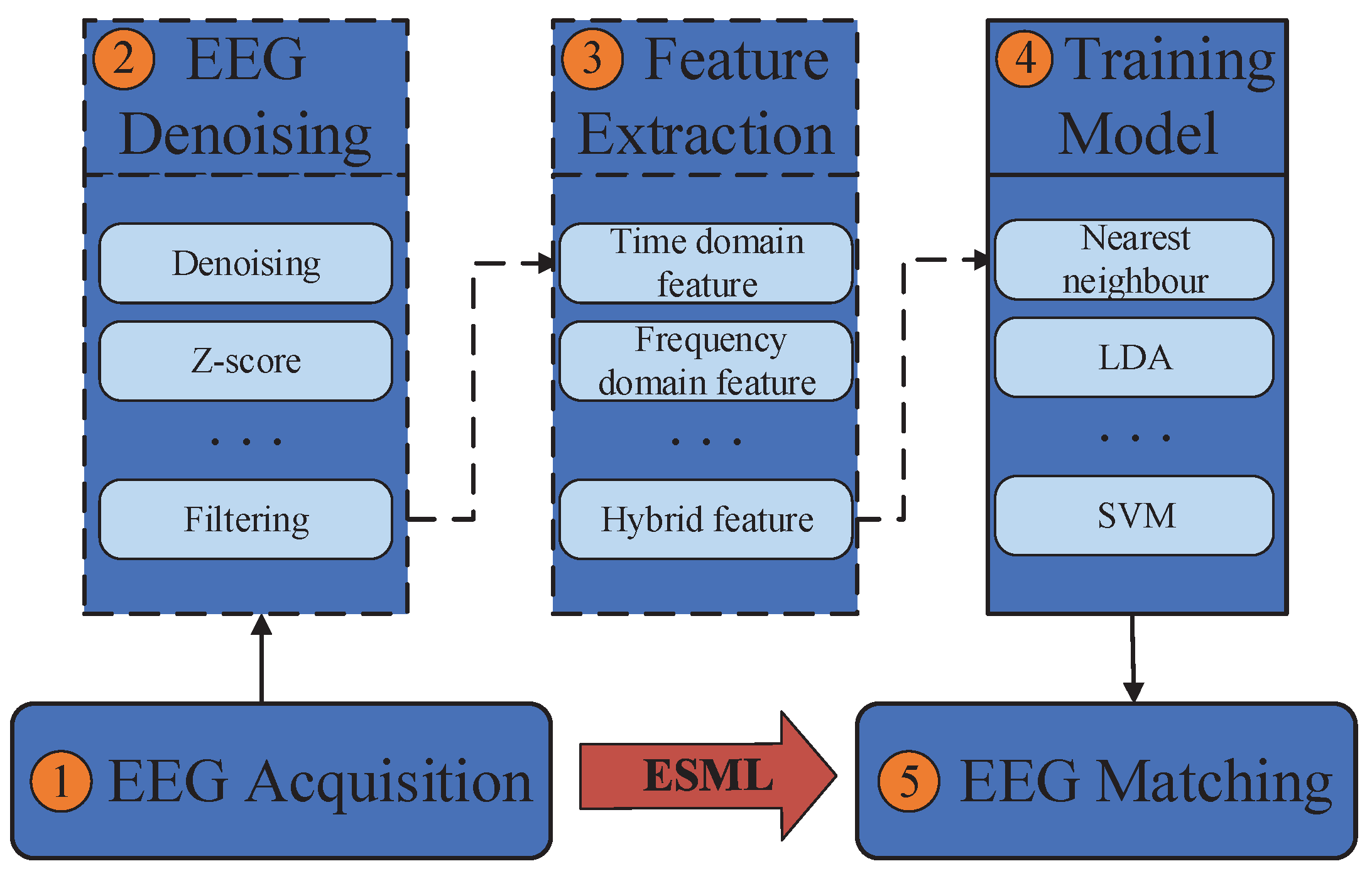
- 1.
- EEG acquisition: It can be collected by electrodes placed on the scalp surface.
- 2.
- EEG denoising: The noise in EEG signals during acquisition can be divided into eight categories: eye electrical (including blink signal), power frequency interference, EEG, electrocardiogram, electrode loosening, sweating, breathing and pulse interference. Brain electrical signal denoising technology mainly includes the use of regression analysis, adaptive filter and direct phase subtraction, principal component analysis method, independent component analysis and wavelet transformation.
- 3.
- Feature extraction: The most typical features used in EEG analysis are time and frequency, which can be obtained through many methods, such as power spectral density, wavelet transform and autoregressive model coefficients.
- 4.
- Model training: Patterns can be learned through various classification models, such as support vector machines, nearest neighbors and naive Bayes.
- 5.
- Model validation: The trained model is used for identity authentication and its performance is measured.
- We introduce a deep learning-based framework called ESML, consisting of two neural networks. is an LSTM-based method used for EEG-based user identification, while is a CNN-based method used for EEG-based task classification.
- The proposed framework is simple, effective and efficient. ESML does not require any restrictions on EEG data collection and eliminates the need for EEG preprocessing operations.
- Experiments were conducted on three public EEG datasets, achieving an accuracy of up to for the largest dataset with 109 users for EEG-user linking. Additionally, it achieved precision in 3-Class task classification and precision in the 5-Class case.
2. Related Work
3. Problem Definition
4. Proposed Framework
4.1. EEG Segmentation
4.2. EEG Characterization
4.2.1. EEG-User Linking
4.2.2. EEG-Task Linking
- Input layer: The processed EEG signal is 1D completed signal data from one channel in 1 min.
- Convolution layer: The convolutional layer tries to analyze each patch of a neural network to obtain more abstract features. ReLu is chosen as activation in the CNN part because of its simplicity and efficiency. We also add dropout operation in the last two layers in CNNs to avoid overfitting.
- Batch-norm layer: It is set up before the input of each convolution layer.
- Max-pooling layer: This operation is used to select the maximum element from the region of the feature map covered by the filter.
4.2.3. Linking
4.3. Optimization
5. Experimental Design
5.1. Datasets
- RSVP: This dataset was originally collected to explore the neural basis of target detection in the human brain, which was collected using a BIOSEMI Active View 2 system with 256 electrodes mounted on a whole-head elastic electrode cap (E-Cap Inc., Winsen, Germany) with a custom near-uniform montage across the scalp, neck and bony parts of the upper face. Computer data acquisition was performed via USB using a customized acquisition driver at a 256 Hz sampling rate with 24-bit digitization.
- Sternberg Task: The purpose of the Sternberg Task was to investigate event-related EEG dynamics through a variation of the Sternberg task. The Sternberg Task data were collected from 71 channels (69 scalp and two periocular electrodes, all referred to as right mastoid) at a sampling rate of 250 Hz with an analog passband of 0.01 to 100 Hz (SA Instrumentation, San Diego, CA, USA). Input impedances were brought under 5 k by careful scalp preparation.
- BCI2000: BCI2000 was created and contributed to PhysioNet by the developers of the BCI2000 instrumentation systems. Users performed different motor/imagery tasks while 64-channel EEGs were recorded using the BCI2000 (http://www.bci2000.org) system.
5.2. Baselines
- SVM: Bashar et al. [49] used SVM to recognize humans from test EEG signals and obtained a true positive rate of . In SVM implementation [49,50,51], the linear kernel is used for solving the EEG-based human recognition problem due to its better performance than other kernels such as RBF kernel and Gaussian kernel in our experiments.
- ConvNets: Robin et al. [3] used deep learning with convolutional neural networks for EEG decoding and visualization; their study thus shows how to design and train ConvNets to decode task-related information from raw EEG without handcrafted features and highlights the potential of deep ConvNets combined with advanced visualization techniques for EEG-based brain mapping. Convolutional Neural Networks are designed to recognize visual patterns directly from pixel images with minimal preprocessing. In machine learning, a ConvNet is a class of deep, feed-forward artificial neural networks that has successfully been applied to analyzing visual imagery.
- LDA: Isuru et al. [35] used linear discriminant analysis as a classification algorithm for their given set of user data, and the maximum accuracy recorded was . The LDA algorithm [35,52] is a generalization of Fisher’s linear discriminant, a method used in statistics, pattern recognition and machine learning to find a linear combination of features that characterizes or separates two or more classes of objects or events.
- NN: Nearest neighbor [53,54] is the optimization problem of finding the point in a given set that is closest (or most similar) to a given point. In a previous work, Lee et al. [54] used Nearest neighbor (NN) classifier to obtain time and frequency characteristics in the EEG signals and achieved an accuracy of up to for a dataset with seven users.
- DTS: Aydemir et al. proposed a decision tree structure-based method that was applied to EEG classification and achieved , and classification accuracy rates on the test data of three subjects [55]. The decision tree is a map of the possible outcomes of a series of related choices and is a type of supervised learning algorithm that is mostly used in classification problems. It works for both categorical and continuous input and output variables.
- Bayesian: Bayesian classification algorithm is a statistical classification method, which is a class of algorithms using probability and statistics knowledge classification. Yu et al. [56] demonstrated that the Bayesian method they proposed achieved a better overall performance than the computing algorithms for EEG classification.
- AdaBoost: Hu [57] used the AdaBoost algorithm to recognize EEG signals, which is an iterative algorithm. The core idea is to train different classifiers on the same training set, and then combine these weak classifiers to form a stronger final classifier.
5.2.1. EEG Denoising
5.2.2. EEG Feature Extraction
5.3. Evaluation Metrics
6. Empirical Results
6.1. EEG-User Linking
6.2. EEG-Task Linking
6.3. Further Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jirayucharoensak, S.; Pan-Ngum, S.; Israsena, P. EEG-based emotion recognition using deep learning network with principal component based covariate shift adaptation. Sci. World J. 2014, 2014, 627892. [Google Scholar] [CrossRef]
- An, X.; Kuang, D.; Guo, X.; Zhao, Y.; He, L. A deep learning method for classification of EEG data based on motor imagery. In Proceedings of the International Conference on Intelligent Computing, Taiyuan, China, 3–6 August 2014; Springer: Berlin/Heidelberg, Germany; pp. 203–210. [Google Scholar]
- Schirrmeister, R.T.; Springenberg, J.T.; Fiederer, L.D.J.; Glasstetter, M.; Eggensperger, K.; Tangermann, M.; Hutter, F.; Burgard, W.; Ball, T. Deep learning with convolutional neural networks for EEG decoding and visualization. Hum. Brain Mapp. 2017, 38, 5391–5420. [Google Scholar] [CrossRef]
- Schons, T.; Moreira, G.J.; Silva, P.H.; Coelho, V.N.; Luz, E.J. Convolutional network for EEG-based biometric. In Proceedings of the Iberoamerican Congress on Pattern Recognition, Valparaiso, Chile, 7–10 November 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 601–608. [Google Scholar]
- Mao, Z.; Yao, W.X.; Huang, Y. EEG-based biometric identification with deep learning. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER) IEEE, Shanghai, China, 25–28 May 2017; pp. 609–612. [Google Scholar]
- Petrantonakis, P.C.; Hadjileontiadis, L.J. Emotion recognition from EEG using higher order crossings. IEEE Trans. Inf. Technol. Biomed. 2009, 14, 186–197. [Google Scholar] [CrossRef]
- Min, W.; Luo, G. Medical applications of EEG wave classification. Chance 2009, 22, 14–20. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, C.H.; Jung, T.P.; Wu, T.L.; Jeng, S.K.; Duann, J.R.; Chen, J.H. EEG-based emotion recognition in music listening. IEEE Trans. Biomed. Eng. 2010, 57, 1798–1806. [Google Scholar]
- Wang, Q.; Sourina, O.; Nguyen, M.K. EEG-based “serious” games design for medical applications. In Proceedings of the 2010 International Conference on Cyberworlds, IEEE, Singapore, 20–22 October 2010; pp. 270–276. [Google Scholar]
- Soufineyestani, M.; Dowling, D.; Khan, A. Electroencephalography (EEG) technology applications and available devices. Appl. Sci. 2020, 10, 7453. [Google Scholar] [CrossRef]
- Lee, W.T.; Nisar, H.; Malik, A.S.; Yeap, K.H. A brain computer interface for smart home control. In Proceedings of the 2013 IEEE International Symposium on Consumer Electronics (ISCE), IEEE, Hsinchu City, Taiwan, 3–6 June 2013; pp. 35–36. [Google Scholar]
- Acharya, U.R.; Sree, S.V.; Swapna, G.; Martis, R.J.; Suri, J.S. Automated EEG analysis of epilepsy: A review. Knowl.-Based Syst. 2013, 45, 147–165. [Google Scholar] [CrossRef]
- Sun, H.; Fu, Y.; Xiong, X.; Yang, J.; Liu, C.; Yu, Z. Identification of EEG induced by motor imagery based on Hilbert-Huang transform. Acta Autom. Sin. 2015, 41, 1686–1692. [Google Scholar]
- Gao, Z.K.; Liu, C.Y.; Yang, Y.X.; Cai, Q.; Dang, W.D.; Du, X.L.; Jia, H.X. Multivariate weighted recurrence network analysis of EEG signals from ERP-based smart home system. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 085713. [Google Scholar] [CrossRef]
- Altenmüller, E.; Gruhn, W.; Parlitz, D.; Liebert, G. The impact of music education on brain networks: Evidence from EEG-studies. Int. J. Music. Educ. 2000, 1, 47–53. [Google Scholar] [CrossRef]
- Bensalem-Owen, M.; Chau, D.F.; Sardam, S.C.; Fahy, B.G. Education research: Evaluating the use of podcasting for residents during EEG instruction: A pilot study. Neurology 2011, 77, e42–e44. [Google Scholar] [CrossRef]
- Prauzner, T. Analysis of the results of the pedagogical research and EEG in the aspect of effective modern teaching aids in the technical education. In Proceedings of the International Scientific Conference, Madrid, Spain, 24–26 June 2015; Volume 4, pp. 480–489. [Google Scholar]
- Nascimento, F.A.; Maheshwari, A.; Chu, J.; Gavvala, J.R. EEG education in neurology residency: Background knowledge and focal challenges. Epileptic Disord. 2020, 22, 769–774. [Google Scholar] [CrossRef]
- Bodda, S.; Chandranpillai, H.; Viswam, P.; Krishna, S.; Nair, B.; Diwakar, S. Categorizing imagined right and left motor imagery BCI tasks for low-cost robotic neuroprosthesis. In Proceedings of the 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), IEEE, Chennai, India, 3–5 March 2016; pp. 3670–3673. [Google Scholar]
- Das, A.; Suresh, S.; Sundararajan, N. A discriminative subject-specific spatio-spectral filter selection approach for EEG based motor-imagery task classification. Expert Syst. Appl. 2016, 64, 375–384. [Google Scholar] [CrossRef]
- Shaari, N.; Syafiq, M.; Amin, M.; Mikami, O. Electroencephalography (EEG) application in neuromarketing-exploring the subconscious mind. J. Adv. Manuf. Technol. 2019, 13, 2. [Google Scholar]
- Lee, J.; Yang, J.H. Analysis of driver’s EEG given take-over alarm in SAE level 3 automated driving in a simulated environment. Int. J. Automot. Technol. 2020, 21, 719–728. [Google Scholar] [CrossRef]
- Hecht, T.; Feldhütter, A.; Radlmayr, J.; Nakano, Y.; Miki, Y.; Henle, C.; Bengler, K. A review of driver state monitoring systems in the context of automated driving. In Proceedings of the Congress of the International Ergonomics Association, Florence, Italy, 26–28 August 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 398–408. [Google Scholar]
- Xiong, H.; Zhang, H.; Sun, J. Attribute-Based Privacy-Preserving Data Sharing for Dynamic Groups in Cloud Computing. IEEE Syst. J. 2018, 13, 2739–2750. [Google Scholar] [CrossRef]
- Jain, A.K.; Flynn, P.; Ross, A.A. Handbook of Biometrics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Wayman, J.; Jain, A.; Maltoni, D.; Maio, D. An introduction to biometric authentication systems. In Biometric Systems: Technology, Design and Performance Evaluation; Springer: London, UK, 2005; pp. 1–20. [Google Scholar]
- Kofanova, O.A.; Mathieson, W.; Thomas, G.A.; Betsou, F. DNA fingerprinting: A quality control case study for human biospecimen authentication. Biopreserv. Biobank. 2014, 12, 151–153. [Google Scholar] [CrossRef]
- Lai, W.K.; Tan, B.G.; Soo, M.S.; Khan, I. User Identification of Keystroke Biometric Patterns with the Cognitive RAM Weightless Neural Net. In Advances in Machine Learning and Signal Processing; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–12. [Google Scholar]
- Damaševičius, R.; Maskeliūnas, R.; Venčkauskas, A.; Woźniak, M. Smartphone user identity verification using gait characteristics. Symmetry 2016, 8, 100. [Google Scholar] [CrossRef]
- Cimato, S.; Gamassi, M.; Piuri, V.; Sana, D.; Sassi, R.; Scotti, F. Personal identification and verification using multimodal biometric data. In Proceedings of the Computational Intelligence for Homeland Security and Personal Safety, IEEE, Alexandria, VA, USA, 16–17 October 2006; pp. 41–45. [Google Scholar]
- Poulos, M.; Rangoussi, M.; Alexandris, N. Neural network based person identification using EEG features. In Proceedings of the Acoustics, Speech, and Signal Processing on 1999 IEEE International Conference, IEEE, Phoenix, AZ, USA, 15–19 March 1999; Volume 2, pp. 1117–1120. [Google Scholar]
- Thorpe, J.; van Oorschot, P.C.; Somayaji, A. Pass-thoughts: Authenticating with our minds. In Proceedings of the 2005 Workshop on New Security Paradigms, ACM, Arrowhead, CA, USA, 20–23 September 2005; pp. 45–56. [Google Scholar]
- Altahat, S.; Huang, X.; Tran, D.; Sharma, D. People identification with RMS-Based spatial pattern of EEG signal. In Proceedings of the Algorithms and Architectures for Parallel Processing: 12th International Conference, Fukuoka, Japan, 4–7 September 2012; Springer: Berlin/Heidelberg, Germany; pp. 310–318. [Google Scholar]
- Poulos, M.; Rangoussi, M.; Chrissikopoulos, V.; Evangelou, A. Person identification based on parametric processing of the EEG. In Proceedings of the Electronics, Circuits and Systems, ICECS’99, the 6th IEEE International Conference on IEEE, Pafos, Cyprus, 5–8 September 1999; Volume 1, pp. 283–286. [Google Scholar]
- Jayarathne, I.; Cohen, M.; Amarakeerthi, S. BrainID: Development of an EEG-based biometric authentication system. In Proceedings of the Information Technology, Electronics and Mobile Communication Conference (IEMCON), 2016 IEEE 7th Annual, IEEE, Vancouver, BC, Canada, 13–15 October 2016; pp. 1–6. [Google Scholar]
- Collura, T.F. History and evolution of electroencephalographic instruments and techniques. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1993, 10, 476–504. [Google Scholar] [CrossRef]
- Jasper, H.H. The ten twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. Suppl. 1958, 10, 371–375. [Google Scholar]
- American Electroencephalographic Society. Guideline thirteen: Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 1994, 11, 111–113. [Google Scholar] [CrossRef]
- Bigdely-Shamlo, N.; Vankov, A.; Ramirez, R.R.; Makeig, S. Brain activity-based image classification from rapid serial visual presentation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Delorme, A.; Makeig, S. Frontal midline EEG dynamics during working memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Alotaiby, T.; El-Samie, F.E.A.; Alshebeili, S.A.; Ahmad, I. A review of channel selection algorithms for EEG signal processing. EURASIP J. Adv. Signal Process. 2015, 2015, 66. [Google Scholar] [CrossRef]
- Baars, B.J.; Gage, N.M. Cognition, Brain, and Consciousness: Introduction to Cognitive Neuroscience; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2007; pp. 635–637. [Google Scholar]
- Li, X.L.; Yang, J.H.; Zhang, L.; Li, S.; Jin, G.; Zhi, S. A new star pattern identification technique using an improved triangle algorithm. Proc. Inst. Mech. Eng. Part G-J. Aerosp. Eng. 2015, 229, 1730–1739. [Google Scholar] [CrossRef]
- Palaniappan, R.; Raveendran, P. Individual identification technique using visual evoked potential signals. Electron. Lett. 2002, 38, 1634–1635. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, F.; Zhang, K.; Trajcevski, G.; Luo, X.; Zhang, F.; Gao, Q.; Zhou, F.; Zhang, K.; Trajcevski, G. Identifying Human Mobility via Trajectory Embeddings. In Proceedings of the Twenty-Sixth International Joint Conference on Artificial Intelligence, Melbourne, Australia, 19–25 August 2017; pp. 1689–1695. [Google Scholar]
- Kingma, D.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Bashar, M.K.; Chiaki, I.; Yoshida, H. Human identification from brain EEG signals using advanced machine learning method EEG-based biometrics. In Proceedings of the Biomedical Engineering and Sciences (IECBES), 2016 IEEE EMBS Conference on IEEE, Kuala Lumpur, Malaysia, 4–8 December 2016; pp. 475–479. [Google Scholar]
- Zhang, Y.; Liu, B.; Ji, X.; Huang, D. Classification of EEG Signals Based on Autoregressive Model and Wavelet Packet Decomposition. Neural Process. Lett. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Zarei, R.; He, J.; Siuly, S.; Zhang, Y. A PCA aided cross-covariance scheme for discriminative feature extraction from EEG signals. Comput. Methods Programs Biomed. 2017, 146, 47. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Gupta, A.; Jain, P.; Rani, A.; Yadav, J. Classification of human emotions from EEG signals using SVM and LDA Classifiers. In Proceedings of the International Conference on Signal Processing and Integrated Networks, Edinburgh, UK, 3–6 July 2016; pp. 180–185. [Google Scholar]
- Parvinnia, E.; Sabeti, M.; Jahromi, M.Z.; Boostani, R. Classification of EEG Signals using adaptive weighted distance nearest neighbor algorithm. J. King Saud Univ.-Comput. Inf. Sci. 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Lee, C.; Kang, J.H.; Kim, S.P. Feature slection using mutual information for EEG-based biometrics. In Proceedings of the Telecommunications and Signal Processing (TSP), 2016 39th International Conference on IEEE, Vienna, Austria, 27–29 June 2016; pp. 673–676. [Google Scholar]
- Aydemir, O.; Kayikcioglu, T. Decision tree structure based classification of EEG signals recorded during two dimensional cursor movement imagery. J. Neurosci. Methods 2014, 229, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Jin, J.; Zhao, Q.; Wang, X.; Cichocki, A. Sparse Bayesian Classification of EEG for Brain-Computer Interface. IEEE Trans. Neural Networks Learn. Syst. 2016, 27, 2256–2267. [Google Scholar] [CrossRef]
- Hu, J. Automated Detection of Driver Fatigue Based on AdaBoost Classifier with EEG Signals. Front. Comput. Neurosci. 2017, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Bandyopadhyay, T. EEG Based Motor Imagery Classification Using SVM and MLP. In Proceedings of the International Conference on Computational Intelligence and Networks, Tehri, India, 23–25 December 2016; pp. 84–89. [Google Scholar]
- Zheng, W.L.; Lu, B.L. Investigating critical frequency bands and channels for EEG-based emotion recognition with deep neural networks. IEEE Trans. Auton. Ment. Dev. 2015, 7, 162–175. [Google Scholar] [CrossRef]
- Adeli, H.; Ghosh-Dastidar, S.; Dadmehr, N. A wavelet-chaos methodology for analysis of EEGs and EEG subbands to detect seizure and epilepsy. IEEE Trans. Biomed. Eng. 2007, 54, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Rundo, F.; Bruni, O.; Terzano, M.G.; Stam, C.J. The functional connectivity of different EEG bands moves towards small-world network organization during sleep. Clin. Neurophysiol. 2008, 119, 2026–2036. [Google Scholar] [CrossRef]
- Del Pozo-Banos, M.; Alonso, J.B.; Ticay-Rivas, J.R.; Travieso, C.M. Electroencephalogram subject identification: A review. Expert Syst. Appl. 2014, 41, 6537–6554. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Hiltunen, J.K.; Ranta-aho, P.O.; Karjalainen, P.A. Estimation of nonstationary EEG with Kalman smoother approach: An application to event-related synchronization (ERS). IEEE Trans. Biomed. Eng. 2004, 51, 516–524. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Hanakawa, T.; Immisch, I.; Toma, K.; Dimyan, M.A.; Van Gelderen, P.; Hallett, M. Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 2003, 89, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, D.; Campisi, P.; Vegso, B.; Cserti, P.; Kozmann, G.; Babiloni, F.; Fallani, F.D.V. Human brain distinctiveness based on EEG spectral coherence connectivity. IEEE Trans. Biomed. Eng. 2014, 61, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, I.; Cohen, M.; Amarakeerthi, S. Person identification from EEG using various machine learning techniques with inter-hemispheric amplitude ratio. PLoS ONE 2020, 15, e0238872. [Google Scholar] [CrossRef]
- Gui, Q.; Jin, Z.; Xu, W. Exploring EEG-based biometrics for user identification and authentication. In Proceedings of the 2014 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), IEEE, Philadelphia, PA, USA, 13 December 2014; pp. 1–6. [Google Scholar]
- Brigham, K.; Kumar, B.V. Subject identification from electroencephalogram (EEG) signals during imagined speech. In Proceedings of the 2010 Fourth IEEE International Conference on Biometrics: Theory, Applications and Systems (BTAS), IEEE, Washington, DC, USA, 23–26 September 2010; pp. 1–8. [Google Scholar]





| Characteristics | EEG | FP | Face | Iris | Voice |
|---|---|---|---|---|---|
| Generality | √ | √ | √ | √ | √ |
| Uniqueness | √ | √ | √ | √ | √ |
| Stability | √ | √ | √ | √ | √ |
| Accessibility | √ | √ | √ | √ | √ |
| Aliveness | √ | × | × | × | × |
| SR | √ | × | × | × | × |
| AC | √ | × | × | × | × |
| Layer | Convolution | Pooling | ||||||
|---|---|---|---|---|---|---|---|---|
| Filters | Kernel Size | Stride | Padding | Output Dim | Pool Size | Strides | Output Dim | |
| 1 | 16 | 3 | 1 | Same | [1,2] | [1,2] | ||
| 16 | 3 | 1 | Same | |||||
| 2 | 32 | 3 | 1 | Same | [1,2] | [1,2] | ||
| 3 | 64 | 3 | 1 | Same | [1,2] | [1,2] | ||
| 4 | 128 | 3 | 1 | Same | [1,2] | [1,2] | ||
| 5 | 128 | 3 | 1 | Same | [1,2] | [1,2] | ||
| Dataset | N | M | F(Hz) | K |
|---|---|---|---|---|
| RSVP | 7 | 2 | 256 | 256 |
| Sternberg Task | 23 | 4 | 256 | 72 |
| BCI2000 | 109 | 14 | 160 | 64 |
| Methods | RSVP | Sternberg Task | BCI2000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Precision | Recall | F1 | Precision | Recall | F1 | Precision | Recall | F1 | |
| 0.37 | 0.30 | 0.29 | 0.75 | 0.74 | 0.71 | 0.96 | 0.96 | 0.96 | |
| SVM | 0.23 | 0.34 | 0.27 | 0.72 | 0.58 | 0.56 | 0.93 | 0.92 | 0.92 |
| ConvNets | 0.28 | 0.26 | 0.27 | 0.71 | 0.67 | 0.70 | 0.93 | 0.93 | 0.93 |
| LDA | 0.15 | 0.19 | 0.16 | 0.45 | 0.44 | 0.44 | 0.42 | 0.36 | 0.36 |
| NN | 0.28 | 0.31 | 0.29 | 0.67 | 0.66 | 0.64 | 0.81 | 0.80 | 0.80 |
| DTS | 0.30 | 0.33 | 0.30 | 0.61 | 0.59 | 0.57 | 0.71 | 0.71 | 0.70 |
| Bayesian | 0.16 | 0.14 | 0.15 | 0.42 | 0.42 | 0.40 | 0.45 | 0.44 | 0.43 |
| AdaBoost | 0.33 | 0.23 | 0.22 | 0.70 | 0.72 | 0.69 | 0.93 | 0.93 | 0.92 |
| MLP | 0.25 | 0.27 | 0.25 | 0.63 | 0.67 | 0.62 | 0.91 | 0.89 | 0.89 |
| Activity ID | Activity Description | Task ID |
|---|---|---|
| Resting state with open eyes | ||
| Resting state with closed eyes | ||
| Open and close left or right fist | ||
| Imagine opening and closing left or right fist | ||
| Open an close both fists or both feet | ||
| Imagine opening and closing both fists or both feet |
| Class ID | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Task ID |
| Class ID | 1 | 2 | 3 |
|---|---|---|---|
| Task ID |
| Method | 3-Class | 5-Class | ||||
|---|---|---|---|---|---|---|
| Precision | Recall | F1 | Precision | Recall | F1 | |
| 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 0.98 | |
| SVM | 0.83 | 0.82 | 0.82 | 0.78 | 0.78 | 0.78 |
| LDA | 0.37 | 0.34 | 0.35 | 0.24 | 0.23 | 0.23 |
| NN | 0.83 | 0.83 | 0.83 | 0.78 | 0.78 | 0.78 |
| DTS | 0.63 | 0.63 | 0.63 | 0.51 | 0.51 | 0.51 |
| Bayesian | 0.44 | 0.42 | 0.26 | 0.16 | 0.21 | 0.20 |
| AdaBoost | 0.78 | 0.76 | 0.76 | 0.70 | 0.69 | 0.69 |
| MLP | 0.79 | 0.79 | 0.35 | 0.76 | 0.75 | 0.75 |
| Research Work | EEG Feature | Method | Number of Users | Performance |
|---|---|---|---|---|
| Polus et al. [31] | FFT | LVQ | 45 | Correct score: to |
| Isuru et al. [67] | IHAR | KNN | 12 | Accuracy: |
| Gui et al. [68] | WT | ANN | 32 | Correct score: |
| Brigham et al. [69] | AR | SVM | 6 | Accuracy: |
| Isuru et al. [35] | CSP | LDA | 12 | Accuracy: |
| Proposed work | × | ESML | 109 | Precision: |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, E.; Qin, Z.; Bi, T.; Qin, Z. Electroencephalogram-Based Subject Matching Learning (ESML): A Deep Learning Framework on Electroencephalogram-Based Biometrics and Task Identification. Behav. Sci. 2023, 13, 765. https://doi.org/10.3390/bs13090765
Xu J, Zhou E, Qin Z, Bi T, Qin Z. Electroencephalogram-Based Subject Matching Learning (ESML): A Deep Learning Framework on Electroencephalogram-Based Biometrics and Task Identification. Behavioral Sciences. 2023; 13(9):765. https://doi.org/10.3390/bs13090765
Chicago/Turabian StyleXu, Jin, Erqiang Zhou, Zhen Qin, Ting Bi, and Zhiguang Qin. 2023. "Electroencephalogram-Based Subject Matching Learning (ESML): A Deep Learning Framework on Electroencephalogram-Based Biometrics and Task Identification" Behavioral Sciences 13, no. 9: 765. https://doi.org/10.3390/bs13090765
APA StyleXu, J., Zhou, E., Qin, Z., Bi, T., & Qin, Z. (2023). Electroencephalogram-Based Subject Matching Learning (ESML): A Deep Learning Framework on Electroencephalogram-Based Biometrics and Task Identification. Behavioral Sciences, 13(9), 765. https://doi.org/10.3390/bs13090765






