The Effect of Induced Regulatory Focus on Frontal Cortical Activity
Abstract
:1. Introduction
1.1. Two Main Models of Frontal Cortical Activity
1.2. Behavioral Activation–Behavioral Inhibition Model and Frontal Cortical Activity
1.3. Regulatory Focus and Frontal Cortical Activity
1.3.1. Promotion and Prevention Focus
1.3.2. Promotion and Prevention Focus and Frontal Cortical Activity
1.4. The Present Research
2. Materials and Methods
2.1. Participants
2.2. Self-Report Measures
2.3. Procedure
2.4. EEG Recording and Preprocessing
2.5. Spectral Analysis
3. Results
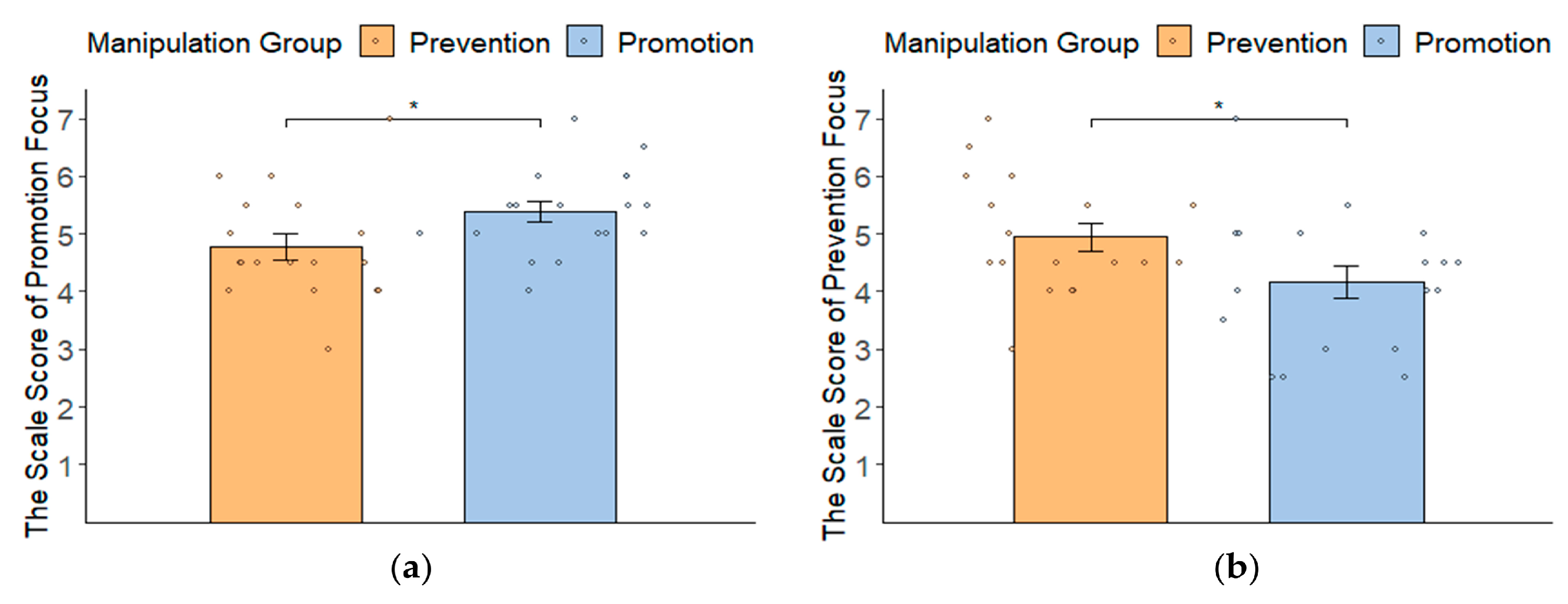
3.1. Manipulation Check: Self-Report Results
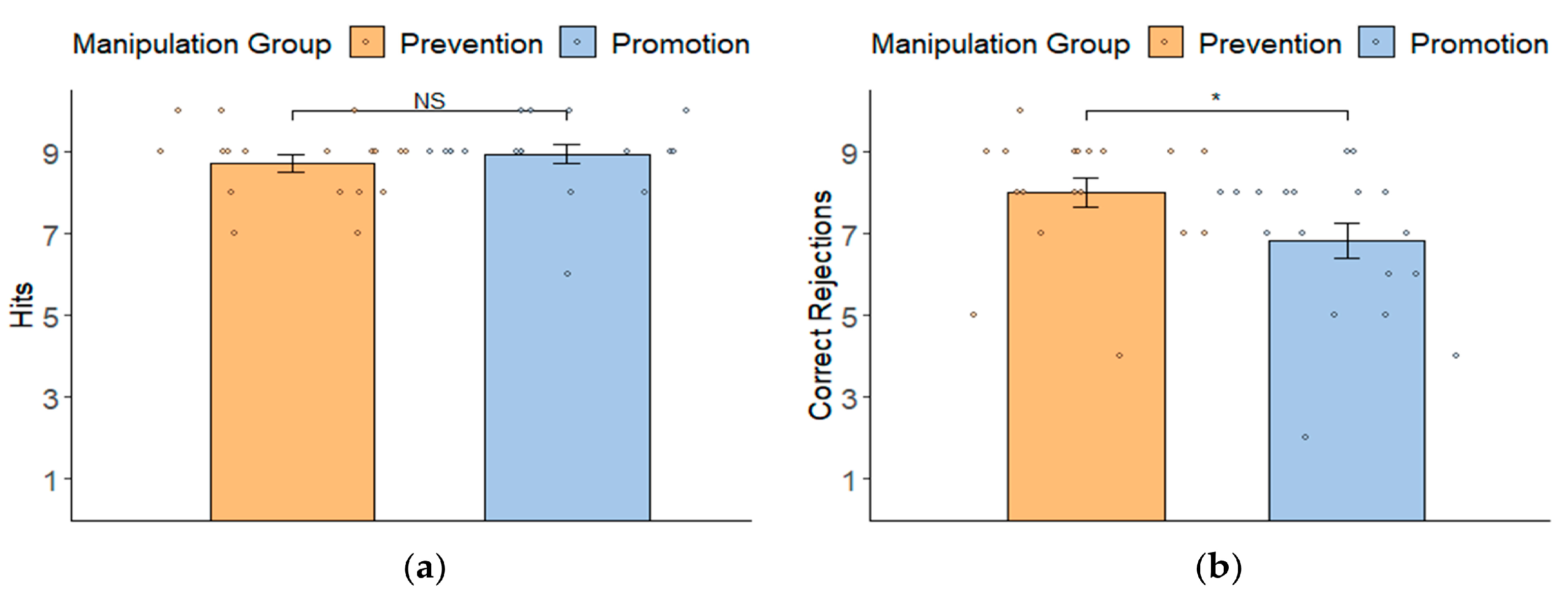
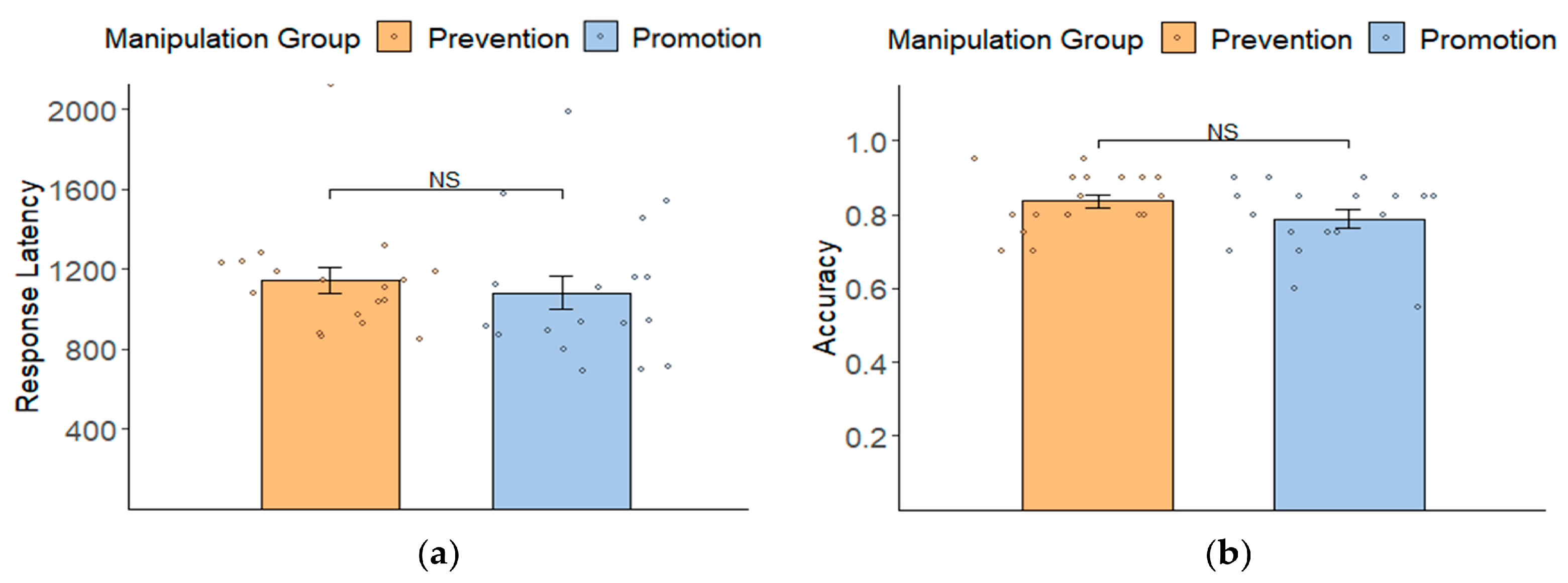
3.2. Manipulation Check: Behavioral Results
3.3. EEG Results
4. Discussion
4.1. Regulatory Focus and Approach and Avoidance Motivation
4.2. Regulatory Focus and Frontal Cortical Activity
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, X.; Zhang, S.; Chen, X.; Coplan, R.J.; Xiao, B.; Ding, X. Links between social avoidance and frontal alpha asymmetry during processing emotional facial stimuli: An exploratory study. Biol. Psychol. 2023, 178, 108516. [Google Scholar] [CrossRef] [PubMed]
- Zsigo, C.; Greimel, E.; Primbs, R.; Bartling, J.; Schulte-Körne, G.; Feldmann, L. Frontal alpha asymmetry during emotion regulation in adults with lifetime major depression. Cogn. Affect. Behav. Neurosci. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, I.; Rasmus, A.; Esmaeilzadeh, S.; Wiłkość-Dębczyńska, M. The Level of Self-Esteem May Influence the Effect of Positive Self-Statements. An EEG Alpha Asymmetry Pilot Study. Symmetry 2021, 13, 1913. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Cui, Y.; Zhao, T.; Xie, X.; Liang, S.; Sha, S.; Zhao, X.; Zhang, L. EEG-based major depressive disorder recognition by neural oscillation and asymmetry. Front. Neurosci. 2024, 18, 1362111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Peng, H.; Liu, H.; Gu, R.; Peng, X.; Wu, J. Alpha frontal asymmetry underlies individual differences in reactivity to acute psychosocial stress in males. Psychophysiology 2021, 58, e13893. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Santesso, D.L.; Segalowitz, S.J.; Ashbaugh, A.R.; Antony, M.M.; McCabe, R.E.; Schmidt, L.A. Frontal EEG asymmetry and sensation seeking in young adults. Biol. Psychol. 2008, 78, 164–172. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, V.; Sommer, K.; Scacchia, P. Resting frontal asymmetry and reward sensitivity theory motivational traits. Sci. Rep. 2018, 8, 13154. [Google Scholar] [CrossRef] [PubMed]
- Monni, A.; Collison, K.L.; Hill, K.E.; Oumeziane, B.A.; Foti, D. The novel frontal alpha asymmetry factor and its association with depression, anxiety, and personality traits. Psychophysiology 2022, 59, e14109. [Google Scholar] [CrossRef]
- Wacker, J.; Chavanon, M.-L.; Leue, A.; Stemmler, G. Is running away right? The behavioral activation-behavioral inhibition model of anterior asymmetry. Emotion 2008, 8, 232. [Google Scholar] [CrossRef]
- Fecteau, S.; Pascual-Leone, A.; Zald, D.H.; Liguori, P.; Théoret, H.; Boggio, P.S.; Fregni, F. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J. Neurosci. 2007, 27, 6212–6218. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.J.; Hortensius, R.; Schutter, D.J.; Harmon-Jones, E. The relationship of approach/avoidance motivation and asymmetric frontal cortical activity: A review of studies manipulating frontal asymmetry. Int. J. Psychophysiol. 2017, 119, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef] [PubMed]
- Hewig, J. Intentionality in frontal asymmetry research. Psychophysiology 2018, 55, e12852. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, M.; Ann Bell, M. Examining Conduct Problems in a Community Sample during Middle Childhood: The Role of Frontal EEG Asymmetry, Temperament, and Working Memory. Res. Child Adolesc. Psychopathol. 2024, 1–15. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Sigelman, J. State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J. Personal. Soc. Psychol. 2001, 80, 797–803. [Google Scholar] [CrossRef]
- Rybak, M.; Crayton, J.W.; Young, I.J.; Herba, E.; Konopka, L.M. Frontal alpha power asymmetry in aggressive children and adolescents with mood and disruptive behavior disorders. Clin. EEG Neurosci. 2006, 37, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E. Anger and the behavioral approach system. Personal. Individ. Differ. 2003, 35, 995–1005. [Google Scholar] [CrossRef]
- Lacey, M.F.; Gable, P.A. Frontal Asymmetry in an approach–avoidance conflict paradigm. Psychophysiology 2021, 58, e13780. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Personal. Soc. Psychol. 1994, 67, 319. [Google Scholar] [CrossRef]
- Gray, J.A. The Psychology of Fear and Stress; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Gray, J.; McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System, 2nd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Fischer, H.; Anderson, J.L.; Furmark, T.; Wik, G.; Fredrikson, M. Right-sided human prefrontal brain activation during acquisition of conditioned fear. Emotion 2002, 2, 233. [Google Scholar] [CrossRef]
- Paus, T. Functional anatomy of arousal and attention systems in the human brain. Prog. Brain Res. 2000, 126, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gable, P.A.; Mechin, N.C.; Hicks, J.A.; Adams, D.L. Supervisory control system and frontal asymmetry: Neurophysiological traits of emotion-based impulsivity. Soc. Cogn. Affect. Neurosci. 2015, 10, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.B.; Gable, P.A. Neurophysiological markers of multiple facets of impulsivity. Biol. Psychol. 2016, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Wacker, J.; Chavanon, M.-L.; Stemmler, G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. J. Res. Personal. 2010, 44, 167–179. [Google Scholar] [CrossRef]
- Gable, P.A.; Neal, L.B.; Threadgill, A.H. Regulatory behavior and frontal activity: Considering the role of revised-BIS in relative right frontal asymmetry. Psychophysiology 2018, 55, e12910. [Google Scholar] [CrossRef] [PubMed]
- Lacey, M.F.; Neal, L.B.; Gable, P.A. Effortful control of motivation, not withdrawal motivation, relates to greater right frontal asymmetry. Int. J. Psychophysiol. 2020, 147, 18–25. [Google Scholar] [CrossRef]
- Amodio, D.M.; Master, S.L.; Yee, C.M.; Taylor, S.E. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology 2008, 45, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.M.; Devine, P.G.; Harmon-Jones, E. A dynamic model of guilt: Implications for motivation and self-regulation in the context of prejudice. Psychol. Sci. 2007, 18, 524–530. [Google Scholar] [CrossRef]
- Wacker, J.; Heldmann, M.; Stemmler, G. Separating emotion and motivational direction in fear and anger: Effects on frontal asymmetry. Emotion 2003, 3, 167. [Google Scholar] [CrossRef]
- Berkman, E.T.; Lieberman, M.D.; Gable, S.L. BIS, BAS, and response conflict: Testing predictions of the revised reinforcement sensitivity theory. Personal. Individ. Differ. 2009, 46, 586–591. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, N.; Corr, P.J. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004, 28, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Smillie, L.D.; Pickering, A.D.; Jackson, C.J. The new reinforcement sensitivity theory: Implications for personality measurement. Personal. Soc. Psychol. Rev. 2006, 10, 320–335. [Google Scholar] [CrossRef]
- Gray, J.; McNaughton, N. The neuropsychology of anxiety; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Kelley, N.J.; Schmeichel, B.J. Noninvasive stimulation over the dorsolateral prefrontal cortex facilitates the inhibition of motivated responding. J. Exp. Psychol. Gen. 2016, 145, 1702. [Google Scholar] [CrossRef] [PubMed]
- Strauman, T.J.; Wilson, W.A. Individual differences in approach and avoidance: Behavioral activation/inhibition and regulatory focus as distinct levels of analysis. Handb. Personal. Self-Regul. 2010, 447–473. [Google Scholar] [CrossRef]
- Kelley, N.J.; Gallucci, A.; Riva, P.; Romero Lauro, L.J.; Schmeichel, B.J. Stimulating self-regulation: A review of non-invasive brain stimulation studies of goal-directed behavior. Front. Behav. Neurosci. 2019, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, W.A.; Raye, C.L.; Johnson, M.K. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn. Affect. Behav. Neurosci. 2005, 5, 202–211. [Google Scholar] [CrossRef]
- Higgins, E.T. Beyond pleasure and pain. Am. Psychol. 1997, 52, 1280. [Google Scholar] [CrossRef] [PubMed]
- Higgins, E.T. Promotion and prevention: Regulatory focus as a motivational principle. In Advances in Experimental Social Psychology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 30, pp. 1–46. [Google Scholar] [CrossRef]
- Higgins, E.T. What distinguishes promotion and prevention? Attaining “+ 1” from “0” as non-gain versus maintaining “0” as non-loss. Pol. Psychol. Bull. 2018, 49, 40–49. [Google Scholar] [CrossRef]
- Crowe, E.; Higgins, E.T. Regulatory focus and strategic inclinations: Promotion and prevention in decision-making. Organ. Behav. Hum. Decis. Process. 1997, 69, 117–132. [Google Scholar] [CrossRef]
- Friedman, R.S.; Förster, J. The effects of promotion and prevention cues on creativity. J. Personal. Soc. Psychol. 2001, 81, 1001. [Google Scholar] [CrossRef] [PubMed]
- Förster, J.; Higgins, E.T.; Bianco, A.T. Speed/accuracy decisions in task performance: Built-in trade-off or separate strategic concerns? Organ. Behav. Hum. Decis. Process. 2003, 90, 148–164. [Google Scholar] [CrossRef]
- Werth, L.; Förster, J. The effects of regulatory focus on braking speed 1. J. Appl. Soc. Psychol. 2007, 37, 2764–2787. [Google Scholar] [CrossRef]
- Detloff, A.M.; Hariri, A.R.; Strauman, T.J. Neural signatures of promotion versus prevention goal priming: fMRI evidence for distinct cognitive-motivational systems. Personal. Neurosci. 2020, 3, e1. [Google Scholar] [CrossRef] [PubMed]
- Eddington, K.M.; Dolcos, F.; Cabeza, R.; R. Krishnan, K.R.; Strauman, T.J. Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. J. Cogn. Neurosci. 2007, 19, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Raye, C.L.; Mitchell, K.J.; Touryan, S.R.; Greene, E.J.; Nolen-Hoeksema, S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc. Cogn. Affect. Neurosci. 2006, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.J.; Cunningham, W.A. Neural correlates of reflection on goal states: The role of regulatory focus and temporal distance. Soc. Neurosci. 2009, 4, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.M.; Shah, J.Y.; Sigelman, J.; Brazy, P.C.; Harmon-Jones, E. Implicit regulatory focus associated with asymmetrical frontal cortical activity. J. Exp. Soc. Psychol. 2004, 40, 225–232. [Google Scholar] [CrossRef]
- Lockwood, P.; Jordan, C.H.; Kunda, Z. Motivation by positive or negative role models: Regulatory focus determines who will best inspire us. J. Personal. Soc. Psychol. 2002, 83, 854. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063. [Google Scholar] [CrossRef]
- Sengupta, J.; Zhou, R. Understanding impulsive eaters’ choice behaviors: The motivational influences of regulatory focus. J. Mark. Res. 2007, 44, 297–308. [Google Scholar] [CrossRef]
- Higgins, E.T.; Shah, J.; Friedman, R. Emotional responses to goal attainment: Strength of regulatory focus as moderator. J. Personal. Soc. Psychol. 1997, 72, 515. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology 2003, 40, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, O.-K.; Kim, D.J. Study of Practical Method for International 10~20 Electrode System. Korean J. Clin. Lab. Sci. 2021, 53, 60–67. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Yue, L.; Zeng, Y.; Zhao, Q.; Gong, Q.; Zhang, J.; Liu, D.; Luo, X.; Xia, X. A novel method to simultaneously record spinal cord electrophysiology and electroencephalography signals. NeuroImage 2021, 232, 117892. [Google Scholar] [CrossRef] [PubMed]
- Mocks, J.; Gasser, T. How to Select Epochs of the Eeg at Rest for Quantitative-Analysis. Electroencephalogr. Clin. Neurophysiol. 1984, 58, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.C.; Howell, B.C.; Salway, S.; Cavanagh, J.F.; Hamilton, D.A.; Claus, E.D.; Frost, M.E. Frontal alpha asymmetry in alcohol-related intimate partner violence. Soc. Cogn. Affect. Neurosci. 2019, 14, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Killeen, L.A.; Teti, D.M. Mothers’ frontal EEG asymmetry in response to infant emotion states and mother–infant emotional availability, emotional experience, and internalizing symptoms. Dev. Psychopathol. 2012, 24, 9–21. [Google Scholar] [CrossRef]
- Kalin, N.H.; Shelton, S.E.; Davidson, R.J. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol. Psychiatry 2007, 62, 1134–1139. [Google Scholar] [CrossRef]
- Harmon-Jones, E. Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology 2006, 43, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cogn. Emot. 1993, 7, 115–138. [Google Scholar] [CrossRef]
- Shah, J.; Higgins, T.; Friedman, R.S. Performance incentives and means: How regulatory focus influences goal attainment. J. Personal. Soc. Psychol. 1998, 74, 285. [Google Scholar] [CrossRef] [PubMed]
- Higgins, E.T.; Roney, C.J.; Crowe, E.; Hymes, C. Ideal versus ought predilections for approach and avoidance distinct self-regulatory systems. J. Personal. Soc. Psychol. 1994, 66, 276. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.; Portugali, J. Urban regulatory focus: A new concept linking city size to human behaviour. R. Soc. Open Sci. 2018, 5, 171478. [Google Scholar] [CrossRef] [PubMed]
- Mungee, A.; Kazzer, P.; Feeser, M.; Nitsche, M.A.; Schiller, D.; Bajbouj, M. Transcranial direct current stimulation of the prefrontal cortex: A means to modulate fear memories. Neuroreport 2014, 25, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A. Revisiting the influence of regulatory focus on eagerness and vigilance in signal detection. Compr. Results Soc. Psychol. 2020, 4, 315–344. [Google Scholar] [CrossRef]
- Banfield, J.F.; van der Lugt, A.H.; Münte, T.F. Juicy fruit and creepy crawlies: An electrophysiological study of the implicit Go/NoGo association task. Neuroimage 2006, 31, 1841–1849. [Google Scholar] [CrossRef]
- Brendl, C.M.; Markman, A.B.; Messner, C. How do indirect measures of evaluation work? Evaluating the inference of prejudice in the Implicit Association Test. J. Personal. Soc. Psychol. 2001, 81, 760. [Google Scholar] [CrossRef]
- McFarland, S.G.; Crouch, Z. A cognitive skill confound on the Implicit Association Test. Soc. Cogn. 2002, 20, 483–510. [Google Scholar] [CrossRef]

| Items | Promotion Focus Manipulation Group (N = 18) | Prevention Focus Manipulation Group (N = 18) | t | p |
|---|---|---|---|---|
| Chronic prevention focus | 5.07 ± 0.98 | 4.55 ± 0.93 | 1.63 | 0.11 |
| Chronic promotion focus | 5.05 ± 0.87 | 4.98 ± 0.75 | 0.25 | 0.80 |
| Positive emotion | 4.02 ± 0.89 | 4.34 ± 0.74 | −1.14 | 0.26 |
| Negative emotion | 2.41 ± 1.01 | 2.11 ± 0.87 | 0.95 | 0.35 |
| Socioeconomic status | 4.56 ± 1.04 | 5.06 ± 1.39 | −1.22 | 0.23 |
| Age (years) | 20.39 ± 2.06 | 20.56 ± 2.33 | −0.23 | 0.82 |
| Gender (male/female) | 2/16 | 4/14 | 0.88 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Sun, X. The Effect of Induced Regulatory Focus on Frontal Cortical Activity. Behav. Sci. 2024, 14, 292. https://doi.org/10.3390/bs14040292
Lin Y, Sun X. The Effect of Induced Regulatory Focus on Frontal Cortical Activity. Behavioral Sciences. 2024; 14(4):292. https://doi.org/10.3390/bs14040292
Chicago/Turabian StyleLin, Yiqin, and Xiaomin Sun. 2024. "The Effect of Induced Regulatory Focus on Frontal Cortical Activity" Behavioral Sciences 14, no. 4: 292. https://doi.org/10.3390/bs14040292





