Terrestrial Carbon Additions to Zooplankton Prey Influence Juvenile Estuarine Fish Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Acclimation
2.2. Artemia and Feeding
2.3. Bacteria and Fish Sampling
2.4. POM and Tissue analyses
2.5. Statistical Analyses
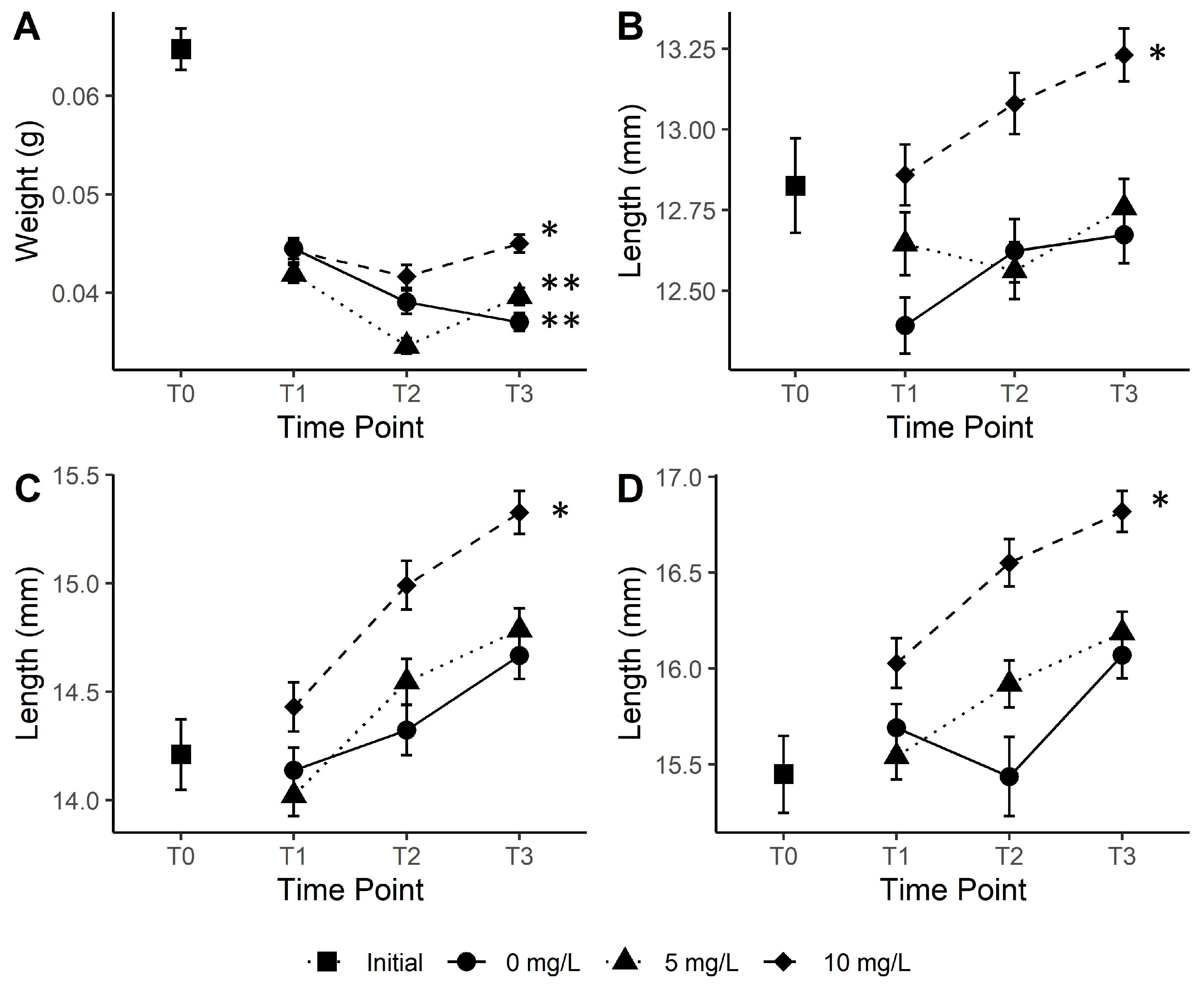
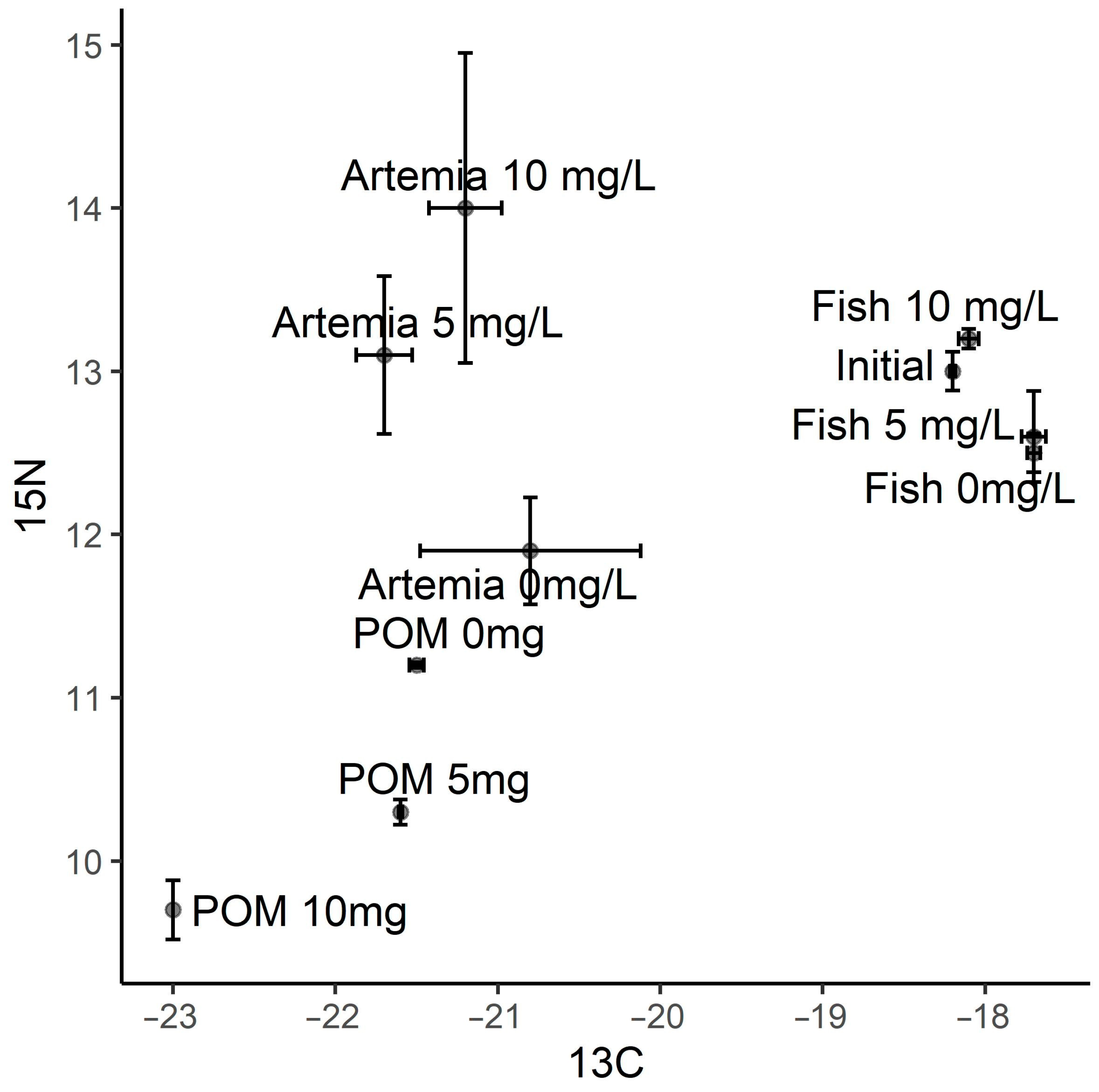
3. Results
4. Discussion
4.1. tDOM Influence on Growth
4.2. tDOM, Inflows and Juvenile Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loneragan, N.R.; Bunn, S.E. River Flows and Estuarine Ecosystems: Implications for Coastal Fisheries from a Review and a Case Study of the Logan River, Southeast Queensland. Aust. J. Ecol. 1999, 24, 431–440. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Bronwyn, M.; Kingsford, M. Impact of Changes in Flow of Freshwater on Estuarine and Open Coastal Habitats and the Associated Organisms. In Oceanography and Marine Biology; Gibson, R.N., Barnes, M., Atkinson, R.J.A., Eds.; Taylor & Francis Ltd.: London, UK, 2002; Volume 40, pp. 233–309. [Google Scholar]
- Connolly, R.M.; Schlacher, T.A.; Gaston, T.F. Stable Isotope Evidence for Trophic Subsidy of Coastal Benthic Fisheries by River Discharge Plumes off Small Estuaries. Mar. Biol. Res. 2009, 5, 164–171. [Google Scholar] [CrossRef] [Green Version]
- ter Morshuizen, L.D.; Whitfield, A.K.; Paterson, A.W. Influence of Freshwater Flow Regime on Fish Assemblages in the Great Fish River and Estuary. S. Afr. J. Aquat. Sci. 1996, 22, 52–61. [Google Scholar] [CrossRef]
- Robins, J.B.B.; Halliday, A.I.; Staunton-Smith, J.; Mayer, G.D.; Sellin, J.M.; Halliday, I.A.; Staunton-Smith, J.; Mayer, D.G.; Sellin, M.J. Freshwater Flow Requirements of Estuarine Fisheries in Tropical Australia: A Review of the State of Knowledge and Application of a Suggested Approach. Mar. Freshw. Res. 2005, 56, 343–360. [Google Scholar] [CrossRef]
- Gillson, J. Freshwater Flow and Fisheries Production in Estuarine and Coastal Systems: Where a Drop of Rain Is Not Lost. Rev. Fish. Sci. 2011, 19, 169–186. [Google Scholar] [CrossRef]
- Salen-Picard, C.; Darnaude, A.M.; Arlhac, D.; Harmelin-Vivien, M.L. Fluctuations of Macrobenthic Populations: A Link between Climate-Driven River Run-off and Sole Fishery Yields in the Gulf of Lions. Oecologia 2002, 133, 380–388. [Google Scholar] [CrossRef]
- Darnaude, A.M.; Salen-Picard, C.; Polunin, N.V.C.; Harmelin-Vivien, M.L. Trophodynamic Linkage between River Runoff and Coastal Fishery Yield Elucidated by Stable Isotope Data in the Gulf of Lions (NW Mediterranean). Oecologia 2004, 138, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.C.; Bronk, D.A.; Olney, J.E. Contribution of Allochthonous Carbon to American Shad Production in the Mattaponi River, Virginia, Using Stable Isotopes. Estuaries Coasts 2007, 30, 1034–1048. [Google Scholar] [CrossRef]
- Abrantes, K.G.; Sheaves, M. Importance of Freshwater Flow in Terrestrial-Aquatic Energetic Connectivity in Intermittently Connected Estuaries of Tropical Australia. Mar. Biol. 2010, 157, 2071–2086. [Google Scholar] [CrossRef]
- Brett, M.T.; Kainz, M.J.; Taipale, S.J.; Seshan, H. Phytoplankton, Not Allochthonous Carbon, Sustains Herbivorous Zooplankton Production. Proc. Natl. Acad. Sci. USA 2009, 106, 21197–21201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, P.T.; Solomon, C.T.; Weidel, B.C.; Jones, S.E. Terrestrial Carbon Is a Resource, but Not a Subsidy, for Lake Zooplankton. Ecology 2014, 95, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Taipale, S.J.; Brett, M.T.; Hahn, M.W.; Martin-Creuzburg, D.; Yeung, S.; Hiltunen, M.; Strandberg, U.; Kankaala, P. Differing Daphnia Magna Assimilation Efficiencies for Terrestrial, Bacterial, and Algal Carbon and Fatty Acids. Ecology 2014, 95, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent Developments in the Essential Fatty Acid Nutrition of Fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. Lipid Nutrition of Marine Fish during Early Development: Current Status and Future Directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; McDowell, W.H.; Neff, J.C. Sources, Production, and Regulation of Allochthonous Dissolved Organic Matter Inputs to Surface Waters. In Aquatic Ecosystems: Interactivity of Dissolved Organic Matter; Findlay, S., Sinsabaugh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 25–70. [Google Scholar]
- Ducklow, H.W.; Purdie, D.A.; Williams, P.J.L.B.; Davies, J.M. Bacterioplankton: A Sink for Carbon in a Coastal Marine Plankton Community. Science 1986, 232, 871–873. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M. Highs and Lows: The Effect of Differently Sized Freshwater Inflows on Estuarine Carbon, Nitrogen, Phosphorus, Bacteria and Chlorophyll a Dynamics. Estuar. Coast. Shelf Sci. 2015, 156, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Asmala, E.; Autio, R.; Kaartokallio, H.; Stedmon, C.A.; Thomas, D.N. Processing of Humic-Rich Riverine Dissolved Organic Matter by Estuarine Bacteria: Effects of Predegradation and Inorganic Nutrients. Aquat. Sci. 2014, 76, 451–463. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Hadwen, W.L.; Growns, I.O.; Rohlfs, A.M. Zooplankton Responses to Freshwater Inflows and Organic-Matter Pulses in a Wave-Dominated Estuary. Mar. Freshw. Res. 2016, 67, 1374–1386. [Google Scholar] [CrossRef]
- Bartels, P.; Ask, J.; Andersson, A.; Karlsson, J.; Giesler, R. Allochthonous Organic Matter Supports Benthic but Not Pelagic Food Webs in Shallow Coastal Ecosystems. Ecosystems 2018, 21, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Berggren, M.; Bengtson, P.; Soares, A.R.A.; Karlsson, J. Terrestrial Support of Zooplankton Biomass in Northern Rivers. Limnol. Ocean 2018, 63, 2479–2492. [Google Scholar] [CrossRef]
- Kostecki, C.; le Loc’h, F.; Roussel, J.M.; Desroy, N.; Huteau, D.; Riera, P.; le Bris, H.; le Pape, O. Dynamics of an Estuarine Nursery Ground: The Spatio-Temporal Relationship between the River Flow and the Food Web of the Juvenile Common Sole (Solea solea, L.) as Revealed by Stable Isotopes Analysis. J. Sea Res. 2010, 64, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Houde, E.D. Patterns and Consequences of Selective Processes in Teleost Early Life Histories. In Early Life History and Recruitment in Fish Populations; Springer Netherlands: Dordrecht, The Netherlands, 1997; pp. 173–196. [Google Scholar]
- Sogard, S.M. Size-Selective Mortality in the Juvenile Stage of Teleost Fishes: A Review. Bull. Mar. Sci. 1997, 60, 1129–1157. [Google Scholar]
- von Biela, V.R.; Zimmerman, C.E.; Cohn, B.R.; Welker, J.M. Terrestrial and Marine Trophic Pathways Support Young-of-Year Growth in a Nearshore Arctic Fish. Polar Biol. 2013, 36, 137–146. [Google Scholar] [CrossRef]
- Arthington, A.H.; Kennen, J.G.; Stein, E.D.; Webb, J.A. Recent Advances in Environmental Flows Science and Water Management—Innovation in the Anthropocene. In Freshwater Biology; Blackwell Publishing Ltd.: Oxford, UK, 2018; Volume 63, pp. 1022–1034. [Google Scholar]
- Hemraj, D.A.; Hossain, A.; Ye, Q.; Qin, J.G.; Leterme, S.C. Anthropogenic Shift of Planktonic Food Web Structure in a Coastal Lagoon by Freshwater Flow Regulation. Sci. Rep. 2017, 7, 44441. [Google Scholar] [CrossRef] [Green Version]
- Degerman, R.; Lefébure, R.; Byström, P.; Båmstedt, U.; Larsson, S.; Andersson, A. Food Web Interactions Determine Energy Transfer Efficiency and Top Consumer Responses to Inputs of Dissolved Organic Carbon. Hydrobiologia 2018, 805, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Growns, I.; Ryder, D.; McInerney, P.; Bond, N.; Holt, G.; Lester, R.; Thompson, R. The Use of Fatty Acids to Identify Food Sources of Secondary Consumers in Wetland Mesocosms. J. Freshw. Ecol. 2020, 35, 173–189. [Google Scholar] [CrossRef]
- Bruesewitz, D.A.; Gardner, W.S.; Mooney, R.F.; Pollard, L.; Buskey, E.J. Estuarine Ecosystem Function Response to Flood and Drought in a Shallow, Semiarid Estuary: Nitrogen Cycling and Ecosystem Metabolism. Limnol. Ocean 2013, 58, 2293–2309. [Google Scholar] [CrossRef]
- Growns, I.; James, M. Relationships between River Flows and Recreational Catches of Australian Bass. J. Fish. Biol. 2005, 66, 404–416. [Google Scholar] [CrossRef]
- Harding, D.J.; Dwyer, R.G.; Mullins, T.M.; Kennard, M.J.; Pillans, R.D.; Roberts, D.T. Migration Patterns and Estuarine Aggregations of a Catadromous Fish, Australian Bass (Percalates Novemaculeata) in a Regulated River System. Mar. Freshw. Res. 2017, 68, 1544–1553. [Google Scholar] [CrossRef] [Green Version]
- Morrongiello, J.R.; Walsh, C.T.; Gray, C.A.; Stocks, J.R.; Crook, D.A. Environmental Change Drives Long-Term Recruitment and Growth Variation in an Estuarine Fish. Glob. Chang. Biol. 2014, 20, 1844–1860. [Google Scholar] [CrossRef]
- Stoessel, D.J.; Morrongiello, J.R.; Raadik, T.A.; Lyon, J.P.; Nicol, M.D. Determinants of Year Class Strength and Growth of Estuary Perch Macquaria Colonorum in a Highly Regulated System. Mar. Freshw. Res. 2018, 69, 1663–1673. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Hadwen, W.L.; Roelke, D.L.; Growns, I.O.; Rohlfs, A.M. Terrestrial Dissolved Organic Carbon Subsidizes Estuarine Zooplankton: An in Situ Mesocosm Study. Limnol. Ocean 2016, 61, 254–267. [Google Scholar] [CrossRef]
- Lennon, J.T.; Pfaff, L.E. Source and Supply of Terrestrial Organic Matter Affects Aquatic Microbial Metabolism. Aquat. Microb. Ecol. 2005, 39, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Fielder, S.; Heasman, M. Hatchery Manual for the Production of Australian Bass, Mulloway and Yellowtail Kingfish; Industry & Investment NSW: Orange, CA, USA, 2011; p. 176. ISBN 978 1 74256 058 8. [Google Scholar]
- van der Wal, E.J.; Nell, J.A. Effect of Food Concentration on the Survival and Growth of Australian Bass (Macquaria Novemaculeata) Larvae. Progress. Fish-Cult. 1986, 48, 202–204. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Dhert, P.; Candreva, P. Use of the Brine Shrimp, Artemia spp., in Marine Fish Larviculture. Aquaculture 2001, 200, 147–159. [Google Scholar] [CrossRef]
- Carney, R.L.; Mitrovic, S.M.; Jeffries, T.; Westhorpe, D.; Curlevski, N.; Seymour, J.R. River bacterioplankton community responses to a high inflow event. Aquat. Microb. Ecol. 2015, 75, 187–205. [Google Scholar] [CrossRef]
- Harris, J.H. Diet of the Australian Bass, Macquaria Novemaculeata (Perciformes: Percichthyidae), in the Sydney Basin. Mar. Freshw. Res. 1985, 36, 219–234. [Google Scholar] [CrossRef]
- Mallen-Cooper, M. Swimming ability of juvenile Australian bass, Macquaria novemaculeata (Steindachner), and juvenile barramundi, Lates calcarifer (Bloch), in an experimental vertical-slot fishway. Mar. Freshw. Res. 1992, 43, 823–833. [Google Scholar] [CrossRef]
- Suthers, I.M.; Cleary, J.J.; Battaglene, S.C.; Evans, R. Relative RNA content as a measure of condition in larval and juvenile fish. Mar. Freshw. Res. 1996, 47, 301–307. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Australia, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. Package ‘Ggpubr’: “ggplot2” Based Publication Ready Plots; R Package Version 0.4.0; 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 January 2023).
- Harris, J.H. Growth of Australian Bass Macquaria Novemaculeata (Perciformes:Percichthyidae) in the Sydney Basin. Mar. Freshw. Res. 1987, 38, 351–361. [Google Scholar] [CrossRef]
- Maceina, M.J.; Shireman, J.V. Grass Carp: Effects of Salinity on Survival, Weight Loss, and Muscle Tissue Water Content. Progress. Fish-Cult. 1979, 41, 69–73. [Google Scholar] [CrossRef]
- Altinok, I.; Galli, S.M.; Chapman, F.A. Ionic and Osmotic Regulation Capabilities of Juvenile Gulf of Mexico Sturgeon, Acipenser Oxyrinchus de Sotoi. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 609–616. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiǧit, M. Effect of Dietary Ulva and Spirulina on Weight Loss and Body Composition of Rainbow Trout, Oncorhynchus Mykiss (Walbaum), during a Starvation Period. J. Anim. Physiol. Anim. Nutr. 2011, 95, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Falahatkar, B.; Akhavan, S.R.; Efatpanah, I.; Meknatkhah, B. Effect of Winter Feeding and Starvation on the Growth Performance of Young-of-Year (YOY) Great Sturgeon, Huso Huso. J. Appl. Ichthyol. 2013, 29, 26–30. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of Diet on the Distribution of Nitrogen Isotopes in Animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in Trophic Shift for Stable Isotope Ratios of Carbon, Nitrogen, and Sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Karlsson, J.; Jonsson, A.; Meili, M.; Jansson, M. Δ15N of Zooplankton Species in Subarctic Lakes in Northern Sweden: Effects of Diet and Trophic Fractionation. Freshw. Biol. 2004, 49, 526–534. [Google Scholar] [CrossRef]
- Colborne, S.F.; Robinson, B.W. Effect of Nutritional Condition on Variation in Δ13C and Δ15N Stable Isotope Values in Pumpkinseed Sunfish (Lepomis Gibbosus) Fed Different Diets. Environ. Biol. Fishes 2013, 96, 543–554. [Google Scholar] [CrossRef]
- Karlsson, J. Different Carbon Support for Respiration and Secondary Production in Unproductive Lakes. Oikos 2007, 116, 1691–1696. [Google Scholar] [CrossRef]
- Meunier, C.L.; Liess, A.; Andersson, A.; Brugel, S.; Paczkowska, J.; Rahman, H.; Skoglund, B.; Rowe, O.F. Allochthonous Carbon Is a Major Driver of the Microbial Food Web—A Mesocosm Study Simulating Elevated Terrestrial Matter Runoff. Mar. Environ. Res. 2017, 129, 236–244. [Google Scholar] [CrossRef]
- Andersson, A.; Brugel, S.; Paczkowska, J.; Rowe, O.F.; Figueroa, D.; Kratzer, S.; Legrand, C. Influence of Allochthonous Dissolved Organic Matter on Pelagic Basal Production in a Northerly Estuary. Estuar. Coast. Shelf Sci. 2018, 204, 225–235. [Google Scholar] [CrossRef]
- Wai, T.-C.; Yeung, J.W.Y.; Lam, V.Y.Y.; Leung, K.M.Y.; Dudgeon, D.; Williams, G.A. Monsoons and Habitat Influence Trophic Pathways and the Importance of Terrestrial-Marine Linkages for Estuary Sharks. Ecosphere 2012, 3, art8. [Google Scholar] [CrossRef] [Green Version]
- Harfmann, J.; Kurobe, T.; Bergamaschi, B.; Teh, S.; Hernes, P. Plant Detritus Is Selectively Consumed by Estuarine Copepods and Can Augment Their Survival. Sci. Rep. 2019, 9, 9076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, H.; Li, Y.; Sheng, Q.; Zhong, L.; Ni, Z. River Damming Affects Energy Flow and Food Web Structure: A Case Study from a Subtropical Large River. Hydrobiologia 2020, 847, 679–695. [Google Scholar] [CrossRef]

| Temperature (°C) | DO (mg/L) | pH | Salinity (ppt) | |
|---|---|---|---|---|
| Average | 19.6 | 9.5 | 8.0 | 20.1 |
| SEM | 0.083 | 0.046 | 0.018 | 0.11 |
| Survival % | |||
|---|---|---|---|
| Treatment | T1 | T2 | T3 |
| 0 mg/L | 97.8 (±1.3) | 76.8 (±13.8) | 79.7 (±1.4) |
| 5 mg/L | 99.8 (±0.1) | 89.2 (±2.4) | 80.2 (±4.0) |
| 10 mg/L | 98 (± 1.2) | 84.9 (± 5.1) | 85.3 (±1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, E.B.; Boys, C.; Hitchcock, J.; Hadwen, W.; Fielder, S.; Facey, J.A.; Mitrovic, S.M. Terrestrial Carbon Additions to Zooplankton Prey Influence Juvenile Estuarine Fish Growth. Environments 2023, 10, 50. https://doi.org/10.3390/environments10030050
Johnson EB, Boys C, Hitchcock J, Hadwen W, Fielder S, Facey JA, Mitrovic SM. Terrestrial Carbon Additions to Zooplankton Prey Influence Juvenile Estuarine Fish Growth. Environments. 2023; 10(3):50. https://doi.org/10.3390/environments10030050
Chicago/Turabian StyleJohnson, Ellery B., Craig Boys, James Hitchcock, Wade Hadwen, Stewart Fielder, Jordan A. Facey, and Simon M. Mitrovic. 2023. "Terrestrial Carbon Additions to Zooplankton Prey Influence Juvenile Estuarine Fish Growth" Environments 10, no. 3: 50. https://doi.org/10.3390/environments10030050
APA StyleJohnson, E. B., Boys, C., Hitchcock, J., Hadwen, W., Fielder, S., Facey, J. A., & Mitrovic, S. M. (2023). Terrestrial Carbon Additions to Zooplankton Prey Influence Juvenile Estuarine Fish Growth. Environments, 10(3), 50. https://doi.org/10.3390/environments10030050








