Immersive Extended Reality (I-XR) in Medical and Nursing for Skill Competency and Knowledge Acquisition: A Systematic Review and Implications for Pedagogical Practices
Abstract
:1. Introduction
1.1. Integration of I-XR in Healthcare Education
1.2. Review of I-XR Studies for Training Effectiveness in Healthcare Education
1.3. Summary
1.4. Purpose
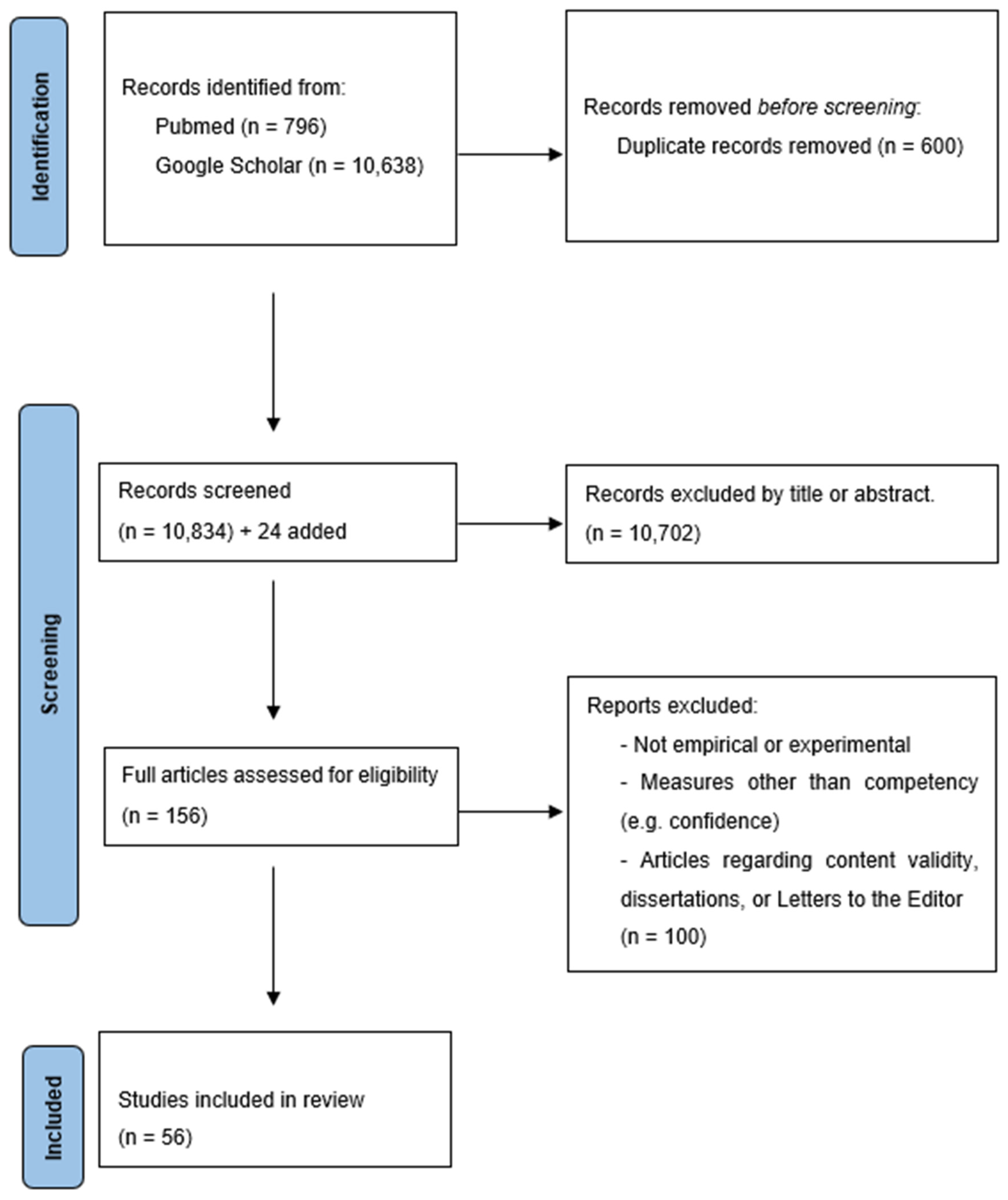
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Coding
2.5. Assessing Article Quality and Bias
3. Results
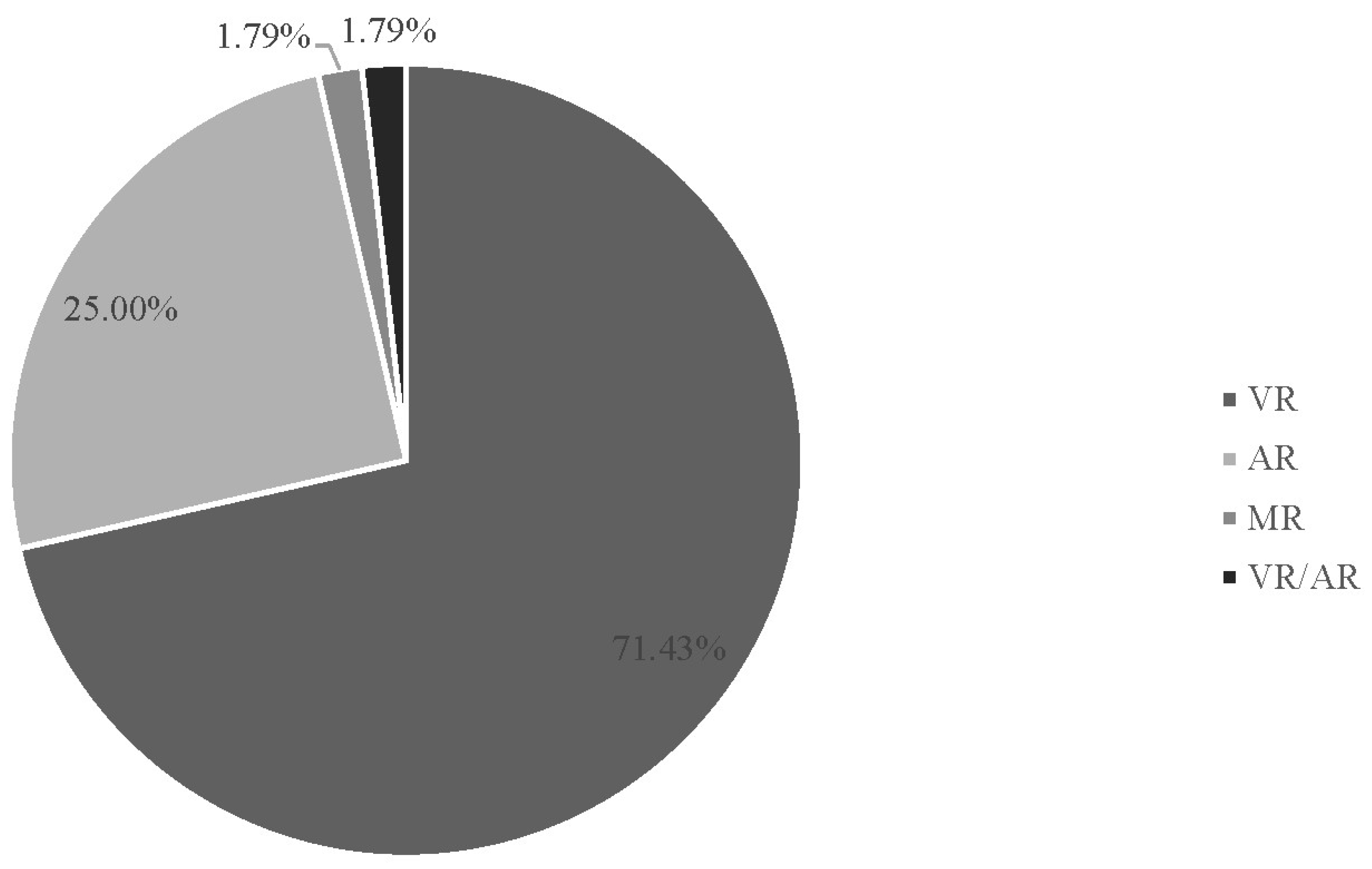
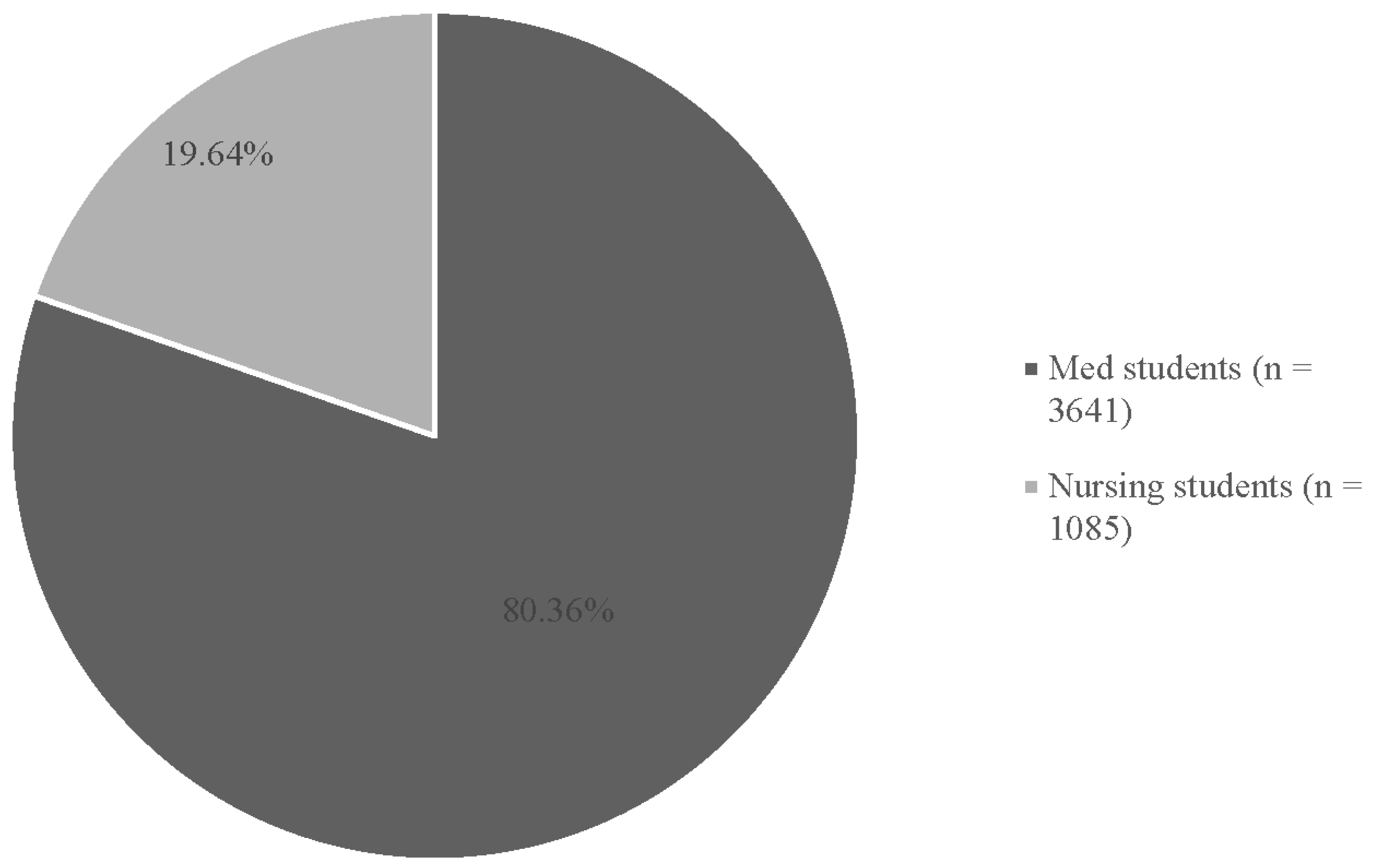
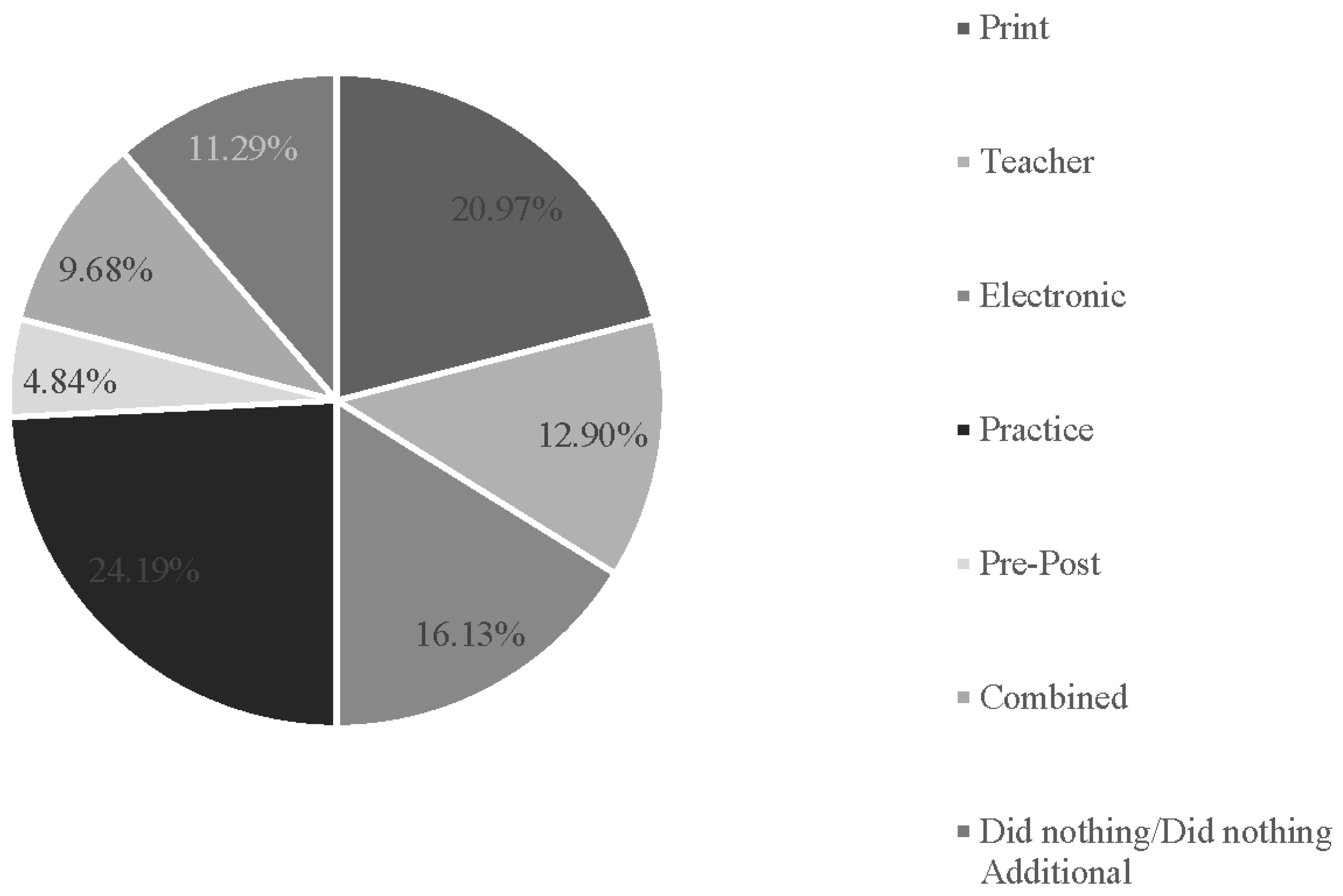
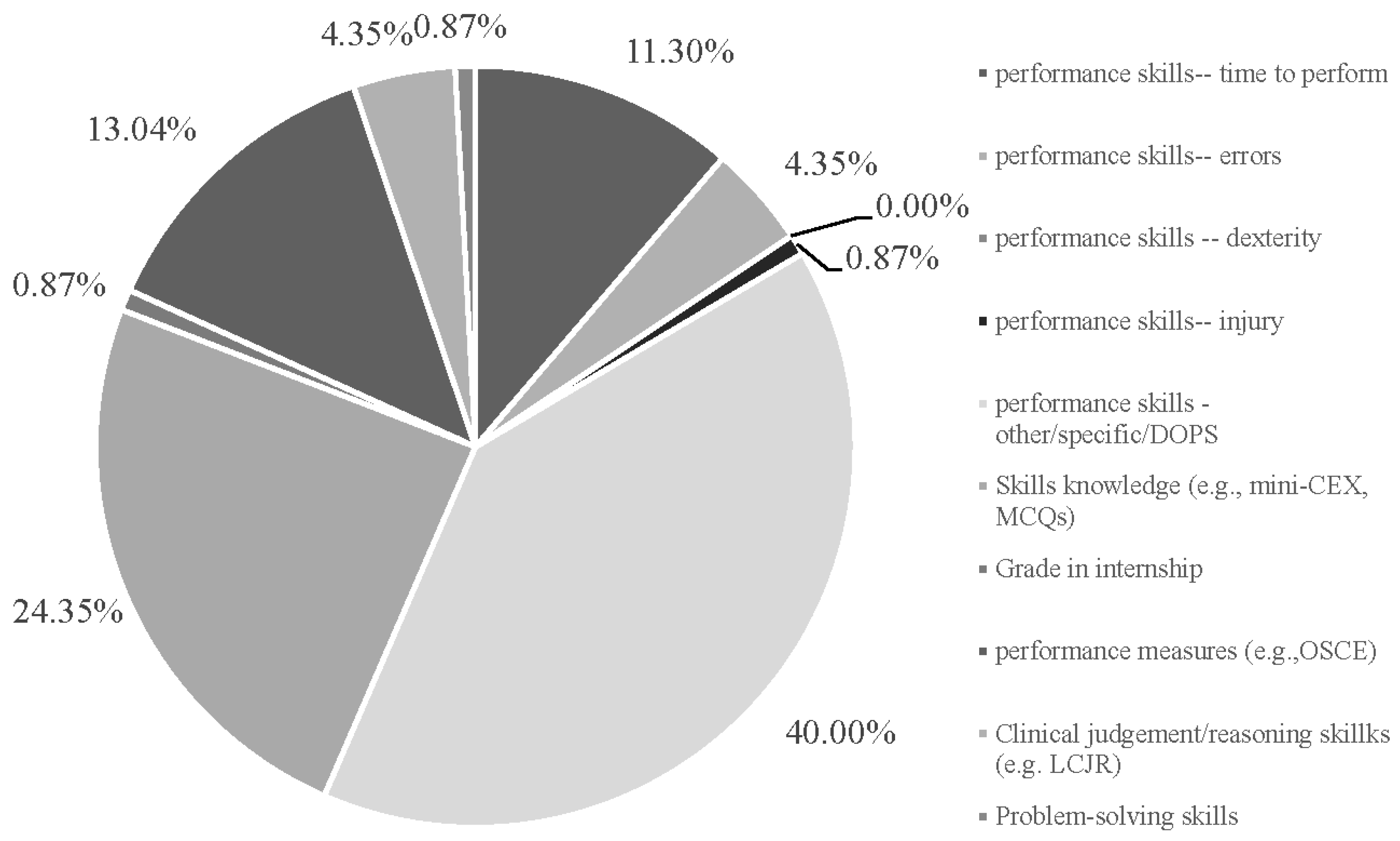
3.1. Descriptive Findings
3.2. Overall Study Assessments of I-XR Skill Competency
4. Discussion
4.1. A New Embodied Learning Model
- Embodied Content Knowledge (ECK): A deep understanding of the subject matter gained through embodied experiences and interactions with the world.
- Embodied Technological Knowledge (ETK): Knowledge and skills related to using tools and technologies in an embodied manner to explore, analyze, and solve problems.
- Embodied Pedagogical Knowledge (EPK): Ability to design and facilitate embodied learning experiences that bridge learners’ tacit understanding with formal knowledge and practices.
- Remembering and Understanding: I-XR can be used to practice interactive simulations and role-playing scenarios that engage learners’ senses and bodily movements, helping them remember and understand key concepts and procedures. For example, learners can practice basic life support skills on virtual patients in an I-XR environment.
- Applying and Analyzing: I-XR can be used to have learners apply and analyze their knowledge and skills in context. For instance, learners can participate in a virtual surgery simulation in an I-XR environment, where they need to make decisions, solve problems, and perform procedures based on their understanding of anatomy and surgical techniques.
- Evaluating and Creating: I-XR can be used to encourage learners to evaluate and create new knowledge and solutions in I-XR. For example, learners can design and conduct virtual experiments in an I-XR to test hypotheses, build virtual models of anatomical structures, and develop new surgical tools and techniques in a virtual environment.
4.2. Limitations
4.3. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Studies Reviewed
- Aebersold, M., Voepel-Lewis, T., Cherara, L., Weber, M., Khouri, C., Levine, R., & Tait, A. R. (2018). Interactive anatomy-augmented virtual simulation training. Clinical Simulation in Nursing, 15, 34–41. https://doi.org/10.1016/j.ecns.2017.09.008
- Andersen, N. L., Jensen, R. O., Posth, S., Laursen, C. B., Jørgensen, R., & Graumann, O. (2021). Teaching ultrasound-guided peripheral venous catheter placement through immersive virtual reality: An explorative pilot study. Medicine, 100(27), e26394. https://doi.org/10.1097/MD.0000000000026394
- Andersen, N. L., Jensen, R. O., Konge, L., Laursen, C. B., Falster, C., Jacobsen, N., Elhakim, M. T., Bojsen, J. A., Riisshede, M., Fransen, M., Rasmussen, B. S. B., Posth, S., Sant, L., & Graumann, O. (2023). Immersive virtual reality in basic point-of-care ultrasound training: A randomized controlled trial. Ultrasound in Medicine and Biology, 49(1), 178–185. https://doi.org/10.1016/j.ultrasmedbio.2022.08.012 [reviewed online before print in 2022; coded as 2022 in current paper]
- Arents, V., De Groot, P. C. M., Struben, V. M. D., & Van Stralen, K. J. (2021). Use of 360° virtual reality video in medical obstetrical education: A quasi-experimental design. BMC Medical Education, 21(1), 202. https://doi.org/10.1186/s12909-021-02628-5
- Azimi, E., Winkler, A., Tucker, E., Qian, L., Doswell, J., Navab, N., & Kazanzides, P. (2018, July). Can mixed-reality improve the training of medical procedures?. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 4065–4068). IEEE. https://doi.org/10.1109/EMBC.2018.8513387.
- Banaszek, D., You, D., Chang, J., Pickell, M., Hesse, D., Hopman, W. M., Borschneck, D., & Bardana, D. (2017). Virtual reality compared with bench-top simulation in the acquisition of arthroscopic skill: A randomized controlled trial. The Journal of Bone and Joint Surgery, 99(7), 34. https://doi.org/10.2106/JBJS.16.00324
- Bayram, S. B., & Caliskan, N. (2019). Effect of a game-based virtual reality phone application on tracheostomy care education for nursing students: A randomized controlled trial. Nurse Education Today, 79, 25–31. https://doi.org/10.1016/j.nedt.2019.05.010
- Blumstein, G., Zukotynski, B., Cevallos, N., Ishmael, C., Zoller, S., Burke, Z., Clarkson, S., Park, H., Bernthal, N., & SooHoo, N. F. (2020). Randomized trial of a virtual reality tool to teach surgical technique for tibial shaft fracture intramedullary nailing. Journal of Surgical Education, 77(4), 969–977. https://doi.org/10.1016/j.jsurg.2020.01.002
- Bogomolova, K., Ham, I. J. M., Dankbaar, M. E. W., Broek, W. W., Hovius, S. E. R., Hage, J. A., & Hierck, B. P. (2020). The effect of stereoscopic augmented reality visualization on learning anatomy and the modifying effect of visual-spatial abilities: A double-center randomized controlled trial. Anatomical Sciences Education, 13(5), 558–567. https://doi.org/10.1002/ase.1941
- Bork, F., Stratmann, L., Enssle, S., Eck, U., Navab, N., Waschke, J., & Kugelmann, D. (2019). The benefits of an augmented reality magic mirror system for integrated radiology teaching in gross anatomy: Anatomical sciences education. Anatomical Sciences Education, 12(6), 585–598. https://doi.org/10.1002/ase.1864
- Brinkmann, C., Fritz, M., Pankratius, U., Bahde, R., Neumann, P., Schlueter, S., Senninger, N., & Rijcken, E. (2016). Box- or virtual-reality trainer: Which tool results in better transfer of laparoscopic basic skills?—A prospective randomized trial. Journal of Surgical Education, 74(4), 724–735. https://doi.org/10.1016/j.jsurg.2016.12.009
- Bube, S., Dagnaes-Hansen, J., Mahmood, O., Rohrsted, M., Bjerrum, F., Salling, L., Hansen, R. B., & Konge, L. (2020). Simulation-based training for flexible cystoscopy—A randomized trial comparing two approaches. Heliyon, 6(1), e0308. https://doi.org/10.1016/j.heliyon.2019.e03086
- Butt, A. L., Kardong-Edgren, S., & Ellertson, A. (2018). Using game-based virtual reality with haptics for skill acquisition. Clinical Simulation in Nursing, 16, 25–32. https://doi.org/10.1016/j.ecns.2017.09.010
- Cevallos, N., Zukotynski, B., Greig, D., Silva, M., & Thompson, R. M. (2022). The utility of virtual reality in orthopedic surgical training. Journal of Surgical Education, 79(6), 1516–1525. https://doi.org/10.1016/j.jsurg.2022.06.007
- Chao, Y. C., Hu, S. H., Chiu, H. Y., Huang, P. H., Tsai, H. T., & Chuang, Y. H. (2021). The effects of an immersive 3d interactive video program on improving student nurses’ nursing skill competence: A randomized controlled trial study. Nurse Education Today, 103, https://doi.org/10.1016/j.nedt.2021.104979
- Chao, Y.-P., Kang, C.-J., Chuang, H.-H., Hsieh, M.-J., Chang, Y.-C., Kuo, T. B. J., Yang, C. C. H., Huang, C.-G., Fang, T.-J., Li, H.-Y., & Lee, L.-A. (2023). Comparison of the effect of 360° versus two-dimensional virtual reality video on history taking and physical examination skills learning among undergraduate medical students: A randomized controlled trial. Virtual Reality(2), 637–650. https://doi.org/10.1007/s10055-022-00664-0 [reviewed online before print in 2022; coded as 2022 in current paper]
- Chen, P. J., & Liou, W. K. (2023). The effects of an augmented reality application developed for paediatric first aid training on the knowledge and skill levels of nursing students: An experimental controlled study. Nurse Education Today, 120, e105629. https://doi.org/10.1016/j.nedt.2022.105629
- Ekstrand, C., Jamal, A., Nguyen, R., Kudryk, A., Mann, J., & Mendez, I. (2018). Immersive and interactive virtual reality to improve learning and retention of neuroanatomy in medical students: A randomized controlled study. CMAJ Open, 6(1), E103–E109. https://doi.org/10.9778/cmajo.20170110
- Fu, Y., Cavuoto, L., Qi, D., Panneerselvam, K., Arikatla, V. S., Enquobahrie, A., De, S., & Schwaitzberg, S. D. (2020). Characterizing the learning curve of a virtual intracorporeal suturing simulator VBLaST-SS©. Surgical Endoscopy, 34, 3135–3144. https://doi.org/10.1007/s00464-019-07081-6
- Haerling, K. A. (2018). Cost-utility analysis of virtual and mannequin-based simulation. Simulation in Healthcare, 13(1), 33–40. https://doi.org/10.1097/SIH.0000000000000280
- Han, S. G., Kim, Y. D., Kong, T. Y., & Cho, J. (2021). Virtual reality-based neurological examination teaching tool(VRNET) versus standardized patient in teaching neurological examinations for the medical students: A randomized, single-blind study. BMC Medical Education, 21(1), 493. https://doi.org/10.1186/s12909-021-02920-4
- Henssen, D. J. H. A., Den Heuvel, L., De Jong, G., Vorstenbosch, M. A. T. M., Cappellen Van Walsum, A., Van Den Hurk, M. M., Kooloos, J. G. M., & Bartels, R. H. M. A. (2020). Neuroanatomy learning: Augmented reality vs. cross-sections. Anatomical Sciences Education, 13(3), 353–365. https://doi.org/10.1002/ase.1912 [reviewed online before print in 2019; coded as 2019 in current paper]
- Hu, K.-C., Salcedo, D., Kang, Y.-N., Lin, C.-W., Hsu, C.-W., Cheng, C.-Y., Suk, F.-M., Huang, W.-C., & Ito, E. (2020). Impact of virtual reality anatomy training on ultrasound competency development: A randomized controlled trial. PLOS ONE, 15(11), e0242731. https://doi.org/10.1371/journal.pone.0242731
- Issleib, M., Kromer, A., Pinnschmidt, H. O., Süss-Havemann, C., & Kubitz, J. C. (2021). Virtual reality as a teaching method for resuscitation training in undergraduate first year medical students: A randomized controlled trial. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 29(1), 27. https://doi.org/10.1186/s13049-021-00836-y
- Jaskiewicz, F., Kowalewski, D., Starosta, K., Cierniak, M., & Timler, D. (2020). Chest compressions quality during sudden cardiac arrest scenario performed in virtual reality: A crossover study in a training environment. Medicine, 99(48), e23374. https://doi.org/10.1097/MD.0000000000023374
- Jung, A. R., & Park, E. A. (2022). The effectiveness of learning to use HMD-based VR technologies on nursing students: Chemoport insertion surgery. International Journal of Environmental Research and Public Health, 19(8), 4823. https://doi.org/10.3390/ijerph19084823
- Kane, D., Ryan, G., Mangina, E., & McAuliffe, F. M. (2022). A randomized control trial of a virtual reality learning environment in obstetric medical student teaching. International Journal of Medical Informatics, 168, 104899. https://doi.org/10.1016/j.ijmedinf.2022.104899
- Kowalewski, K. F., Minassian, A., Hendrie, J. D., Benner, L., Preukschas, A. A., Kenngott, H. G., Fischer, L., Müller-Stich, B. P., & Nickel, F. (2019). One or two trainees per workplace for laparoscopic surgery training courses: results from a randomized controlled trial. Surgical Endoscopy, 33, 1523–1531. https://doi.org/10.1007/s00464-018-6440-5
- Küçük, S., Kapakin, S., & Göktaş, Y. (2016). Learning anatomy via mobile augmented reality: Effects on achievement and cognitive load: Learning anatomy. Anatomical Sciences Education, 9(5), 411–421. https://doi.org/10.1002/ase.1603
- Lau, S. T., Liaw, S. Y., Loh, W. L., Schmidt, L. T., Yap, J., Lim, F. P., Ang, E., Jiat, C., & Siah, R. (2023). Mid-career switch nursing students’ perceptions and experiences of using immersive virtual reality for clinical skills learning: A mixed methods study. Nurse Education Today, 124, 105760. https://doi.org/10.1016/j.nedt.2023.105760
- Lemke, M., Lia, H., Gabinet-Equihua, A., Sheahan, G., Winthrop, A., Mann, S., Fichtinger, G., & Zevin, B. (2020). Optimizing resource utilization during proficiency-based training of suturing skills in medical students: a randomized controlled trial of faculty-led, peer tutor-led, and holography-augmented methods of teaching. Surgical Endoscopy, 34, 1678–1687. https://doi.org/10.1007/s00464-019-06944-2.
- Logishetty, K., Western, L., Morgan, R., Iranpour, F., Cobb, J. P., & Auvinet, E. (2019). Can an augmented reality headset improve accuracy of acetabular cup orientation in simulated THA? A randomized trial. Clinical Orthopaedics & Related Research, 477(5), 1190–1199. https://doi.org/10.1097/CORR.0000000000000542
- Maresky, H. S., Oikonomou, A., Ali, I., Ditkofsky, N., Pakkal, M., & Ballyk, B. (2019). Virtual reality and cardiac anatomy: Exploring immersive three-dimensional cardiac imaging, a pilot study in undergraduate medical anatomy education. Clinical Anatomy, 32(2), 238–243. https://doi.org/10.1002/ca.23292 [reviewed online before print in 2018; coded as 2018 in current paper]
- Moll-Khosrawi, P., Falb, A., Pinnschmidt, H., Zöllner, C., & Issleib, M. (2022). Virtual reality as a teaching method for resuscitation training in undergraduate first year medical students during COVID-19 pandemic: A randomised controlled trial. BMC Medical Education, 22(1), 483. https://doi.org/10.1186/s12909-022-03533-1
- Moro, C., Štromberga, Z., Raikos, A., & Stirling, A. (2017). The effectiveness of virtual and augmented reality in health sciences and medical anatomy: VR and AR in health sciences and medical anatomy. Anatomical Sciences Education, 10(6), 549–559. https://doi.org/10.1002/ase.1696
- Nagayo, Y., Saito, T., & Oyama, H. (2022). Augmented reality self-training system for suturing in open surgery: A randomized controlled trial. International Journal of Surgery, 102, 106650. https://doi.org/10.1016/j.ijsu.2022.106650
- Neumann, E., Mayer, J., Russo, G. I., Amend, B., Rausch, S., Deininger, S., Harland, N., Da Costa, I. A., Hennenlotter, J., Stenzl, A., Kruck, S., & Bedke, J. (2019). Transurethral resection of bladder tumors: Next-generation virtual reality training for surgeons. European Urology Focus, 5(5), 906–911. https://doi.org/10.1016/j.euf.2018.04.011
- Nielsen, M. R., Kristensen, E. Q., Jensen, R. O., Mollerup, A. M., Pfeiffer, T., & Graumann, O. (2021). Clinical ultrasound education for medical students: Virtual reality versus e-learning, a randomized controlled pilot trial. Ultrasound Quarterly, 37(3), 292–296.
- Noll, C., von Jan, U., Raap, U., & Albrecht, U.-V. (2017). Mobile augmented reality as a feature for self-oriented, blended learning in medicine: Randomized controlled trial. JMIR mHealth and uHealth, 5(9), e7943. https://doi.org/10.2196/mhealth.7943
- Orland, M. D., Patetta, M. J., Wieser, M., Kayupov, E., & Gonzalez, M. H. (2020). Does virtual reality improve procedural completion and accuracy in an intramedullary tibial nail procedure? A randomized control trial. Clinical Orthopaedics & Related Research, 478(9), 2170–2177. https://doi.org/10.1097/CORR.0000000000001362
- Plotzky, C., Loessl, B., Kuhnert, B., Friedrich, N., Kugler, C., König, P., & Kunze, C. (2023). My hands are running away - learning a complex nursing skill via virtual reality simulation: A randomised mixed methods study. BMC Nursing, 22(1), 222. https://doi.org/10.1186/s12912-023-01384-9
- Price, M. F., Tortosa, D. E., Fernandez-Pacheco, A. N., Alonso, N. P., Madrigal, J. J. C., Melendreras-Ruiz, R., García-Collado, A. J., Manuel, P. R., & Rodriguez, L. J. (2018). Comparative study of a simulated incident with multiple victims and immersive virtual reality. Nurse Education Today, 71, 48–53. https://doi.org/10.1016/j.nedt.2018.09.006
- Ros, M., Debien, B., Cyteval, C., Molinari, N., Gatto, F., & Lonjon, N. (2020). Applying an immersive tutorial in virtual reality to learning a new technique. Neurochirurgie, 66(4), 212–218. https://doi.org/10.1016/j.neuchi.2020.05.006
- Ros, M., Neuwirth, L. S., Ng, S., Debien, B., Molinari, N., Gatto, F., & Lonjon, N. (2021). The effects of an immersive virtual reality application in first person point-of-view (IVRA-FPV) on the learning and generalized performance of a lumbar puncture medical procedure. Educational Technology Research and Development, 69, 1529–1556. https://doi.org/10.1007/s11423-021-10003-w
- Schoeb, D. S., Schwarz, J., Hein, S., Schlager, D., Pohlmann, P. F., Frankenschmidt, A., Gratzke, C., & Miernik, A. (2020). Mixed reality for teaching catheter placement to medical students: A randomized single-blinded, prospective trial. BMC Medical Education, 20(1), 510. https://doi.org/10.1186/s12909-020-02450-5
- Shao, X., Yuan, Q., Qian, D., Ye, Z., Chen, G., Le Zhuang, K., Jiang, X., Jin, Y., & Qiang, D. (2020). Virtual reality technology for teaching neurosurgery of skull base tumor. BMC Medical Education, 20(1), 3. https://doi.org/10.1186/s12909-019-1911-5
- Smith, S. J., Farra, S. L., Ulrich, D. L., Hodgson, E., Nicely, S., & Mickle, A. (2018). Effectiveness of two varying levels of virtual reality Simulation. Nursing Education Perspectives, 39(6), E10–E15. https://doi.org/10.1097/01.NEP.0000000000000369
- Stepan, K., Zeiger, J., Hanchuk, S., Del Signore, A., Shrivastava, R., Govindaraj, S., & Iloreta, A. (2017). Immersive virtual reality as a teaching tool for neuroanatomy: Immersive VR as a neuroanatomy teaching tool. International Forum of Allergy & Rhinology, 7(10), 1006–1013. https://doi.org/10.1002/alr.21986
- Sultan, L., Abuznadah, W., Al-Jifree, H., Khan, M. A., Alsaywid, B., & Ashour, F. (2019). An experimental study on usefulness of virtual reality 360° in undergraduate medical education. Advances in Medical Education and Practice, 10, 907–916. https://doi.org/10.2147/AMEP.S219344
- Watari, T., Tokuda, Y., Owada, M., & Onigata, K. (2020). The utility of virtual patient simulations for clinical reasoning education. International Journal of Environmental Research and Public Health, 17(15), 5325. https://doi.org/10.3390/ijerph17155325
- Wolf, J., Wolfer, V., Halbe, M., Maisano, F., Lohmeyer, Q., & Meboldt, M. (2021). Comparing the effectiveness of augmented reality-based and conventional instructions during single ECMO cannulation training. International Journal of Computer Assisted Radiology and Surgery, 16(7), 1171–1180. https://doi.org/10.1007/s11548-021-02408-y
- Yang, S.-Y., & Oh, Y.-H. (2022). The effects of neonatal resuscitation gamification program using immersive virtual reality: A quasi-experimental study. Nurse Education Today, 117, 105464. https://doi.org/10.1016/j.nedt.2022.105464
- Yeo, C. T., Ungi, T., Leung, R., Moult, E., Sargent, D., McGraw, R., & Fichtinger, G. (2018). Augmented reality assistance in training needle insertions of different levels of difficulty. In Medical Imaging 2018: Image-Guided Procedures, Robotic Interventions, and Modeling, 10576, 266–271. SPIE.
- Yu, P., Pan, J., Wang, Z., Shen, Y., Li, J., Hao, A., & Wang, H. (2022). Quantitative influence and performance analysis of virtual reality laparoscopic surgical training system. BMC Medical Education, 22(1), 92. https://doi.org/10.1186/s12909-022-03150-y
- Zackoff, M. W., Real, F. J., Sahay, R. D., Fei, L., Guiot, A., Lehmann, C., Tegtmeyer, K., & Klein, M. (2020). Impact of an immersive virtual reality curriculum on medical students’ clinical assessment of infants with respiratory distress. Pediatric Critical Care Medicine, 21(5), 477–485. https://doi.org/10.1097/PCC.0000000000002249
- Zhou, G., Nagle, A., Takahashi, G., Hornbeck, T., Loomis, A., Smith, B., Duerstock, B., & Yu, D. (2022). Bringing patient mannequins to life: 3D projection enhances nursing simulation. In Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems (pp. 1–15). https://doi.org/10.1145/3491102.3517562
References
- Abbas, J. R., Chu, M. M., Jeyarajah, C., Isba, R., Payton, A., McGrath, B., Tolley, N., & Bruce, I. (2023). Virtual reality in simulation-based emergency skills training: A systematic review with a narrative synthesis. Resuscitation Plus, 16, 100484. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, M., & Dunbar, D. M. (2021). Virtual and augmented realities in nursing education: State of the science. Annual Review of Nursing Research, 39, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, M., Voepel-Lewis, T., Cherara, L., Weber, M., Khouri, C., Levine, R., & Tait, A. R. (2018). Interactive anatomy-augmented virtual simulation training. Clinical Simulation in Nursing, 15, 34–41. [Google Scholar] [CrossRef]
- Ajemba, M. N., Ikwe, C., & Iroanya, J. C. (2024). Effectiveness of simulation-based training in medical education: Assessing the impact of simulation-based training on clinical skills acquisition and retention: A systematic review. World Journal of Advanced Research and Reviews, 21, 1833–1843. [Google Scholar] [CrossRef]
- Andersen, N. L., Jensen, R. O., Konge, L., Laursen, C. B., Falster, C., Jacobsen, N., Elhakim, M. T., Bojsen, J. A., Riisshede, M., Fransen, M., Rasmussen, B. S. B., Posth, S., Sant, L., & Graumann, O. (2023). Immersive virtual reality in basic point-of-care ultrasound training: A randomized controlled trial. Ultrasound in Medicine and Biology, 49(1), 178–185. [Google Scholar] [CrossRef]
- Andersen, N. L., Jensen, R. O., Posth, S., Laursen, C. B., Jørgensen, R., & Graumann, O. (2021). Teaching ultrasound-guided peripheral venous catheter placement through immersive virtual reality: An explorative pilot study. Medicine, 100(27), e26394. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L. W., & Krathwohl, D. R. (Eds.). (2001). A taxonomy for learning, teaching, and assessing: A revision of bloom’s taxonomy of educational objectives. Addison Wesley Longman Inc. ISBN 080131903X. [Google Scholar]
- Arents, V., De Groot, P. C. M., Struben, V. M. D., & Van Stralen, K. J. (2021). Use of 360° virtual reality video in medical obstetrical education: A quasi-experimental design. BMC Medical Education, 21(1), 202. [Google Scholar] [CrossRef]
- Ayub, S. M. (2022). See one, do one, teach one: Balancing patient care and surgical training in an emergency trauma department. Journal of Global Health, 12, 03051. [Google Scholar] [CrossRef]
- Azimi, E., Winkler, A., Tucker, E., Qian, L., Doswell, J., Navab, N., & Kazanzides, P. (2018, July 18–21). Can mixed-reality improve the training of medical procedures? 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 4065–4068), Honolulu, HI, USA. [Google Scholar] [CrossRef]
- Banaszek, D., You, D., Chang, J., Pickell, M., Hesse, D., Hopman, W. M., Borschneck, D., & Bardana, D. (2017). Virtual reality compared with bench-top simulation in the acquisition of arthroscopic skill: A randomized controlled trial. The Journal of Bone and Joint Surgery, 99(7), 34. [Google Scholar] [CrossRef]
- Bankar, M. N., Bankar, N. J., Singh, B. R., Bandre, G. R., Shelke, Y. P., Bankar, M., & Shelke, Y. P. (2023). The role of E-content development in medical teaching: How far have we come? Cureus, 15, e43208. [Google Scholar] [CrossRef]
- Barsalou, L. W. (2008). Grounded cognition. Annual Review of Psychology, 59, 617–645. [Google Scholar] [CrossRef]
- Baskaran, R., Mukhopadhyay, S., & Ganesananthan, S. (2023). Enhancing medical students’ confidence and performance in integrated structured clinical examinations (ISCE) through a novel near-peer, mixed model approach during the COVID-19 pandemic. BMC Medical Education, 23, 128. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S. B., & Caliskan, N. (2019). Effect of a game-based virtual reality phone application on tracheostomy care education for nursing students: A randomized controlled trial. Nurse Education Today, 79, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Becker, L. R., & Hermosura, B. A. (2019). Comprehensive healthcare simulation: Obstetrics and gynecology (pp. 11–24). Springer. ISBN 978-3-319-98994-5. [Google Scholar]
- Beilock, S. L., & Goldin-Meadow, S. (2010). Gesture changes thought by grounding it in action. Psychological Science, 21, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, G., Zukotynski, B., Cevallos, N., Ishmael, C., Zoller, S., Burke, Z., Clarkson, S., Park, H., Bernthal, N., & SooHoo, N. F. (2020). Randomized trial of a virtual reality tool to teach surgical technique for tibial shaft fracture intramedullary nailing. Journal of Surgical Education, 77(4), 969–977. [Google Scholar] [CrossRef]
- Bogomolova, K., Ham, I. J. M., Dankbaar, M. E. W., Broek, W. W., Hovius, S. E. R., Hage, J. A., & Hierck, B. P. (2020). The effect of stereoscopic augmented reality visualization on learning snatomy and the modifying effect of visual-spatial abilities: A double-center randomized controlled trial. Anatomical Sciences Education, 13(5), 558–567. [Google Scholar] [CrossRef]
- Bork, F., Stratmann, L., Enssle, S., Eck, U., Navab, N., Waschke, J., & Kugelmann, D. (2019). The benefits of an augmented reality magic mirror system for integrated radiology teaching in gross anatomy: Anatomical sciences education. Anatomical Sciences Education, 12(6), 585–598. [Google Scholar] [CrossRef]
- Brinkmann, C., Fritz, M., Pankratius, U., Bahde, R., Neumann, P., Schlueter, S., Senninger, N., & Rijcken, E. (2017). Box-or virtual-reality trainer: Which tool results in better transfer of laparoscopic basic skills?-A prospective randomized trial. Journal of Surgical Education, 74(4), 724–735. [Google Scholar] [CrossRef]
- Bube, S., Dagnaes-Hansen, J., Mahmood, O., Rohrsted, M., Bjerrum, F., Salling, L., Hansen, R. B., & Konge, L. (2020). Simulation-based training for flexible cystoscopy–A randomized trial comparing two approaches. Heliyon, 6(1), e0308. [Google Scholar] [CrossRef]
- Butt, A. L., Kardong-Edgren, S., & Ellertson, A. (2018). Using game-based virtual reality with haptics for skill acquisition. Clinical Simulation in Nursing, 16, 25–32. [Google Scholar] [CrossRef]
- Cevallos, N., Zukotynski, B., Greig, D., Silva, M., & Thompson, R. M. (2022). The utility of virtual reality in orthopedic surgical training. Journal of Surgical Education, 79(6), 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y. C., Hu, S. H., Chiu, H. Y., Huang, P. H., Tsai, H. T., & Chuang, Y. H. (2021). The effects of an immersive 3d interactive video program on improving student nurses’ nursing skill competence: A randomized controlled trial study. Nurse Education Today, 103, 104979. [Google Scholar] [CrossRef]
- Chao, Y.-P., Kang, C.-J., Chuang, H.-H., Hsieh, M.-J., Chang, Y.-C., Kuo, T. B. J., Yang, C. C. H., Huang, C.-G., Fang, T.-J., Li, H.-Y., & Lee, L.-A. (2023). Comparison of the effect of 360° versus two-dimensional virtual reality video on history taking and physical examination skills learning among undergraduate medical students: A randomized controlled trial. Virtual Reality, 27(2), 637–650. [Google Scholar] [CrossRef]
- Chen, C. Y., Huang, T. W., Kuo, K. N., & Tam, K. W. (2017). Evidence-based health care: A roadmap for knowledge translation. Journal of the Chinese Medical Association, 80, 747–749. [Google Scholar] [CrossRef]
- Chen, F. Q., Leng, Y. F., Ge, J. F., Wang, D. W., Li, C., Chen, B., & Sun, Z. L. (2020). Effectiveness of virtual reality in nursing education: Meta-analysis. Journal of Medical Internet Research, 22(9), e18290. [Google Scholar] [CrossRef]
- Chen, P. J., & Liou, W. K. (2023). The effects of an augmented reality application developed for paediatric first aid training on the knowledge and skill levels of nursing students: An experimental controlled study. Nurse Education Today, 120, e105629. [Google Scholar] [CrossRef]
- Chengoden, R., Victor, N., Huynh-The, T., Yenduri, G., Jhaveri, R. H., Alazab, M., & Gadekallu, T. R. (2023). Metaverse for healthcare: A survey on potential applications, challenges, and future directions. IEEE Access, 11, 12765–12795. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Program. (2024). Available online: http://www.casp-uk.net/ (accessed on 25 April 2024).
- Crogman, H. T., Cano, V. D., Pacheco, E., Sonawane, R. B., & Boroon, R. (2025). Virtual reality, augmented reality, and mixed reality in experiential learning: Transforming educational paradigms. Education Sciences, 15, 303. [Google Scholar] [CrossRef]
- Dodson, T. M., Reed, J. M., & Cleveland, K. (2023). Exploring undergraduate nursing students’ ineffective communication behaviors in simulation: A thematic analysis. Teaching and Learning in Nursing, 18, 480–485. [Google Scholar] [CrossRef]
- Ekstrand, C., Jamal, A., Nguyen, R., Kudryk, A., Mann, J., & Mendez, I. (2018). Immersive and interactive virtual reality to improve learning and retention of neuroanatomy in medical students: A randomized controlled study. CMAJ Open, 6(1), E103–E109. [Google Scholar] [CrossRef]
- Fokides, E., Atsikpasi, P., & Arvaniti, P. A. (2021). Lessons learned from a project examining the learning outcomes and experiences in 360o videos. Journal of Educational Studies and Multidisciplinary Approaches, 1, 50–70. [Google Scholar] [CrossRef]
- Fu, Y., Cavuoto, L., Qi, D., Panneerselvam, K., Arikatla, V. S., Enquobahrie, A., De, S., & Schwaitzberg, S. D. (2020). Characterizing the learning curve of a virtual intracorporeal suturing simulator VBLaST-SS©. Surgical Endoscopy, 34, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V. (2006). Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Research, 1079, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Haerling, K. A. (2018). Cost-utility analysis of virtual and mannequin-based simulation. Simulation in Healthcare, 13(1), 33–40. [Google Scholar] [CrossRef]
- Han, S. G., Kim, Y. D., Kong, T. Y., & Cho, J. (2021). Virtual reality-based neurological examination teaching tool(VRNET) versus standardized patient in teaching neurological examinations for the medical students: A randomized, single-blind study. BMC Medical Education, 21(1), 493. [Google Scholar] [CrossRef]
- Hashemiparast, M., Negarandeh, R., & Theofanidis, D. (2019). Exploring the barriers of utilizing theoretical knowledge in clinical settings: A qualitative study. International Journal of Nursing Sciences, 6(4), 399–405. [Google Scholar] [CrossRef]
- Henssen, D. J. H. A., Den Heuvel, L., De Jong, G., Vorstenbosch, M. A. T. M., Cappellen Van Walsum, A., Van Den Hurk, M. M., Kooloos, J. G. M., & Bartels, R. H. M. A. (2020). Neuroanatomy learning: Augmented reality vs. cross-sections. Anatomical Sciences Education, 13(3), 353–365. [Google Scholar] [CrossRef]
- Hong, Q. N., Gonzalez-Reyes, A., & Pluye, P. (2018). Improving the usefulness of a tool for appraising the quality of qualitative, quantitative, and mixed methods studies, the Mixed Methods Appraisal Tool (MMAT). Journal of Evaluation in Clinical Practice, 24(3), 459–467. [Google Scholar] [CrossRef]
- Horowitz, M. L., Stone, D. S., Sibrian, J., DuPee, C., & Dang, C. (2022). An innovative approach for graduate nursing student achievement of leadership, quality, and safety competencies. Journal of Professional Nursing, 43, 134–139. [Google Scholar] [CrossRef]
- Hu, K.-C., Salcedo, D., Kang, Y.-N., Lin, C.-W., Hsu, C.-W., Cheng, C.-Y., Suk, F.-M., Huang, W.-C., & Ito, E. (2020). Impact of virtual reality anatomy training on ultrasound competency development: A randomized controlled trial. PLoS ONE, 15(11), e0242731. [Google Scholar] [CrossRef]
- Huai, P., Li, Y., Wang, X., Zhang, L., Liu, N., & Yang, H. (2024). The effectiveness of virtual reality technology in student nurse education: A systematic review and meta-analysis. Nurse Education Today, 138, 106189. [Google Scholar] [CrossRef] [PubMed]
- Hultquist, B. L., & Bradshaw, M. J. (Eds.). (2016). Innovative teaching strategies in nursing and related health professions (7th ed.). Jones & Bartlett Publishers. ISBN 978-1284107074. [Google Scholar]
- Immordino-Yang, M. H., & Damasio, A. (2007). We feel, therefore we learn: The relevance of affective and social neuroscience to education. Mind, Brain, and Education, 1, 3–10. [Google Scholar] [CrossRef]
- Issa, T., Isaias, P. T., & Kommers, P. (2013). Guest editors’ introduction—Special issue on digital society and e-technologies. Pacific Asia Journal of the Association for Information Systems, 5, 1. [Google Scholar] [CrossRef]
- Issenberg, B. S., Mcgaghie, W. C., Petrusa, E. R., Lee, G. D., & Scalese, R. J. (2005). Features and uses of high-fidelity medical simulations that lead to effective learning: A BEME systematic review. Medical Teacher, 27, 10–28. [Google Scholar] [CrossRef]
- Issleib, M., Kromer, A., Pinnschmidt, H. O., Süss-Havemann, C., & Kubitz, J. C. (2021). Virtual reality as a teaching method for resuscitation training in undergraduate first year medical students: A randomized controlled trial. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 29(1), 27. [Google Scholar] [CrossRef] [PubMed]
- Jallad, S. T., & Işık, B. (2022). The effectiveness of virtual reality simulation as a learning strategy in the acquisition of medical skills in nursing education: A systematic review. Irish Journal of Medical Science, 191, 1407–1426. [Google Scholar] [CrossRef]
- Jaros, S., & Dallaghan, G. B. (2024). Medical education research study quality instrument: An objective instrument susceptible to subjectivity. Medical Education Online, 29, 2308359. [Google Scholar] [CrossRef]
- Jaskiewicz, F., Kowalewski, D., Starosta, K., Cierniak, M., & Timler, D. (2020). Chest compressions quality during sudden cardiac arrest scenario performed in virtual reality: A crossover study in a training environment. Medicine, 99(48), e23374. [Google Scholar] [CrossRef]
- Jiang, J., & Fryer, L. K. (2024). The effect of virtual reality learning on students’ motivation: A scoping review. Journal of Computer Assisted Learning, 40, 360–373. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. (2014). Reviewers’ manual: 2014 edition. Available online: http://www.joannabriggs.org (accessed on 7 April 2024).
- Johnson-Glenberg, M. C. (2019). The necessary nine: Design principles for embodied VR and active stem education. Springer. ISBN 978-981-13-8265-9. [Google Scholar]
- Johnson-Glenberg, M. C. (2022). Evaluating embodied immersive STEM VR using the quality of education in virtual reality rubric (QUIVRR). MIT Press. ISBN 9780262368995. [Google Scholar]
- Johnson-Glenberg, M. C., & Megowan-Romanowicz, C. (2017). Embodied science and mixed reality: How gesture and motion capture affect physics education. Cognitive Research: Principles and Implications, 2(2017), 1–28. [Google Scholar] [CrossRef]
- Jung, A. R., & Park, E. A. (2022). The effectiveness of learning to use HMD-based VR technologies on nursing students: Chemoport insertion surgery. International Journal of Environmental Research and Public Health, 19(8), 4823. [Google Scholar] [CrossRef]
- Kane, D., Ryan, G., Mangina, E., & McAuliffe, F. M. (2022). A randomized control trial of a virtual reality learning environment in obstetric medical student teaching. International Journal of Medical Informatics, 168, 104899. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D. M., & Mann, K. V. (2010). Teaching and learning in medical education: How theory can inform practice. In T. Swanwick (Ed.), Understanding medical education: Evidence, theory, and practice (pp. 16–36). Wiley Online Library. ISBN 978-1-405-19680-2. [Google Scholar] [CrossRef]
- Khan, R., Plahouras, J., Johnston, B. C., Scaffidi, M. A., Grover, S. C., & Walsh, C. M. (2018). Virtual reality simulation training for health professions trainees in gastrointestinal endoscopy. Cochrane Database of Systematic Reviews, 8, CD008237. [Google Scholar] [CrossRef] [PubMed]
- Kivuti-Bitok, L. W., Cheptum, J. J., Mutwiri, M., Wanja, S., & Ngune, I. (2022). Virtual reality and serious gaming in re-engineering clinical teaching: A review of literature of the experiences and perspectives of clinical trainers. African Journal of Health, Nursing and Midwifery, 6, 53–86. [Google Scholar] [CrossRef]
- Kolb, D. A. (1984). Experiential learning: Experience as the source of learning and development. Prentice-Hall. ISBN 0132952610. [Google Scholar]
- Kolb, D. A. (2015). Experiential learning: Experience as the source of learning and development (2nd ed.). Pearson Education Inc. ISBN 0-13-389240-9. [Google Scholar]
- Kolcun, K., Zellefrow, C., Karl, J., Ulloa, J., Zehala, A., Zeno, R., & Tornwall, J. (2023). Identifying best practices for virtual nursing clinical education: A scoping review. Journal of Professional Nursing, 48, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Kovoor, J. G., Gupta, A. K., & Gladman, M. A. (2021). Validity and effectiveness of augmented reality in surgical education: A systematic review. Surgery, 170, 88–98. [Google Scholar] [CrossRef]
- Kowalewski, K. F., Minassian, A., Hendrie, J. D., Benner, L., Preukschas, A. A., Kenngott, H. G., Fischer, L., Müller-Stich, B. P., & Nickel, F. (2019). One or two trainees per workplace for laparoscopic surgery training courses: Results from a randomized controlled trial. Surgical Endoscopy, 33, 1523–1531. [Google Scholar] [CrossRef]
- Küçük, S., Kapakin, S., & Göktaş, Y. (2016). Learning anatomy via mobile augmented reality: Effects on achieve-ment and cognitive load: Learning anatomy. Anatomical Sciences Education, 9(5), 411–421. [Google Scholar] [CrossRef]
- Lau, S. T., Liaw, S. Y., Loh, W. L., Schmidt, L. T., Yap, J., Lim, F. P., Ang, E., Jiat, C., & Siah, R. (2023). Mid-career switch nursing students’ perceptions and experiences of using immersive virtual reality for clinical skills learning: A mixed methods study. Nurse Education Today, 124, 105760. [Google Scholar] [CrossRef]
- Lebert, A., & Vilarroya, Ó. (2024). The links between experiential learning and 4E cognition. Annals of the New York Academy of Sciences, 1541, 37–52. [Google Scholar] [CrossRef]
- Le Cook, B., Manning, W., & Alegria, M. (2013). Measuring disparities across the distribution of mental health care expenditures. The Journal of Mental Health Policy and Economics, 16(1), 3. [Google Scholar] [PubMed Central]
- Lemke, M., Lia, H., Gabinet-Equihua, A., Sheahan, G., Winthrop, A., Mann, S., Fichtinger, G., & Zevin, B. (2020). Optimizing resource utilization during proficiency-based training of suturing skills in medical students: A randomized controlled trial of faculty-led, peer tutor-led, and holography-augmented methods of teaching. Surgical Endoscopy, 34, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Li, X., Wei, R., Slatyer, S., & Rappa, N. A. (2025). Extended reality for enhancing communication skills in nursing education: A scoping review. Teaching and Learning in Nursing, in press. [Google Scholar] [CrossRef]
- Logeswaran, A., Munsch, C., Chong, Y. J., Ralph, N., & McCrossnan, J. (2021). The role of extended reality technology in healthcare education: Towards a learner-centered approach. Future Healthcare Journal, 8(1), e79–e84. [Google Scholar] [CrossRef]
- Logishetty, K., Western, L., Morgan, R., Iranpour, F., Cobb, J. P., & Auvinet, E. (2019). Can an augmented reality headset improve accuracy of acetabular cup orientation in simulated THA? A randomized trial. Clinical Orthopaedics & Related Research, 477(5), 1190–1199. [Google Scholar] [CrossRef]
- Lu, F., & Bowman, D. A. (2021, March 27–April 1). Evaluating the potential of glanceable AR interfaces for authentic everyday uses. 2021 IEEE Virtual Reality and 3D User Interfaces (pp. 768–777), Lisboa, Portugal. [Google Scholar] [CrossRef]
- Macrine, S. L., & Fugate, J. M. B. (2021). Translating embodied cognition for embodied learning in the classroom. Frontiers in Education, 6, 712626. [Google Scholar] [CrossRef]
- Macrine, S. L., & Fugate, J. M. B. (Eds.). (2022). Movement matters: How embodied cognition informs teaching and learning. MIT Press. [Google Scholar] [CrossRef]
- Macrine, S. L., Fugate, J. M. B., Hou, Y., & Walkington, C. (2025). Engaging the senses: A framework for embodied learning in STEM and healthcare education [in preparation]. [Google Scholar]
- Mann, K. V. (2011). Theoretical perspectives in medical education: Past experience and future possibilities. Medical Education, 45, 60–68. [Google Scholar] [CrossRef]
- Maresky, H. S., Oikonomou, A., Ali, I., Ditkofsky, N., Pakkal, M., & Ballyk, B. (2018). Virtual reality and cardiac anatomy: Exploring immersive three-dimensional cardiac imaging, a pilot study in undergraduate medical anatomy education. Clinical Anatomy, 32(2), 238–243. [Google Scholar] [CrossRef]
- McGrath, J. L., Tekman, J. M., Dev, P., Danforth, D. R., Mohan, D., Kman, N., Crichlow, A., & Bond, W. F. (2018). Using virtual reality simulation environments to assess competence for emergency medicine learners. Academic Emergency Medicine, 25, 186–195. [Google Scholar] [CrossRef]
- Mishra, P., & Koehler, M. J. (2006). Technological pedagogical content knowledge: A framework for teacher knowledge. Teachers College Record, 108, 1017–1054. [Google Scholar] [CrossRef]
- Moll-Khosrawi, P., Falb, A., Pinnschmidt, H., Zöllner, C., & Issleib, M. (2022). Virtual reality as a teaching method for resuscitation training in undergraduate first year medical students during COVID-19 pandemic: A randomised controlled trial. BMC Medical Education, 22(1), 483. [Google Scholar] [CrossRef]
- Moro, C., Štromberga, Z., Raikos, A., & Stirling, A. (2017). The effectiveness of virtual and augmented reality in health sciences and medical anatomy: VR and AR in health sciences and medical anatomy. Anatomical Sciences Education, 10(6), 549–559. [Google Scholar] [CrossRef] [PubMed]
- Nagayo, Y., Saito, T., & Oyama, H. (2022). Augmented reality self-training system for suturing in open surgery: A randomized controlled trial. International Journal of Surgery, 102, 106650. [Google Scholar] [CrossRef]
- Neumann, E., Mayer, J., Russo, G. I., Amend, B., Rausch, S., Deininger, S., Harland, N., Da Costa, I. A., Hennenlotter, J., Stenzl, A., Kruck, S., & Bedke, J. (2019). Transurethral resection of bladder tumors: Next-generation virtual reality training for surgeons. European Urology Focus, 5(5), 906–911. [Google Scholar] [CrossRef] [PubMed]
- Newen, A., De Bruin, L., & Gallagher, S. (Eds.). (2018). The Oxford handbook of 4E cognition. Oxford University Press. ISBN 978-0-19-873541-0. [Google Scholar]
- Nielsen, M. R., Kristensen, E. Q., Jensen, R. O., Mollerup, A. M., Pfeiffer, T., & Graumann, O. (2021). Clinical ultrasound education for medical students: Virtual reality versus e-learning, a randomized controlled pilot trial. Ultrasound Quarterly, 37(3), 292–296. [Google Scholar] [CrossRef] [PubMed]
- Noll, C., von Jan, U., Raap, U., & Albrecht, U.-V. (2017). Mobile augmented reality as a feature for self-oriented, blended learning in medicine: Randomized controlled trial. JMIR mHealth and uHealth, 5(9), e7943. [Google Scholar] [CrossRef]
- Orland, M. D., Patetta, M. J., Wieser, M., Kayupov, E., & Gonzalez, M. H. (2020). Does virtual reality improve procedural completion and accuracy in an intramedullary tibial nail procedure? A randomized control trial. Clinical Orthopae-dics & Related Research, 478(9), 2170–2177. [Google Scholar] [CrossRef]
- Pfeifer, R., & Bongard, J. (2007). How the body shapes the way we think: A new view of intelligence. MIT Press. ISBN 9780262281553. [Google Scholar]
- Plotzky, C., Loessl, B., Kuhnert, B., Friedrich, N., Kugler, C., König, P., & Kunze, C. (2023). My hands are running away—Learning a complex nursing skill via virtual reality simulation: A randomised mixed methods study. BMC Nursing, 22(1), 222. [Google Scholar] [CrossRef]
- Pottle, J. (2019). Virtual reality and the transformation of medical education. Future Healthcare Journal, 6, 181–185. [Google Scholar] [CrossRef]
- Price, M. F., Tortosa, D. E., Fernandez-Pacheco, A. N., Alonso, N. P., Madrigal, J. J. C., Melendreras-Ruiz, R., García-Collado, A. J., Manuel, P. R., & Rodriguez, L. J. (2018). Comparative study of a simulated incident with multiple victims and immersive virtual reality. Nurse Education Today, 71, 48–53. [Google Scholar] [CrossRef]
- Radianti, J., Majchrzak, T. A., Fromm, J., & Wohlgenannt, I. (2020). A systematic review of immersive virtual reality applications for higher education: Design elements, lessons learned, and research agenda. Computers & Education, 147, 103778. [Google Scholar] [CrossRef]
- Ribeiro, K. R. B., do Prado, M. L., Backes, V. M. S., Mendes, N. P. D. N., & Mororó, D. D. (2020). Teaching in health residencies: Knowledge of preceptors under Shulman’s analysis. Revista Brasileira de Enfermagem, 73. [Google Scholar] [CrossRef]
- Rohrich, R. J. (2006). See one, do one, teach one: An old adage with a new twist. Plastic Reconstructive Surgery, 118, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Ros, M., Debien, B., Cyteval, C., Molinari, N., Gatto, F., & Lonjon, N. (2020). Applying an immersive tutorial in virtual reality to learning a new technique. Neurochirurgie, 66(4), 212–218. [Google Scholar] [CrossRef]
- Ros, M., Neuwirth, L. S., Ng, S., Debien, B., Molinari, N., Gatto, F., & Lonjon, N. (2021). The effects of an immersive virtual reality application in first person point-of-view (IVRA-FPV) on the learning and generalized performance of a lumbar puncture medical procedure. Educational Technology Research and Development, 69, 1529–1556. [Google Scholar] [CrossRef]
- Saleem, M., & Khan, Z. (2023). Healthcare Simulation: An effective way of learning in health care. Pakistan Journal of Medical Sciences, 39, 1185–1190. [Google Scholar] [CrossRef]
- Schoeb, D. S., Schwarz, J., Hein, S., Schlager, D., Pohlmann, P. F., Frankenschmidt, A., Gratzke, C., & Miernik, A. (2020). Mixed reality for teaching catheter placement to medical students: A randomized single-blinded, prospective trial. BMC Medical Education, 20(1), 510. [Google Scholar] [CrossRef]
- Sezgin, M. G., & Bektas, H. (2023). Effectiveness of interprofessional simulation-based education programs to improve teamwork and communication for students in the healthcare profession: A systematic review and meta-analysis of randomized controlled trials. Nurse Education Today, 120, 105619. [Google Scholar] [CrossRef] [PubMed]
- Shah, N. K., Taunk, N. K., Maxwell, R., Wang, X., Hubley, E., Anamalayil, S., Trotter, J. W., & Li, T. (2022). Comparison of virtual reality platforms to enhance medical education for procedures. Frontiers in Virtual Reality, 3, 1000035. [Google Scholar] [CrossRef]
- Shao, X., Yuan, Q., Qian, D., Ye, Z., Chen, G., Le Zhuang, K., Jiang, X., Jin, Y., & Qiang, D. (2020). Virtual reality technology for teaching neurosurgery of skull base tumor. BMC Medical Education, 20(1), 3. [Google Scholar] [CrossRef]
- Shapiro, L., & Spaulding, S. (2019). Embodied cognition and sport. In Handbook of embodied cognition and sport psychology (pp. 3–21). The MIT Press. ISBN 9780262038508. [Google Scholar]
- Shulman, L. S. (1986). Those who understand: Knowledge growth in teaching (pp. 4–14). Educational Researcher. [Google Scholar]
- Smith, S. J., Farra, S. L., Ulrich, D. L., Hodgson, E., Nicely, S., & Mickle, A. (2018). Effectiveness of two varying levels of virtual reality Simulation. Nursing Education Perspectives, 39(6), E10–E15. [Google Scholar] [CrossRef]
- Stepan, K., Zeiger, J., Hanchuk, S., Del Signore, A., Shrivastava, R., Govindaraj, S., & Iloreta, A. (2017). Immersive virtual reality as a teaching tool for neuroanatomy: Immersive VR as a neuroanatomy teaching tool. International Forum of Allergy & Rhinology, 7(10), 1006–1013. [Google Scholar] [CrossRef]
- Su, Y., & Zeng, Y. (2023). Simulation-based training versus non-simulation-based training in anesthesiology: A meta-analysis of randomized controlled trials. Heliyon, 9, e18249. [Google Scholar] [CrossRef] [PubMed]
- Sultan, L., Abuznadah, W., Al-Jifree, H., Khan, M. A., Alsaywid, B., & Ashour, F. (2019). An experimental study on usefulness of virtual reality 360° in undergraduate medical education. Advances in Medical Education and Practice, 10, 907–916. [Google Scholar] [CrossRef]
- Tang, K. S., Cheng, D. L., Mi, E., & Greenberg, P. B. (2020). Augmented reality inmedical education: Asystematic review. Canadian Medical Education Journal, 11, 81–96. [Google Scholar] [CrossRef]
- Tonteri, T., Holopainen, J., Lumivalo, J., Tuunanen, T., Parvinen, P., & Laukkanen, T. (2023, January 3–6). Immersive virtual reality in experiential learning: A value co-creation and co-destruction approach. 56th Hawaii International Conference on System Sciences (pp. 1313–1322), Maui, HI, USA. Available online: https://scholarspace.manoa.hawaii.edu/server/api/core/bitstreams/cb46cecd-c8e2-4f8e-9c37-c44af3ae0d32/content (accessed on 6 May 2024).
- Uslu-Sahan, F., Bilgin, A., & Ozdemir, L. (2023). Effectiveness of virtual reality simulation among BSN students: A meta-analysis of randomized controlled trials. Computers, Informatics, Nursing, 41, 921–929. [Google Scholar] [CrossRef]
- Van Merriënboer, J. J., & Sweller, J. (2010). Cognitive load theory in health professional education: Design principles and strategies. Medical Education, 44, 85–93. [Google Scholar] [CrossRef]
- Velev, D., & Zlateva, P. (2017). Virtual reality challenges in education and training. International Journal of Learning and Teaching, 3, 33–37. [Google Scholar] [CrossRef]
- Watari, T., Tokuda, Y., Owada, M., & Onigata, K. (2020). The utility of virtual patient simulations for clinical reasoning education. International Journal of Environmental Research and Public Health, 17(15), 5325. [Google Scholar] [CrossRef]
- Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2014). Newcastle-Ottawa quality assessment scale cohort studies. University of Ottawa. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 April 2024).
- Whiting, P. F., Rutjes, A. W., Westwood, M. E., Mallett, S., Deeks, J. J., Reitsma, J. B., Leeflang, M. M., Sterne, J. A., & Bossuyt, P. M. (2011). QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine, 155, 529–536. [Google Scholar] [CrossRef]
- Wilson, M. (2002). Six views of embodied cognition. Psychonomic Bulletin and Review, 9, 625–636. [Google Scholar] [CrossRef]
- Wilson, R. A., & Foglia, L. (2017). Embodied cognition. The Stanford Encyclopedia of Philosophy. ISBN 1095-5054. [Google Scholar]
- Wohlgenannt, I., Simmon, A., & Stieglitz, S. (2020). Virtual reality. Business & Information Systems Engineering, 62, 455–461. [Google Scholar] [CrossRef]
- Wolf, J., Wolfer, V., Halbe, M., Maisano, F., Lohmeyer, Q., & Meboldt, M. (2021). Comparing the effectiveness of augmented reality-based and conventional instructions during single ECMO cannulation training. International Journal of Com-puter Assisted Radiology and Surgery, 16(7), 1171–1180. [Google Scholar] [CrossRef]
- Wu, A. W., & Norvell, M. (2022). To improve patient safety, lean in. Journal of Patient Safety and Risk Management, 27, 3–5. [Google Scholar] [CrossRef]
- Yang, S.-Y., & Oh, Y.-H. (2022). The effects of neonatal resuscitation gamification program using immersive virtual reality: A quasi-experimental study. Nurse Education Today, 117, 105464. [Google Scholar] [CrossRef]
- Yeo, C. T., Ungi, T., Leung, R., Moult, E., Sargent, D., McGraw, R., & Fichtinger, G. (2018). Augmented reality assistance in training needle insertions of different levels of difficulty. In Medical Imaging 2018: Image-Guided Procedures, Robotic Interventions, and Modeling (vol. 10576, pp. 266–271). SPIE. [Google Scholar]
- Yu, P., Pan, J., Wang, Z., Shen, Y., Li, J., Hao, A., & Wang, H. (2022). Quantitative influence and performance analysis of virtual reality laparoscopic surgical training system. BMC Medical Education, 22(1), 92. [Google Scholar] [CrossRef]
- Zackoff, M. W., Real, F. J., Sahay, R. D., Fei, L., Guiot, A., Lehmann, C., Tegtmeyer, K., & Klein, M. (2020). Impact of an immersive virtual reality curriculum on medical students’ clinical assessment of infants with respiratory distress. Pediatric Critical Care Medicine, 21(5), 477–485. [Google Scholar] [CrossRef]
- Zhang, J., Lu, V., & Khanduja, V. (2023). The impact of extended reality on surgery: A scoping review. International Orthopaedics, 47, 611–621. [Google Scholar] [CrossRef]
- Zhou, G., Nagle, A., Takahashi, G., Hornbeck, T., Loomis, A., Smith, B., Duerstock, B., & Yu, D. (2022, April 29–May 5). Bringing patient mannequins to life: 3D projection enhances nursing simulation. 2022 CHI Conference on Human Factors in Computing Systems (pp. 1–15), New Orleans, LA, USA. [Google Scholar] [CrossRef]
- Ziv, A., Wolpe, P. R., Small, S. D., & Glick, S. (2003). Simulation-based medical education: An ethical imperative. Academic Medicine, 78, 783–788. [Google Scholar] [CrossRef]
- Zwaan, R., & Pecher, D. (Eds.). (2005). Grounding cognition: The role of perception and action in memory, language, and thought (pp. 224–245). Cambridge University Press. ISBN 0-521-83464-3. [Google Scholar]
- Zweifach, S. M., & Triola, M. M. (2019). Extended reality in medical education: Driving adoption through provider-centered design. Digital Biomarkers, 3, 14–21. [Google Scholar] [CrossRef]

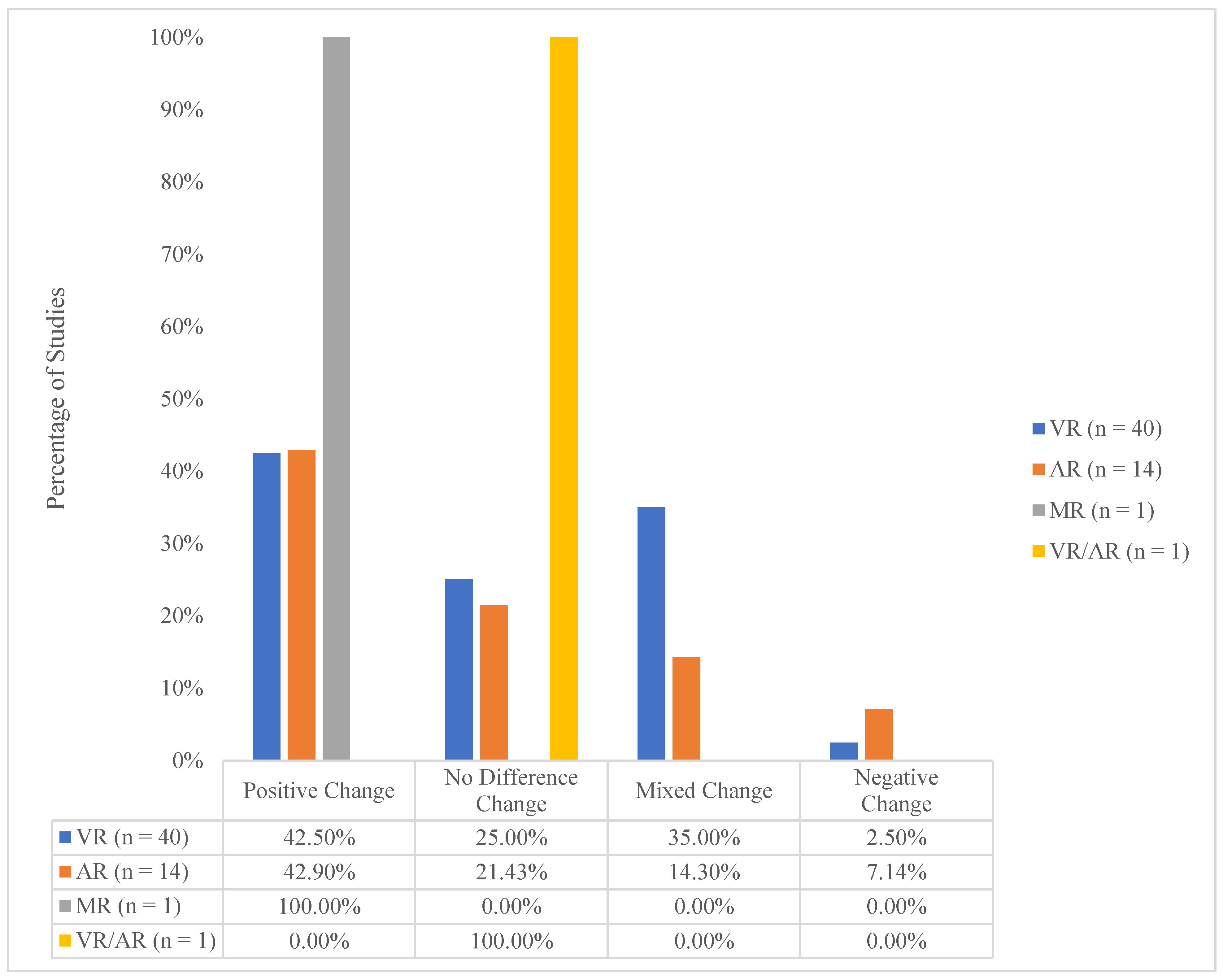
| Authors | Location | Type of I-XR | Population Studied/Setting | Number of Participants | Control Group | Basic Experimental Design/Description | Results | DVs | Outcome Evidence for I-XR | Study Evidence for I-XR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Aebersold et al. (2018) | United States | AR | nursing − tube placement | 69 nursing students | 1 (Combined) | Nursing students were tested on their ability to place a nasogastric tube. They were randomly assigned to either usual training (which included both video and didactic content) or an iPad anatomy-augmented virtual simulation training module. | The AR group was able to more accurately and successfully place the NGT, p = 0.011. CONTROL: n = 34, M = 15.39 (SD = 1.01). EXPERIMENAL: n = 35, M = 15.96 (SD = 0.75). | 1 (performance skills − specific) | + Performance skills | Positive |
| 2. Andersen et al. (2021) | Denmark | VR + Electronic * | Medical − catheter placement | 19 medical students | 1 (Did nothing additional) | Students were split into two different training groups: immersive virtual reality versus the control group. Both groups viewed videos showing ultrasound-guided peripheral venous cannulation placement. The control group was given no further training. | The immersive VR group was significantly more successful at peripheral venous cannulation placement in comparison to the control group, p ≤ 0.001. CONTROL: n = 9, M = 22.2% placement [0.11, 0.41]. EXPERIMENTAL: n = 10, M = 73.3% placement [0.56, 0.86]. | 1 (performance skills − specific) | + Performance skills | Positive |
| 3. Andersen et al. (2023) | Denmark | VR | medical − Ultrasound skills | 104 medical students | 1 (Teacher) | Medical students were divided into two groups to learn Point-of-care ultrasound (POCUS) skills: a self-directed immersive virtual reality (IVR) group versus an instructor-led learning group. US skills were then assessed according to an OSAUS test. | There were no significant differences between the self-directed IVR and instructor-led groups in terms of OSAUS scoring or any other subgroup objectives. Overall effect, p = 0.36. EXPERIMENTAL: n = 51, M = 10.3 [9.0, 11.5]. CONTROL: n = 53, M = 11.0 [9.8, 12.2]. | 1 (performance measures − OSCE) | X No difference in performance measures | No Difference |
| 4. Arents et al. (2021) | Netherlands | VR | medical − Obstetrics training | 89 medical students | 1 (Print) | Two weeks prior to medical students’ OB/Gyn internship, students underwent teaching on gentle Casarean Sections (gash) and general obstetric knowledge. Students were divided into either a control group that underwent conventional study or an experimental group that watched 360-degree videos using VR. After the internship, the authors analyzed the grades received for the internship, as well as administered both open-ended and multiple-choice question tests. | No significant difference in internship grade between groups, p = 0.66 (adjusted 0.68). EXPERIMENTAL: n = 53, M = 7.75 CONTROL: n = 48, M = 7.83 Mean difference CI [−0.33, 0.16]. No significant difference in multiple-choice testing (skills knowledge) between the groups, p = 0.91 (adjusted 0.68). EXPERIMENTAL: n = 53, M = 6.63. CONTROL: n = 48, M = 6.67 Mean difference CI [−0.61, 0.55]. | 2 (Grade internship) (Skills knowledge based on MCQs) | X No difference Grade internship X No difference Skills knowledge | No Difference |
| 5. Azimi et al. (2018) | United States | AR | nursing − IV placement & Chest compression | 20 nursing students | 1 (Practice) | Students underwent either standard training or training with AR via a head-mounted display for learning needle chest decompression and IV-line placement skills. The students’ skills were measured with a post-assessment both immediately after training and 3 weeks later. | Results are assessed with respect to control immediately after. The AR head-mounted group displayed better needle chest decompression skills. No p value or M, SD reported. No significant difference in IV placement performance was found between groups. No p value or M or SD was reported. EXPERIMENTAL: n = 10 CONTROL: n = 10 | 2 (performance skills − specific chest) (performance skills- other IV) | + Performance skills X No difference in performance skills | Mixed Positive |
| 6. Banaszek et al. (2017) | Canada | VR | Medical − surgery | 40 medical students | 2 (Practice) (Did nothing) | Medical students underwent five weeks of independent training sessions in one of three groups: a high-fidelity virtual reality arthroscopic simulator, a bench-top arthroscopic simulator, or an untrained group (control). To measure post-test skill acquisition, students performed a diagnostic arthroscopy on both simulators and were tested in a simulated intraoperative environment using a cadaveric knee. A more difficult surprise skills transfer test was also administered. Students were evaluated using the Global Rating Scale (GRS) and a timer to determine efficiency. | Results are not reported for crossover group post- training. Both the high-fidelity VR simulator and bench-top arthroscopic simulator groups showed significant improvement in arthroscopic skills compared to the control, p < 0.05 for both. The VR simulation group showed the greatest improvement in performance in the diagnostic arthroscopy crossover tests using the GRS), p < 0.001. CONTROL: n = not reported, MD = 0.75 (SD only reflected in error bars). EXPERIMENTAL VR: n = not reported, MD = 12.6 (SD only reflected in error bars). VR group showed the fastest improvement in simulated cadaveric setup with timer, p < 0.001. CONTROL: n = not reported, MD = 9.1 (SD only reflected in error bars). EXPERIMENTAL VR: n = not reported, MD = 17.3 (SD only reflected in error bars). | 2 (performance measures GRS) (performance skills-time) | + Performance measures + Performance skills | Positive |
| 7. Bayram & Caliskan (2019) | United States | VR | nursing − tracheostomy care | 172 nursing students | 1 (Combined) | Nursing students were divided into control and VR groups for tracheostomy care and skill knowledge. Both groups completed a theoretical class, labs, and small group study. The experimental group was provided with a game-based virtual reality phone application. Skills knowledge was assessed using the FEMA IS-346 exam, and performance skills were assessed using the Decontamination Checklist for performance. | Results for the less immersive VR are not reported. Only the immersive experimental group is compared to the control group. Both groups increased their skills performance after training but did not differ from one another, p = 0.443. CONTROL: n = 58, M = 13.48 (SD = 0.30). EXPERIMENTAL: n = 59, M = 14.24 (SD = 0.29). Both groups increased their skills and knowledge after training but did not differ from one another, p = 1.00. CONTROL: n = 58, M = 16.07 (SD = 0.30). EXPERIMENTAL: n = 59, M = 16.25 (SD = 0.29). | 2 (performance skills − specific suctioning) (skills knowledge) | X No difference performance skills X No difference skills knowledge | No Difference |
| 8. Blumstein et al. (2020) | United States | VR | medical − surgery | 20 medical students | 1 (Print) | Medical students were randomized into either standard guide (SG) or virtual reality (VR) learning groups to learn intramedullary nailing (IMN) of the tibia. Students then performed a simulated tibia IMN procedure immediately following their training and were evaluated by an attending surgeon using a procedure-specific checklist and a 5-point global assessment scale. Students returned 2 weeks later for repeat training and testing. | The VR groups showed significantly higher global assessment scores, p < 0.001. CONTROL: n = 10, M = 7.5, SD = not reported. EXPERIMENTAL: n = 10, M = 17.5, SD = not reported. The VR also completed a higher percentage of steps correctly according to the procedure-specific checklist, p < 0.002. CONTROL: n = 10, M = 25, SD = not reported. EXPERIMENTAL: n = 10, M = 63, SD = not reported. | 2 (performance skills − specific) (performance measures) | + Performance skills + Performance measures | Positive |
| 9. Bogomolova et al. (2020) | Netherlands | AR | BIO medical students − anatomy | 58 (bio)medical students | 2 (Print) (Practice) | Students were divided into three groups: (1) the stereoscopic 3D augmented-reality (AR) group, (2) the monoscopic 3D desktop model group, or (3) the 2D anatomical atlas group. Students were told what the learning goals consisted of and were given instructions for the session. Visual-spatial abilities were measured before the learning session began. Post-session learning was measured using a 30-question knowledge test that tested the factual, functional, and spatial organization of anatomical structures. | All groups performed equally well on the knowledge test, p = 1.00. Results are between the AR and the atlas control. CONTROL: n = 18, M = 50.9 (SD = 13.8). EXPERIMENTAL: n = 20, M = 47.8 (SD = 9.8). | 1 (skills knowledge) | X No difference in skills knowledge | No Difference |
| 10. Bork et al. (2019) | Germany | AR | medical − anatomy | 749 medical students | 2 (Print) (Practice) | Medical students were divided into one of three groups: (1) the control group using radiology atlases, (2) a virtual dissection table, or (3) AR Magic Mirror. A pre and post-test was taken about anatomy questions. | Pre-post not evaluated for final assessment. Both the AR Magic Mirror group and the Theory (control) group showed significantly increased post-test scores but did not differ from one another. No p value for comparison between change in improvement was given. Results are from the post scores between the AR and the theory from the print control group. CONTROL: n = 24, M = 50.60 (SD = 12.53). EXPERIMENTAL: n = 24, M = 48.00 (SD = 13.07). | 1 (skills knowledge) | X No difference in skills knowledge | No Difference |
| 11. Brinkmann et al. (2017) | Germany | VR | med medical − surgery | 36 medical students | 1 (Practice) | Medical students underwent a 5-day laparoscopic basic skills training course using either a box trainer or virtual reality (VR) training curriculum. Skills were measured by students’ performance of an ex-situ laparoscopic cholecystectomy on a pig liver using RT and errors. The performance was evaluated by the Global Operative Assessment of Laparoscopic Skills (GOALS) score. | Both groups showed significant improvement in their acquisition of laparoscopic basic skills, and the two groups did not differ in improvement on the peg transfer, p = 0.311. CONTROL: n = 18, M = 53 (SD = 21.3). EXPERIMENTAL: n = 18, M = 44.4 (SD = 14.9). The two groups also did not differ in their pattern cutting, p = 0.088. CONTROL: n = 18, M = 31.6 (SD = 17.3). EXPERIMENTAL: n = 18, M = 42.6 (SD = 16.9). The two groups did not differ on loop placement, p = 0.174. CONTROL: n = 18, M = 46.3 (SD = 54). EXPERIMENTAL: n = 18, M = 53.1 (SD = 32.5). The two groups did not differ in their knot tying, p = 0.174. CONTROL: n = 18, M = 37.2 (SD = 11.9). EXPERIMENTAL: n = 18, M = 42.6 (SD = 16.4). The GOALS scores on four of the five items were significantly higher in the box-trained group compared to the VR-trained group (individual comparisons in Table 5 of the original publication). | 5 (performance skills − other peg) (performance skills − other cutting) (performance skills − other loop) (performance skills − other knot) (performance measures- GOALS) | X No difference in performance skills X No difference in performance skills X No difference in performance skills X No difference in performance skills − Performance measures | Mixed Negative |
| 12. Bube et al. (2020) | Denmark | VR | medical- cystoscopy | 32 medical students | 1 (Teacher) | Two groups of medical students completed endoscopic procedure training. The control group underwent traditional lecture-based training, whereas the experimental group used VR and other self-directed simulation training methods. Three weeks after the training, participants performed cystoscopies on two patients, and performance was measured using a Global Rating Scale (GRS). | No significant difference in performance between the two groups was found after training, p = 0.63. CONTROL: n = 12, M = 14.3. EXPERIMENTAL: n = 13, M = 13.6. CI of the difference only reported: [−2.4, 3.9]. | 1 (performance measures − GRS) | X No difference in performance measures | No Difference |
| 13. Butt et al. (2018) | United States | VR | nursing − catheter | 20 nursing students | 1 (Combined) | Nursing students were assigned to either a control group (traditional learning with a task trainer) or an experimental group (VR software/game) to learn catheter insertion skills. Skills were assessed approximately two weeks after completion of the training session. | VR group completed more procedures than the traditional group, p < 0.001. CONTROL: n = 10, M = 1.8 (SD = 0.42). EXPERIMENTAL: n = 10, M = 3.0 (SD = 1.3). Pass rates at two weeks were identical; no p value was given. | 2 (performance skills − other number of procedures completed) (performance skills − other specific pass rates) | + Performance skills X No difference in performance skills | Mixed Positive |
| 14. Cevallos et al. (2022) | United States | VR | medical − surgery | 20 medical students and orthopedic residents | 1 (Combined) | Medical students and orthopedic residents were randomized into either standard guide (SG) or virtual reality (VR) learning groups to learn pinning of a slipped capital femoral epiphysis (SCFE), a pediatric orthopedic surgery procedure. All participants watched a technique video, and the VR group completed additional training on the Osso VR surgical trainer. Participants were then asked to achieve “ideal placement”, and performed a SCFE guidewire placement on Sawbones model 1161. Evaluation was based on time, number of pins “in-and-outs”, articular surface penetration, the angle between the pin and physis, distance from pin tip to the subchondral bone, and distance from the center-center point of the epiphysis. | The VR group showed superiority across multiple domains but were not statistically different from the control in the following: time to final pin placement, p = 0.26. CONTROL: n = 10, M = 706 (SD shown in Figure 1 in the original publication). EXPERIMENTAL: n = 10, M = 573 (SD shown in Figure 1 in the original publication). VR performed better compared to the control for pin in and out p = 0.26. CONTROL: n = 10, M = 1.7 (SD shown in Figure 1 in the original publication). EXPERIMENTAL: n = 10, M = 0.5 (SD shown in Figure 1 in the original publication). VR group performed fewer surface penetrations, p = 0.36. CONTROL: n = 10, M = 0.2 (SD shown in Figure 1 in the original publication). EXPERIMENTAL: n = 10, M = 0.4 (SD shown in Figure 1 in the original publication). VR group had a smaller distance pin to tip to subchondral bone, p = 0.49. CONTROL: n = 10, M = 5.8 (SD = 3.36). EXPERIMENTAL: n = 10, M = 7.2 (SD = 6.5). VR group had a lower angle deviation between the pin and physis, p < 0.05. CONTROL: n = 10, M = 4.9 (SD = 3.0). EXPERIMENTAL: n = 10, M = 2.5 (SD = 1.42). | 5 (performance skills − time) (performance skills − other specific pin in and outs) (performance skills − errors) (performance skills − other specific pin tips to bone) (performance skills − other specific angle) | X No difference in performance skills X No difference in performance skills X No difference in performance skills X No difference in performance skills + Performance skills | Mixed Positive |
| 15. Y. C. Chao et al. (2021) | Taiwan | VR | nursing − tube placement | 45 nursing students | 1 (Electronic) | Nursing students were randomly assigned into two groups to learn nasogastric (NG) tube feeding: (1) an immersive 3D interactive video program group or (2) a regular demonstration video. Students completed a pre- and post-intervention questionnaire, which included a nasogastric tube feeding quiz (NGFQ) to study NG tube feeding knowledge. Students were assessed after intervention and 1 mo. Later. | Knowledge scores on NG tube feeding improved significantly in both groups; however, there was no significant difference in the knowledge scores after treatment, p = 0.77 CONTROL: n = 23, M = 11.7 (SD = 1.86). EXPERIMENTAL: n = 22, M = 11.9 (SD = 2.04). | 1 (skills knowledge) | X No difference in skill knowledge | No Difference |
| 16. Y.-P. Chao et al. (2023) | Taiwan | VR | Medical − intake skills | 64 medical students | 1 (Electronic) | Students were randomized into two groups and received either a 10 min immersive 360-degree virtual reality or a 2D virtual reality instructional video on history taking and physical examination skills. Within 60 min of watching the video, students performed a focused history and physical on a patient. The Direct Observation of Procedural Skills (DOPS) was used to measure physical exam skills, and the Mini-CEX was used to measure general history and physical exam skills. | The average DOPS-total score was significantly higher in the VR video group compared to the 2D video group, p = 0.01. CONTROL: n = 32, M = 85.8 (SD = 3.2). EXPERIMENTAL: n = 32, M = 88.4 (SD = 4.0). No significant differences in the average Mini-CEX scores were found between the groups, p = 0.75. CONTROL: n = 32, M = 39.8 (SD = 5.2). EXPERIMENTAL: n = 32, M = 40.1 (SD = 4.1). | 2 (performance skills − other/DOPS) (skills knowledge − Mini-CEX) | + Performance skills X No difference in skills knowledge | Mixed Positive |
| 17. P. J. Chen & Liou (2023) | Taiwan | AR | Nursing students − first aid | 95 nursing students | 1 (Practice) | Nursing students were divided into two groups for pediatric first-aid training. The control group performed a simulation using a traditional Resusci Annie, whereas the experimental group used an interactive Resusci Anne that overlaid AR. Pre and post-tests were given to evaluate participant knowledge and skills. Knowledge was assessed using a 20-question test. Skill level was assessed using a graded evaluation checklist. | The AR intervention group showed significantly higher post-test knowledge, p < 0.001. CONTROL: n = 49, M = 18.08 (SD = 1.6). EXPERIMENTAL: n = 46, M = 18.78 (SD = 1.1). The AR group also showed improved skill in first-aid level scoring compared to the control group post-test, p < 0.001. CONTROL: n = 49, M = 29.71 (SD = 1.5). EXPERIMENTAL: n = 46, M = 32.52 (SD = 1.3). | 2 (skills knowledge) (performance measures − other specific first aid) | + Skills knowledge + Performance measures | Positive |
| 18. Ekstrand et al. (2018) | Canada | VR + Print * | medical − neuroanatomy | 64 medical students | 1 (did nothing additional) | Medical students neuroanatomy learning with VR. Pre and post-intervention tests were given, including a post-test immediately after the study completion and one 5–9 days later. | Both groups showed significant improvement between pre- and post-test scores but no significant differences on the neuroanatomy test between the groups on either of the post-test results, p = 0.5. Means and SDs are not reported: T-statistic reported for control (n = 33) vs. VR (n = 31) post-training, t(62) = − 0.38. | 1 (skills knowledge) | X No difference in skills knowledge | No Difference |
| 19. Fu et al. (2020) | United States | VR | medical − suturing | 14 medical students | 1 (Practice) | Students were assigned to one of two training groups: (1) the VBLaST-SS (virtual simulator) training group or (2) the FLS training group. Students then watched a video that taught the intracorporal suturing task they were going to be practicing. Students then performed the task on both systems to measure baseline performance. Students then practiced once a day, five days a week, for three weeks. Performance scoring was based on the original FLS scoring system. | Both training modalities showed significant performance improvement, but there were no significant differences in the group x time interaction, p = 0.20. Learning curves for both learning modalities were also similar. Means and SD are only shown in Figure 3 in original publication. | 1 (performance skills − specific FLS) | X No difference in performance skills | No Difference |
| 20. Haerling (2018) | United States | VR | Nursing − case evaluation for COPD | 81 nursing students | 1 (Practice) | This study placed students in two groups: those using mannequin-based simulations and those using VR simulations. Participants completed a standardized patient encounter of a complex case involving a patient with COPD. Pre and post-intervention knowledge assessments were also performed using the LCJR and the C-SEI. | Students in both groups showed significant improvement in post-test knowledge assessment. Scores between the groups were not significantly different in the post-test knowledge assessment, p = 0.48. CONTROL: n = 14, M = 79.82 (SD = 17.63). EXPERIMENTAL: n = 14, M = 82.16 (SD = 11.76). There was no statistical difference post-intervention for either group for the LCJR, p = 0.374. CONTROL: n = 14, M = 82.69 (SD = 13.65). EXPERIMENTAL: n = 14, M = 78.18 (SD = 12.71). There was also no statistical difference post-intervention on the C-SEI between groups. CONTROL: n = 14, M = 84.62 (SD = 14.91). EXPERIMENTAL: n = 14, M = 81.93 (SD = 16.41). | 3 (skills knowledge) (clinical reasoning − LCJR) (clinical reasoning − C-SEI) | X No difference in skills knowledge X No difference in clinical reasoning X No difference in clinical reasoning | No Difference |
| 21. Han et al. (2021) | Republic of Korea | VR + SP | medical − neurological | 95 medical students | 1 (did nothing additional) | Medical students were divided into two groups: a standardized patient (SP) group that was provided neurological findings using conventional methods (verbal description, pictures, videos) versus an SP with Virtual Reality-based Neurological Examination Teaching Tool (VRNET) group. A researcher measured student performance using the Neurologic Physical Exam (NPE) score. | The SP + VR group had significantly higher NPE scores compared to the SP group, p = 0.043. CONTROL: n = 39, M = 3.40 (SD = 1.01). EXPERIMENTAL n = 59, M = 3.81 (SD = 0.92). | 1 (skills knowledge − NPE score) | + Skills knowledge | Positive |
| 22. Henssen et al. (2020) | Netherlands | AR | medical and Biomedical- neuroanatomy | 31 medical and biomedical students | 1 (Print) | Students were assigned to one of two groups for learning neuroanatomy. The control group underwent learning with cross-sections of the brain, whereas the experimental group underwent AR learning. | Results are assessed with respect to control. The control group showed improved post-test scoring compared to the AR, p = 0.035. Results for adapted test scores: CONTROL: n = 16, M = 60.6 (SD = 12.4). EXPERIMENTAL: n = 15, M = 50.0 (SD = 10.2). | 1 (skills knowledge) | − Skills knowledge | Negative |
| 23. Hu et al. (2020) | Taiwan | VR + workshop in ultrasound * | medical − Ultrasound skills | 101 medical students | 1 (Print) | Medical students took place in an ultrasonography (US) training program. They were divided into either the virtual reality (VR) intervention group or the control group. Both groups participated in an ultrasound workshop; however, the intervention group used a self-directed VR-enhanced anatomy review and used VR to complete additional review sessions during the US hands-on practice. After the US workshop was completed, participant competency was measured using a standardized practical US test, which focused on the identification of various anatomical structures, and a 10-Q MCQ on anatomy. | Participants in the intervention group showed significantly higher scores on US task performance overall, p < 0.01. The results below are for mean rank. No variability was given. CONTROL: n = 54, MR = 38.52. EXPERIMENTAL: n = 47, MR = 65.34. The VR group also showed significantly better scores on the knowledge test, p < 0.05. CONTROL: n = 54, median = 2 (IQR = 3). EXPERIMENTAL: n = 47, Median = 3 (IQR = 3). | 2 (skills knowledge) (performance skills − other practical US) | + Skills knowledge + Performance skills | Positive |
| 24. Issleib et al. (2021) | Germany | VR | medical − CPR | 160 medical students | 1 (Practice) | Medical students were randomized into an intervention or control group. The intervention group completed a BLS course in virtual reality, whereas the control group underwent standard BLS training. At the end of training, all students performed a 3 min practical test using the Leardal Mannequin to record no flow time on the task. | The control group had significantly shorter no-flow time compared to the VR, p < 0.0001. CONTROL: n = 104, M = 82.03 (SD = not reported). EXPERIMENTAL: n = 56, M = 92.96 (SD = not reported). | 1 (performance skills − specific, no flow time) | - Performance skills | Negative |
| 25. Jaskiewicz et al. (2020) | Poland | VR + Practice * | medical − CPR | 91 medical students | 1 (Teacher) | Both the control and experimental groups completed a 3-h BLS course including background training and practice on a CPR mannequin. Students then participated in either a traditional teaching or VR scenario where hands-only CPR was completed. The quality of the chest compressions (rate and depth) was then tested and analyzed. | There were no significant differences in chest rate compression performance between the control and virtual reality groups, p = 0.48. CONTROL: n = 45, Median = 114 (IQR 108–122). EXPERIMENTAL: n = 45, Median = 115 (IQR 108–122). There was also no significant difference in chest rate depth between groups, p > 0.05. CONTROL: n = 45, Median = 48 (IQR = 44–55). EXPERIMENAL: n = 45, Median = 49 (IQR = 43–53). Finally, there was a significant increase in the percentage of chest compression relaxation for the control group compared to the VR group, p < 0.01. CONTROL: n = 45, Median = 97 (IQR = 85–100). EXPERIMENTAL: n = 45, Median = 69 (IQR = 26–98). | 3 (performance skills − specific rate) (performance skills − specific depth) (performance skills − specific relaxation) | X No difference in performance skills X No difference in performance skills - Performance skills | Mixed Negative |
| 26. Jung & Park (2022) | Republic of Korea | VR | Nursing students − chemport insertion surgery | 60 nursing students | 1 (Print) | Nursing students were divided into two groups to learn chemoport insertion surgery. The control group’s learning consisted of instruction by an operating nursing instructor, learning via a handout, and time for self-study. The experimental group used VR. Pre and post-test knowledge was assessed using a 10-point questionnaire about key knowledge of insertion. | The VR group showed significantly higher post-test knowledge scores compared to the control group after training, p = 0.001. CONTROL: n = 30, M = 4.80 (SD = 1.65). EXPERIMETNAL: n = 30, M = 6.97 (SD = 1.35). | 1 (skills knowledge) | + Skills knowledge | Positive |
| 27. Kane et al. (2022) | Ireland | VR | med students − obstetrics | 69 medical students | 1 (Electronic) | Medical students were placed into one of two groups to help them learn and conceptualize fetal lies and presentation. The interventional group was immersed in a virtual reality learning environment (VRLE) to explore fetal lie, and the control group used traditional 2D images. After their sessions, clinical exam skills were tested using an obstetric abdominal model. Knowledge was assessed by students’ ability to determine fetal lies and presentation on this model. Time taken to complete the test was also measured. | No significant differences were found between the two groups in terms of knowledge assessment, although the authors note that there was a noticeable trend of higher success rates in the intervention group in the VR group compared to the control group) for a combined lie and presentation scores, p = not reported. CONTROL: n = 34, M = 70.0 (SD = not reported). EXPERIMENTAL: n = 33), M = 56 (SD = not reported). However, the time to complete the task was significantly less in the intervention group compared to the control group, p = 0.012. CONTROL: n = 34, M = 38 (SD = 10.83). EXPERIMENTAL: n = 33, M = 45 (SD = 12.95). | 2 (performance skills − time) (performance skills -specific success) | X No difference in performance skills + Performance skills | Mixed Positive |
| 28. Kowalewski et al. (2019) | Germany | VR | medical − surgery | 100 medical students | 1 (Did nothing) | Medical students were divided into three groups to complete laparoscopic training: (1) the control group, which received no training; (2) the “alone” group, and (3) the dyad group. Intervention groups completed box and VR training, after which performance was measured with a cadaveric porcine laparoscopic cholecystectomy (LC), and the objective structured assessment of technical skills (OSATS) was used. Global operative assessment of laparoscopic skills (GOALS), time to complete LC, and VR performances were also measured. | Results are reported for improvement between the VR and control groups only. The VR group and the control group did not differ on the OSATS, p = 0.548. CONTROL: n = 20, M = 37.1 (SD = 7.4). EXPERIMENTAL: n = 40, M = 40.2 (SD = 9.8). The two groups did not differ on the GOALS either, p = 0.998. CONTROL: n = 20, M = 10.1 (SD = 3.0). EXPERIMENTAL: n = 40, M = 10.6 (SD = 3.0). The VR groups were faster than the control group in completion time, p < 0.001. CONTROL: n = 20, M = 13.5 [11.8, 17.5]. EXPERIMENTAL: n = 40, M = 10.2 [7.9, 11.3]. The VR group also had fewer movements, p = 0.002. CONTROL: n = 20, M = 871 [637, 1105]. EXPERIMENTAL: n = 40, M = 683 [468, 898]. The VR group also had a shorter path length, p = 0.004. CONTROL: n = 20, M = 1640 [1174, 2106]. EXPERIMENTAL: n = 40, M = 1316 [948, 1684]. | 5 (performance measures -OSATS) (performance measures − GOALS) (performance skills − time) (performance skills − other specific path) (performance skills − other specific length) | X No difference in performance measures X No difference in performance measures + Performance skills + Performance skills + Performance skills | Mixed Positive |
| 29. Küçük et al. (2016) | Turkey | AR | medical − anatomy | 70 medical students | 1 (Print) | Medical students were placed into a control group, which used traditional teaching methods (textbook), or an experimental group, which used mobile augmented reality (mAR) technology (MagicBook) to learn neuroanatomy. Post-intervention knowledge was measured using an Academic Achievement Test (AAT), a 30-question multiple-choice test. | The experimental mAR group showed significantly superior performance on the AAT test, p < 0.05. CONTROL: n = 34, M = 68.34 (SD = 12.83). EXPERIMENTAL: n = 36, M = 78.14 (SD = 16.19). | 1 (performance measures − AAT) | + Performance measures | Positive |
| 30. Lau et al. (2023) | Singapore | VR | Nursing students | 34 nursing students | 1 (Nothing) (pre-post) | Nursing students participated in pre-test and post-test knowledge questionnaires regarding both subcutaneous insulin injection and intravenous therapy. After completing the pre-test, participants underwent learning using immersive VR before completing the post-test questionnaire. | There was a significant improvement in knowledge test scores (IV and subcutaneous injection) after training, p = 0.075. Only z-scores were reported with ranks. No M or SDs are reported pre vs. post. | 1 (skills knowledge) | + Skills knowledge | Positive |
| 31. Lemke et al. (2020) | Canada | AR | medical − suturing | 44 medical students | 2 (Teacher) (Combined) | Students were randomized into one of three intervention groups to learn suturing skills: (1) faculty-led, (2) peer tutor-led, or (3) holography-augmented intervention arms (Suture Tutor). Outcomes measured include the number of simple interrupted sutures that were placed to achieve proficiency, the total number of full-length sutures used, and the time to achieve proficiency. | Results are reported among three groups. No significant differences among the intervention groups in the number of full-length sutures used, p = 0.376. Means and SD are reported for Teacher control vs. AR CONTROL: N = 16, M = 80.0 (SD = 47.2). EXPERIMENTAL: n = 14, M = 107.4 (SD = 61.3). No significant differences among the intervention groups in the number of simple interrupted sutures placed, p = 0.735. CONTROL: n = 16, M = 9.3 (SD = 5.3). EXPERIMETNAL: n = 14, M = 11.0 (SD = 6.0). No significant differences among the intervention groups in the time to achieve proficiency, p = 0.390. CONTROL: n = 16, M = 158.1 (SD = 89.2). EXPERIMETNAL: n = 14, M = 205.0 (SD = 113.2). | 3 (performance skills − other specific number placed) (performance skills − other specific interruptions) (performance skills − other specific # used) | X No difference in performance skills X No difference in performance skills X No difference in performance skills | No Difference |
| 32. Logishetty et al. (2019) | United Kingdom | AR | Medical − surgery | 24 medical students | 1 (Practice) | This study simulated total hip arthroplasty (THA) and placed students in one of two groups to determine what training is more effective at improving the accuracy of acetabular component positioning: (1) augmented reality (AR) training (with live holographic orientation feedback) or (2) hands-on training with a hip arthroplasty surgeon. Students participated in one baseline assessment, training session, and reassessment a week for four weeks and were recorded on the target angle (inclination − anteversion). | AR intervention group showed smaller average errors than the control practice with the surgeon group after training, p < 0.0001. CONTROL: n = 12, M = 6 (SD = 4). EXPERIMENTAL: n = 12, M = 1 (SD = 1). In the final session, both groups showed improvement on the target angle, but there was no significant difference in performance between groups, p = 0.281. Means and SD are reported as differences from pre. CONTROL: n = 12, MD = −8.4 [−7.0, −9.8]. EXPERIMENTAL: n = 12, MD = −7.8 [−5.5, −10.2]. | 2 (performance skills − errors) (performance skills − other specific target angles) | + Performance skills X No difference in Performance skills | Mixed Positive |
| 33. Maresky et al. (2018) | Canada | VR immersive + VR simulation* | medical − anatomy | 42 medical students | 1 (Print) | Before learning cardiac anatomy, medical students underwent a VR simulation of the subject. Students then were separated into either a control group that continued independent anatomy study or an experimental group that underwent an immersive VR experience. Pre and post-test scores were obtained, which measured both conventional and visual-spatial (VS) cardiac anatomy questions. | Students in the immersive VR intervention group scored significantly higher overall after the intervention, p < 0.001. Mean differences between the control (n = 14) and experimental group (n = 28) are only reported: MD = 24.8 (SD = 3.89). This included both subsections of VS and conventional content. | 1 (skills knowledge) | + Skills knowledge | Positive |
| 34. Moll-Khosrawi et al. (2022) | Germany | VR | medical − BLS | 88 medical students | 1 (Electronic) | Medical students were placed into one of two groups to compare a control group that received web-based basic life support (BLS) training to an intervention group that underwent additional individual virtual reality (VR) training. The quality of BLS skills was assessed after training with a no-flow-time indicator. Overall, BLS performance was also assessed using an adapted observational checklist, graded by experts. | The VR intervention group showed significantly lower no-flow-time compared to the control, p = 0.009. CONTROL: n = 42, M = 11.05 (SD = 10.765). EXPERIMENTAL: n = 46, M = 6.46 (SD = 3.49). The VR group also showed significantly superior overall (lower penalty point) for their BLS performance in comparison to the control group, p < 0.001. CONTROL: n = 42, M = 29.19 (SD = 16.31). EXPERIMENTAL: n = 46, M = 13.75 (SD = 9.66). | 2 (performance skills − other specific flow time) (performance measures − checklist) | + Performance skills + Performance measures | Positive |
| 35. Moro et al. (2017) | Australia | AR/VR | medical − anatomy | 59 medical and health science students | 1 (Electronic) | Medical students completed a lesson on skull anatomy using one of three learning modalities: (1) virtual reality (VR), (2) augmented reality (AR), or (3) tablet-based (TB). After their 10min anatomy lesson using their respective learning modality, students completed a 20-question multiple-choice anatomy test to measure their knowledge. | No significant differences in the anatomy test scores were found between the three groups; AR and VR intervention did not show superior knowledge acquisition in comparison to tablet-based skull anatomy learning after the intervention, p = 0.874. Means are for the VR group and control group only. Standard deviations are depicted only in Figure 1 in the original publication. CONTROL: n = 22, M = 61. EXPERIMETNAL: n = 20, M = 59. | 1 (skills knowledge) | X No difference in skills knowledge | No Difference |
| 36. Nagayo et al. (2022) | Japan | AR | medical − surgery | 38 medical students | 1 (Electronic) | Medical students were randomized into one of two groups for self-training suturing learning: (1) the augmented reality (AR) training group or (2) the instructional video group. Both groups watched an instructional video on subcuticular interrupted suturing and took a pre-test. They then practiced the suture 10 times using their assigned learning modality before completing a post-test. Pre- and post-tests were performed on a skin pad and were graded using global rating and task-specific subscales. | Both groups showed significant improvement between pre-test and post-test scores in both global rating and task-specific subscales on suturing performance; however, no significant difference in performance was found between the AR and instruction video training groups using the global rating, p = 0.38. CONTROL: n = 19, M = 15.11 (SD = 2.84). EXPERIMENTAL: n = 19, M 15.03 (SD = 1.94). | 1 (performance skills − other specific suturing performance) | + Performance skills | Positive |
| 37. Neumann et al. (2019) | Germany | VR | medical − surgery | 51 medical students | 1 (Electronic) | Medical students were randomized into two groups for cystoscopy (UC) and transurethral bladder tumor resection (TURBT) training. The control group watched video tutorials by an expert. After completion of the training, students performed a VR-UC and VR-TURBT performance task and 12 measures of performance were recorded. | Both groups improved on three variables after training, including significantly lower average procedure length, lower resectoscope movement, and accidental bladder injury, but there was only one significant difference in the improved performances for the VR compared to the control (procedure time, p = 0.04). All Means and SD are listed in Table 2 of the original publication. | 12 (performance skills − other specific × 9) (performance skills − injury) (performance skills − time × 2) | X No difference in performance skills (for 11 of the 12 variables) | No Difference |
| 38. Nielsen et al. (2021) | Denmark | VR | medical − Ultrasound skills | 20 medical students | 1 (Electronic) | Medical students were randomized into either a virtual reality (VR) or e-learning group for ultrasound education and training. Performance was scored using the OSAUS. | The VR group showed significantly higher scoring on the OSAUS compared to the e-learning group after intervention, p ≤ 0.001. CONTROL: n = 9, M = 125.7 (SD = 16.2). EXPERIMENTAL: n = 11, M = 142.6 (SD = 11.8). | 1 (performance measures − OSAUS) | + Performance measures | Positive |
| 39. Noll et al. (2017) | Germany | AR | medical − dermatology | 44 medical students | 1 (Print) | Medical students were randomized into one of two groups for learning dermatological knowledge. Group A’s training involved the use of a mobile augmented reality (mAR) application, whereas Group B’s training involved textbook-based learning. Baseline and post-test knowledge were assessed using a 10-question single choice (SC) test, which was repeated after 14 days to assess longer-term retention. | The initial SC post-test showed significant knowledge gain in both groups, but the VR group showed a marginally significant memory for correct answers after two weeks, compared to the control group, p = 0.10. CONTROL: n = 22, M = 0.33 (SD = 1.62). EXPERIMENTAL: n = 22, M = 1.14 (SD = 1.30). | 1 (skills knowledge) | + (marginal) Skills knowledge | Positive |
| 40. Orland et al. (2020) | United States | VR+ | Medical − surgery | 25 medical students | 1 (Print) | Medical students were randomized into one of three groups for learning intramedullary tibial nail insertion: (1) the technique guide-only control group, (2) the virtual reality (VR) only group, or (3) the VR plus technique guide group. The experimental groups participated in three separate VR simulations, 3–4 days apart. After 10–14 days of preparation, students performed an intramedullary tibial nail insertion simulation into a bone-model tibia. Completion and accuracy were assessed. | Overall assessments are made with comparisons to both VR groups compared to the control group. Both experimental groups that involved VR training showed higher completion rates, p = 0.01 Both groups also had fewer incorrect steps, p = 0.02, in comparison to the control group. The Means and SD below are for VR+ and the control group only. ERRORS CONTROL: n = 8, M = 5.7 (SD = 0.2). EXPERIMENTAL: n = 9, M = 3.1 (SD = 0.1). COMPLETION TIME CONTROL: n = 8, M = 24 (SD = 4). EXPERIMENTAL: n = 9, M = 18 (SD = 8). | 2 (performance skills − errors) (performance skills − time) | + Performance skills + Performance skills | Positive |
| 41. Plotzky et al. (2023) | Germany | VR ‘high’ | Nursing students- endotracheal suctioning skills | 131 nursing students | 2 (low VR) + Electronic | Nursing students were split into one of three groups for learning of endotracheal suctioning skills. The control group’s intervention was a video tutorial. The intervention groups consisted of a VR low group and a VR high group. The VR ‘low’ group’s intervention consisted of basic VR technology, including head tracking and controller-based controls. The VR ‘high’ group’s intervention consisted of more advanced VR technology, including head and hand tracking, allowing users to interact with their actual hands, as well as supplementing with real-world video clips. Participants were assessed using a knowledge test and a skill demonstration test on a manikin using an objective structured clinical examination (OSCE). The knowledge test was given immediately after intervention and 3 weeks later. | Each of the three groups showed a significant increase in knowledge acquisition; however, there was no significant difference among them for skills knowledge, p = 0.730. Means and SDs are for the VR+ vs. control group after intervention. CONTROL: n = 43, M = 7.16 (SD = 2.29). EXPERIMENTAL: n = 47, M = 7.06 (SD = 1.42). There was a significant difference among groups on the OSCE after intervention, p < 0.001. Means and SDs are for the VR+ vs. control group after intervention. CONTROL: n = 43, M = 11.95 (SD = 1.65). EXPERIMENTAL: n = 47, M = 9.41 (SD = 2.70). | 2 (skills knowledge) (performance measures – OSCE) | X No difference in skills knowledge − Performance measures | Mixed Negative |
| 42. Price et al. (2018) | Spain | VR | nursing − triage skills | 67 nursing students | 1 (Practice) | This study compared the use of VR to clinical simulation to determine the efficiency in executing the START (Simple Triage and Rapid Treatment) triage. | There were no significant differences between the VR and clinical simulation groups in the percentage of victims that were correctly triaged, p = 0.612 CONTROL: n = 35, M = 88.3 (SD = 9.65). EXPERIMENTAL: n = 32, M = 87.2 (SD = 7.2). | 1 (performance skills − specific triage variables) | X PNo difference in performance skills | No difference |
| 43. Ros et al. (2020) | France | VR + Print | medical − surgery | 176 medical students | 1 (Did nothing additional) | All students were given a technical note detailing an external ventricular drainage (neurosurgical) technique. Students were randomized into two groups, one of which received no additional training and one which used immersive virtual reality (VR) as supplemental teaching. Knowledge was assessed with a multiple-choice test immediately after training and six months later. | VR training showed significantly superior knowledge gain after both initial assessment and at the six-month mark, p = 0.01. Means and SD are reported after the initial training. CONTROL: n = 88, M = 4.59, (SD = 1.4). EXPERIMENTAL: n = 85, M = 5.17 (SD = 1.29). | 1 (skills knowledge) | + Skills knowledge | Positive |
| 44. Ros et al. (2021) | France | VR | Medical − lumbar puncture | 89 medical students | 1 (Teacher) | Medical students were randomized into one of two groups to complete training on how to perform a lumbar puncture. The control group participated in traditional lecture learning, whereas Group 2 participated in immersive VR 3D video filmed from a first-person point of view (IVRA-FPV). After training, students performed a simulated lumbar puncture on a mannequin to analyze their applied learning skillset. An oral examination was also included as an assessment. | The group that participated in the traditional lecture showed significantly superior scoring in the oral examination, p < 0.001. CONTROL: n = 44, M = 4.97, SE = 0.10. EXPERIMENTAL: n = 45, M = 4.06, SE = 0.12. The VR group took less time to perform the simulated lumbar puncture compared to the control, p < 0.01. CONTROL: n = 55, M = 73, SE = not reported. EXPERIMENTAL: n = 36, M = 50, SE = not reported. The VR group also had reduced errors compared to the control, p < 0.01. Means are reported as latency of errors. CONTROL: n = 43, M = 227.50, SE = 34.34. EXPERIMENTAL: n = 44, M = 153.26, SE = 11.19 | 3 (skills knowledge) (performance skills − time) (performance skills − errors) | − Skills knowledge + Performance skills + Performance skills | Mixed Positive |
| 45. Schoeb et al. (2020) | Germany | MR + Practice | medical − catheter placement | 164 medical students | 1 (Did nothing additional) | Medical students were randomized into one of two groups to undergo bladder catheter placement learning. One group underwent learning with an instructor, while the other group received mixed reality (MR) training using Microsoft HoloLens. Both groups were able to participate in hands-on training before undergoing a standardized objective structured clinical examination (OSCE) for performance assessment. | The MR intervention group showed significantly superior bladder catheter placement simulation in comparison to the control group, p = 0.000. CONTROL: n = 107, M = 19.96, (SD = 2.42). EXPERIMENTAL: n = 57, M = 21.49, (SD = 2.27) | 1 (performance measures − OSCE) | + Performance measures | Positive |
| 46. Shao et al. (2020) | China | VR | Medical − anatomy | 30 medical students | 1 (Combined) | Students were divided into either a traditional teaching group, or a virtual reality (VR) teaching group for teaching skull base tumors and skull anatomy. The traditional teaching group used literature-based learning, problem-based teaching, and case-based teaching, whereas the VR groups used real case images and Hololens (VR) glasses after the completion of their intervention. | VR group had a higher total scoring on the basic knowledge assessment compared to the traditional control group, p < 0.001. CONTROL: n = 15, M = 63.6 (SD = 3.81). EXPERIMENTAL: n = 15, M = 77.07 (SD = 4.00). | 1 (skills knowledge) | + Skills knowledge | Positive |
| 47. Smith et al. (2018) | Turkey | VR immersive or VR computer | nursing − decontamination skills | 172 nursing students | 1 (Print) | Nursing students were divided into three groups for decontamination skills training. The control group used a traditional written instructions learning method, whereas the experimental groups underwent immersive VR or computer-based VR training. Post-learning competency was measured using a Decontamination Checklist in which students performed skills on a mannequin. Cognitive test scores, performance scores, and time to complete skills were measured immediately post-training and 6 months later. | Results are reported for the immediate follow-up post-intervention. There were no significant differences among groups for the cognitive scores, p = 0.568. Means and SD are shown both VR groups combined and the traditional control group only. CONTROL: n = 43, M = 19 [8, 23]. EXPERIMENTAL: n = 43, M = 19 [13, 23]. There was no difference among the groups for time to completion on the OSCE, p = 0.723. Medians and ranges are shown in both VR groups combined and the traditional control group only. CONTROL: n = 43, Median = 260 (range: 190, 360). EXPERIMENTAL: n = 43, Median = 260 (range: 180, 360). The computer/mouse VR groups showed superior performance measures compared to the control group on the immediate post-test, p = 0.017. Medians and ranges are shown in both VR groups combined and the traditional control group only. CONTROL: n = 43, Median = 54 (range: 46, 57). EXPERIMENTAL: n = 43, Median = 55 (range: 46, 57). | 3 (skills knowledge) (performance skills − time) (performance skills - other specific decontainment mannikin) | X No difference in skills knowledge X No difference in performance skills − Performance skills | Mixed Negative |
| 48. Stepan et al. (2017) | United States | VR | medical − neuroanatomy | 66 medical students | 1 (Electronic) | Medical students were assigned to either a control (online textbook) or an experimental (3D imaging VR interactive model) group for the learning of neuroanatomy. | Students completed preintervention, post-intervention, and retention tests for assessment of knowledge. No significant differences in anatomy knowledge assessments were found between the control and VR groups, p = 0.87. CONTROL: n = 33, M = 0.76 (SD = 0.14) EXPERIMENTAL: n = 33, M = 75 (SD = 0.16) | 1 (skills knowledge) | X No difference in skills knowledge | No difference |
| 49. Sultan et al. (2019) | Saudi Arabia | VR | Medical − tube placement | 169 medical students | 1 (Practice) | Medical students were split into two groups to participate in a learning workshop regarding communication and collaboration. The workshop was a half-day, once a week, for 6 months. VR group received VR instruction, whereas the control group received conventional learning (simulated patients, lectures). Post-intervention assessment included an MCQs score and an Objective Structured Clinical Examinations (OSCE) score. | The VR intervention groups showed significantly higher MCQs compared to the control group after training, p < 0.001. CONTROL: n = 112, M = 15.9 (SD = 2.9). EXPERIMENTAL: n = 57, M = 17.4 (SD = 2.1). The VR group also showed improved OSCE after training compared to the conventional learning group, p < 0.001. CONTROL: n = 112, M = 9.8 (SD = 4.2). EXPERIMENTAL: n = 57, M = 12.9 (SD = 4.1). | 2 (skills knowledge) (performance measures-OSCE) | + Skills knowledge + Performance measures | Positive |
| 50. Watari et al. (2020) | Japan | VR | medical students − basic clinical knowledge | 210 medical students | 1 Nothing (pre-post) | Medical students participated in a lecture that used Virtual Patient Simulations (VPSs). Pre- and post-test 20-item multiple-choice questionnaires were taken and involved both knowledge and clinical reasoning items. | Students showed a significant increase in post-test scoring on both knowledges, p = 0.003. EXPERIMENTALpre: n = 169, M = 4.78 [4.55, 5.01]. EXPERIMENTALpost: n = 169, M = 5.12 [4.90, 5.43]. Students also had increased clinical reasoning after training, p < 0.001. EXPERIMENTALpre: n = 169, M = 5.3 [4.98, 5.58] EXPERIMENTALpost: n = 169, M = 7.81 [7.57, 8.05]. | 2 (skills knowledge) (clinical reasoning) | + Skills knowledge + Clinical reasoning | Positive |
| 51. Wolf et al. (2021) | Switzerland | AR | Medical − surgery | 21 medical students | 1 (Print) | Medical students were recruited for training in extracorporeal membrane oxygenation (ECMO) cannulation. They were split into two groups: (1) conventional training instructions for the first procedure and AR instructions for the second; (2) reverse order (AR instructions for the first procedure). Participants performed the two ECMO cannulation procedures on a simulator. Training times and a detailed error protocol were used for assessment. | AR group showed minimally higher training times compared to the control group, with no p values given. Means and SD in Figure 5 in original publication. AR group had significantly fewer errors when performing the second (more complex) simulation procedure, no p values given. Means and SD in Figure 5 in original publication. | 2 (performance skills − error) (performance skills − time) | + Performance skills - Performance skills | Mixed |
| 52. Yang & Oh (2022) | Republic of Korea | VR | nursing − infant respiration | 83 nursing students | 2 (Teacher) (Practice) | Nursing students were separated into three groups to undergo neonatal resuscitation training. These groups included a virtual reality group, a high-fidelity simulation group, and a control (online lectures only) group. Pre and post-test scores were analyzed on neonatal resuscitation knowledge, problem-solving ability, and clinical reasoning ability. | Knowledge scores increased for all groups post-intervention, but the VR and simulation groups showed significantly higher knowledge after intervention compared to the control group, p = 0.004. Means and SDs are reported for the VR and control groups only. CONTROL: n = 26, M = 11.85 (SD = 5.43). EXPERIMENTAL: n = 29, M = 18.00 (SD = 2.55). The VR group showed significantly improved problem-solving ability scores in comparison to both the simulation and control groups, p = 0.038. CONTROL: n = 26, M = 106.24 (SD = 24.52). EXPERIMENTAL: n = 29, M = 122.72 (SD = 15.68). Clinical reasoning ability showed significantly improved performance, but none of the groups differed statistically, p = 0.123. CONTROL: n = 26, M = 53.69 (SD = 12.02). EXPERIMENTAL: n = 29, M = 59.66 (SD = 9.44). | 3 (skills knowledge − other specific resuscitation) (problem-solving) (clinical reasoning) | + Skills knowledge + Problem-solving X No difference in clinical reasoning | Mixed Positive |
| 53. Yeo et al. (2018) | Canada | AR + Ultrasound | medical- lumbar puncture and facet joint injection | 36 medical students | 1 (Practice) | Medical students were randomized into either a control group (ultrasound use only), or an experimental group that involved training with ultrasound and AR using Perk Tutor software. The training involved learning lumbar puncture and facet joint injection skills on five different tasks for two tasks, a simpler and hard one (ultrasound-guided facet joint injection). | Results are reported for the harder task only. The AR group had more successful injections p = 0.04. CONTROL: n = 10, M = 37.5, SD = not reported. EXPERIMETNAL: n = 10, M = 62.5, SD = not reported. AR was also better than control post-training for total time, p < 0.001. CONTROL: n = 10, M = 103 (SD = 13). EXPERIMETNAL: n = 10, M = 47 (SD = 3). AR was also better than control post-training for time inside the phantom body, p < 0.01. CONTROL: n = 10, M = 31 (SD = 5). EXPERIMETNAL: n = 10, M = 14 (SD = 2). AR was also better than the control post-training for path distance inside the phantom body, p < 0.01. CONTROL: n = 10, M = 266 (SD = 76). EXPERIMETNAL: n = 182, M = 47 (SD = 36). AR was also better than control post-training for potential tissue damage, p = 0.03. CONTROL: n = 10, M = 3217 (SD = 1173). EXPERIMETNAL: n = 10, M = 2376 (SD = 673). | 5 (performance skills − time) (performance skills − other specific time inside) (performance skills − other specific path) (performance skills − other specific damage) (performance skills − other specific success) | + Performance skills + Performance skills + Performance skills + Performance skills + Performance skills | Positive |
| 54. Yu et al. (2022) | China | VR + Practice (BOX) * | Medical − surgery | 51 medical students | 1 Nothing (pre-post) | Medical students completed four pre and post-experiments with a box trainer-based laparoscopic surgery simulators (VRLS). Students were assessed by expert surgeons using the Global Operative Assessment of Laparoscopic Skills (GOALS) standards on performance and time on two tasks, the fundamental task (FT) and the color resection task (CRT) | Post-test assessments showed a significantly faster task completion post-training on both tasks, p < 0.01. Means and SDs are shown pre- and post-for-one- task (FT). EXPERIMENTALpre: n = 51, M = 21.95, SD = not reported. EXPERIMETNALpost: n = 51, M = 14.04, SD = not reported. Post-training also had improved performance on the GOALS (heart rate) for both tasks, p < 0.05. Means and SDs are shown pre- and post-for-one-tasks (FT). EXPERIMENTALpre: n = 51, M = 94.96, (SD = 11.14). EXPERIMENTALpost: n = 51, M = 92.71, (SD = 11.67). | 2 (performance measures -GOALS) (performance skills − time) | + Performance skills + Performance measures | Positive |
| 55. Zackoff et al. (2020) | United States | VR + Teacher + Practice | Med students- infant respiratory distress | 168 medical studemmininntmiminns | 1 (Teacher) | Medical students completed standard respiratory distress training using traditional didactic material, as well as a mannequin simulation. A randomized group of students also completed an additional 30 min training using immersive virtual reality with various infant simulations (no distress, respiratory distress, and impending respiratory failure). After training, all students completed a free-response test regarding various video questions, including mental status, breathing, breadth sounds, and vital signs for three different cases: no distress, respiratory distress, and respiratory failure. In addition, the need for escalation of care was assessed in each case. | Students who underwent additional VR intervention showed significantly superior status interpretation across all assessed dimensions and all cases, p < 0.01. Means and SDs for one case are shown (no distress). CONTROL: n = 90, M = 104 (SD = 61.2). EXPERIMENTAL: n = 78, M = 124 (SD = 80). The additional VR status groups also had a higher recognition of the need for increased care, p = 0.0004. Means and SDs for one case are shown (respiratory failure). CONTROL: n = 90, M = 41, (SD = 45.6). EXPERIMENTAL: n = 78, M = 56, (SD = 72.7). | 2 (skills knowledge- respiratory) (skills knowledge − level of care) | + Skills knowledge + Skills knowledge | Positive |
| 56. Zhou et al. (2022) | United States | AR | Nursing − stroke | 36 nursing students | 1 (Practice) | Nursing students were divided into two groups: a mannequin-based simulation only (control) or a mannequin-based simulation with AR. They were assessed for clinical judgment with the LCJR. | The AR group spent less time than the control group in the critical phase of the stroke simulation, p < 0.05. CONTROL: n = 18, M = 99.57 (SD = 79.00). EXPERIMENTAL: n = 18, M = 46.61 (SD = 27.92). AR outperformed the control group on the LCJR for one of three sections (noticing), p < 0.05. Means and SDs are only shown in Figure 14 in the original publication. | 2 (performance skills − time) (clinical reasoning − LCJR) | + Performance skills + Clinical Reasoning | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fugate, J.M.B.; Tonsager, M.J.; Macrine, S.L. Immersive Extended Reality (I-XR) in Medical and Nursing for Skill Competency and Knowledge Acquisition: A Systematic Review and Implications for Pedagogical Practices. Behav. Sci. 2025, 15, 468. https://doi.org/10.3390/bs15040468
Fugate JMB, Tonsager MJ, Macrine SL. Immersive Extended Reality (I-XR) in Medical and Nursing for Skill Competency and Knowledge Acquisition: A Systematic Review and Implications for Pedagogical Practices. Behavioral Sciences. 2025; 15(4):468. https://doi.org/10.3390/bs15040468
Chicago/Turabian StyleFugate, Jennifer M. B., Michaela J. Tonsager, and Sheila L. Macrine. 2025. "Immersive Extended Reality (I-XR) in Medical and Nursing for Skill Competency and Knowledge Acquisition: A Systematic Review and Implications for Pedagogical Practices" Behavioral Sciences 15, no. 4: 468. https://doi.org/10.3390/bs15040468
APA StyleFugate, J. M. B., Tonsager, M. J., & Macrine, S. L. (2025). Immersive Extended Reality (I-XR) in Medical and Nursing for Skill Competency and Knowledge Acquisition: A Systematic Review and Implications for Pedagogical Practices. Behavioral Sciences, 15(4), 468. https://doi.org/10.3390/bs15040468







