Current to Biomass: Media Optimization and Strain Selection from Cathode-Associated Microbial Communities in a Two-Chamber Electro-Cultivation Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation
2.2. Chemicals and Medium
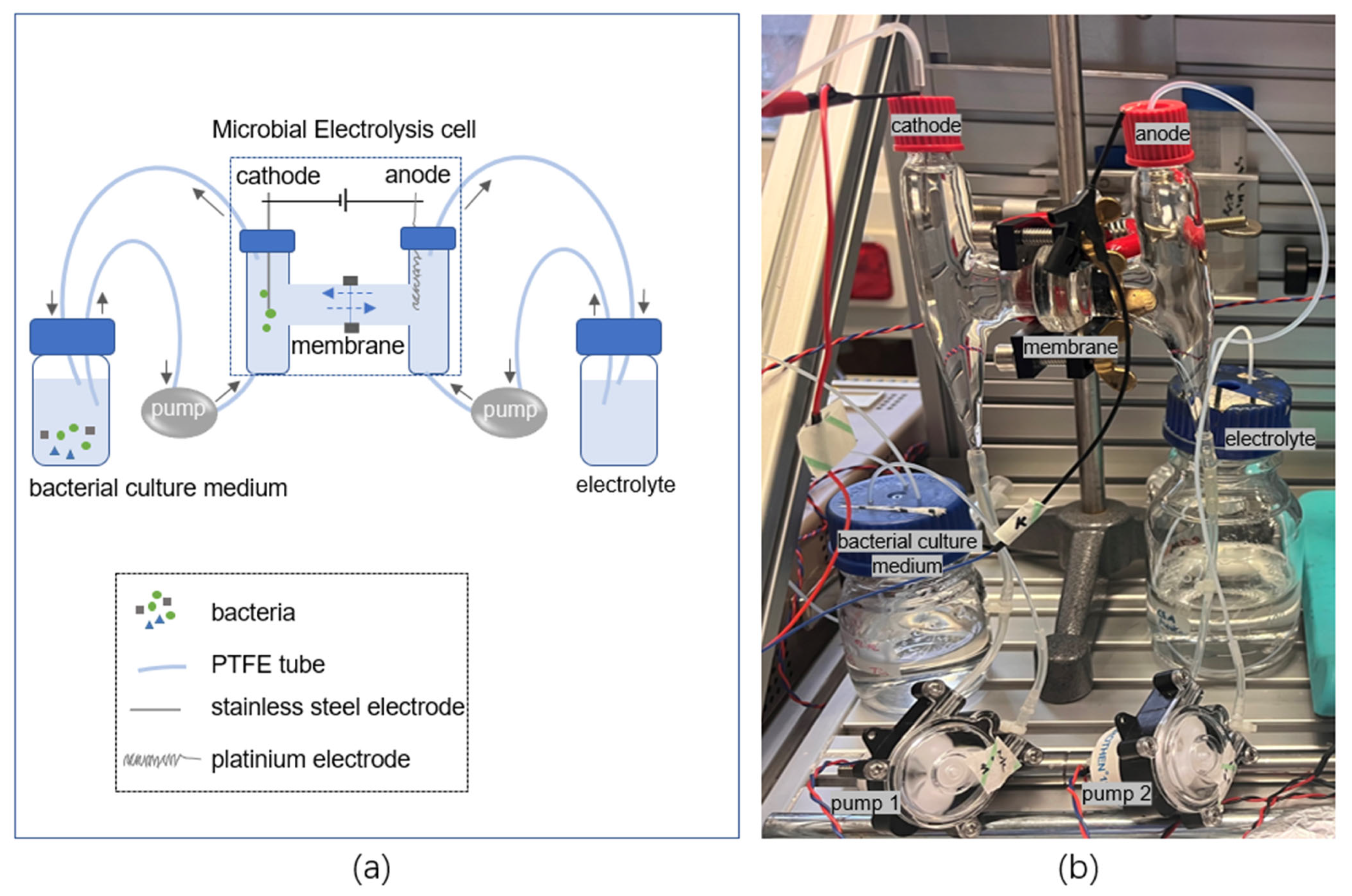
2.3. Electrochemical Experiment Concept and Arrangement
2.4. Next-Generation Gene Sequencing and Data Processing
2.5. Single Strain Isolation and Identification
2.6. Confirmation of the Dry Weight of the Microbial Biomass
3. Results
3.1. Growth Kinetics and pH Development during Electrochemical Incubation with Soil Sample HB35
3.2. Comparison of the Microbial Community Abundance of HB35
3.2.1. With and without Electrochemical Incubation
3.2.2. Comparison at the Genus Level
3.2.3. Comparison of the Unique OTUs of HB35
3.3. Comparison of the Community Abundances in HB35 Related to Different Culture Agar Media
3.3.1. Comparison at the Phylum Level
3.3.2. Comparison at the Genus Level
3.4. Electrochemical Incubation of Isolated Strains
| Name | Culture Condition | Agar Medium | Phylum | Identification Result |
|---|---|---|---|---|
| TX166 | A | CMM1 | Bacteroidota | Epilithonimonas bovis DSM 19482 |
| TX167 | A | CMM1 | Bacteroidota | Epilithonimonas bovis DSM 19482 |
| TX168 | A | CMM1 | Bacteroidota | Epilithonimonas bovis DSM 19482 |
| TX169 | A | TSA | Actinobacteriota | Arthrobacter humicola |
| TX183 | B | AM1 | Proteobacteria | Pseudomonas alcaliphila |
| TX184 | Ref. B | AM1 | Proteobacteria | Pseudomonas stutzeri |
| TX185 | B | CMM1 | Proteobacteria | Pseudomonas azotoformans |
| TX186 | Ref. A | LB | Actinobacteriota | Arthrobacter humicola |
| TX187 | Ref. A | TSA | Proteobacteria | Lysobacter caeni |
| TX188 | Ref. B | TSA | Actinobacteriota | Pseudarthrobacter oxydans |
| TX190 | A | AM2 | Proteobacteria | Sphingopyxis chilensis |
| TX191 | A | TSA | Proteobacteria | Acinetobacter johnsonii |
| TX296 | Ref. A | CMM1 | Actinobacteriota | Arthrobacter humicola |
| TX297 | Ref. A | LB | Actinobacteriota | Arthrobacter humicola |
| TX298 | B | TSA | Proteobacteria | Pseudomonas alcaliphila |
| TX299 | B | TSA | Proteobacteria | Pseudomonas sp. J380 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Ibrahim, M.N.M.; Yaqoob, A.A.; Setapar, S.H.M. Microbial Fuel Cells for Environmental Remediation; Springer Nature: Singapore, 2022; Volume 1, p. 450. [Google Scholar]
- He, K.; Li, W.; Tang, L.; Lv, S.; Xing, D. Suppressing Methane Production to Boost High-Purity Hydrogen Production in Microbial Electrolysis Cells. Environ. Sci. Technol. 2021, 56, 11931–11951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2020, 53, 107682. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef] [PubMed]
- González-Paz, J.R.; Becerril-Varela, K.; Guerrero-Barajas, C. Iron reducing sludge as a source of electroactive bacteria: Assessing iron reduction in biofilm bacteria, planktonic cells and isolates from a microbial fuel cell. Arch. Microbiol. 2022, 204, 632. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Stams, A.J.M. Electricity-Assisted Biological Hydrogen Production from Acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 2011, 45, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, G.; Zhang, R.; Lu, Y.; Luo, H. Acetate production and electron utilization facilitated by sulfate-reducing bacteria in a microbial electrosynthesis system. Bioresour. Technol. 2017, 241, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Askitosari, T.D.; Berger, C.; Tiso, T.; Harnisch, F.; Blank, L.M.; Rosenbaum, M.A. Coupling an Electroactive Pseudomonas putida KT2440 with Bioelectrochemical Rhamnolipid Production. Microorganisms 2020, 8, 1959. [Google Scholar] [CrossRef]
- Choi, O.; Sang, B.-I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Rosenbaum, M.A. Boosting mediated electron transfer in bioelectrochemical systems with tailored defined microbial cocultures. Biotechnol. Bioeng. 2018, 115, 2183–2193. [Google Scholar] [CrossRef]
- Paquete, C.M.; Rosenbaum, M.A.; Bañeras, L.; Rotaru, A.E.; Puig, S. Let’s chat: Communication between electroactive microorganisms. Bioresour. Technol. 2022, 347, 126705. [Google Scholar] [CrossRef]
- Chabert, N.; Ali, O.A.; Achouak, W. All ecosystems potentially host electrogenic bacteria. Bioelectrochemistry 2015, 106, 88–96. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X. Bacterial Succession in Salt Marsh Soils Along a Short-term Invasion Chronosequence of Spartina alterniflora in the Yellow River Estuary, China. Microb. Ecol. 2020, 79, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wang, Y.; Liu, H.; Fan, G.; Sahu, S.K.; Jin, T.; Chen, J.; Zhang, P.; Gram, L.; Strube, M.L.; et al. Deciphering the Microbial Taxonomy and Functionality of Two Diverse Mangrove Ecosystems and Their Potential Abilities to Produce Bioactive Compounds. mSystems 2020, 5, e00851-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, Y.; Ren, W.; Li, Y.; Yang, T.; Chen, Y.; Zhao, L.; Zhang, H.; Kuramae, E.E. Variations of Bacterial and Diazotrophic Community Assemblies throughout the Soil Profile in Distinct Paddy Soil Types and Their Contributions to Soil Functionality. Msystems 2022, 7, e01047-21. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Schmidt, H.; Fuchslueger, L. Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat. Commun. 2021, 12, 5308. [Google Scholar] [CrossRef]
- Köhler, J.M.; Kalensee, F.; Cao, J.; Günther, P.M. Hadesarchaea and other extremophile bacteria from ancient mining areas of the East Harz region (Germany) suggest an ecological long-term memory of soil. SN Appl. Sci. 2019, 1, 839. [Google Scholar] [CrossRef] [Green Version]
- Ehrhardt, L.; Günther, P.M.; Böhme, M.; Köhler, J.M.; Cao, J. Three Soil Bacterial Communities from an Archaeological Excavation Site of an Ancient Coal Mine near Bennstedt (Germany) Characterized by 16S r-RNA Sequencing. Environments 2022, 9, 115. [Google Scholar] [CrossRef]
- Araújo, A.S.F.; Lima, L.M.; Santos, V.M.; Schmidt, R. Repeated application of composted tannery sludge affects differently soil microbial biomass, enzymes activity, and ammonia-oxidizing organisms. Environ. Sci. Pollut. Res. 2016, 23, 19193–19200. [Google Scholar] [CrossRef]
- Miranda, A.R.L.; Mendes, L.W.; Lemos, L.N.; Antunes, J.E.L.; Amorim, M.R.; Melo, V.M.M.; de Melo, W.J.; Brink, P.J.V.D.; Araujo, A.S.F. Dynamics of archaeal community in soil with application of composted tannery sludge. Sci. Rep. 2019, 9, 7347. [Google Scholar] [CrossRef] [Green Version]
- Leigh, J.A.; Albers, S.-V.; Atomi, H.; Allers, T. Model organisms for genetics in the domain Archaea: Methanogens, halophiles, Thermococcales and Sulfolobales. FEMS Microbiol. Rev. 2011, 35, 577–608. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucky, B.J. SeqTrace: A Graphical Tool for Rapidly Processing DNA Sequencing Chromatograms. J. Biomol. Tech. JBT 2012, 23, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drancourt, M.; Berger, P.; Raoult, D. Systematic 16S rRNA Gene Sequencing of Atypical Clinical Isolates Identified 27 New Bacterial Species Associated with Humans. J. Clin. Microbiol. 2004, 42, 2197–2202. [Google Scholar] [CrossRef] [Green Version]
- Li, E.; de Orduña, R.M. A rapid method for the determination of microbial biomass by dry weight using a moisture analyser with an infrared heating source and an analytical balance. Lett. Appl. Microbiol. 2010, 50, 283–288. [Google Scholar] [CrossRef]
- Stanbury, P.; Whittaker, A.; Hall, S. Principles of Fermentation Technology (Chap. 6); Butterworth-Heinemann: Oxford, UK, 1995. [Google Scholar]
- Peppler, H.J.; Perlman, D. Microbial Technology: Microbial Processes; Academic Press: Cambridge, MA, USA, 1979; Volume 1. [Google Scholar]
- Kim, J.; Lee, J.Y.; Ahting, C.; Johnstone, R.; Lu, T. Growth of Chlorella vulgaris using sodium bicarbonate under no mixing condition. Asia-Pac. J. Chem. Eng. 2014, 9, 604–609. [Google Scholar]
- Yuan, Y.; Cai, X.; Wang, Y.; Zhou, S. Electron transfer at microbe-humic substances interfaces: Electrochemical, microscopic and bacterial community characterizations. Chem. Geol. 2017, 456, 1–9. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kondaveeti, S.; Min, B.; Park, H.-D. Enrichment of Clostridia during the operation of an external-powered bio-electrochemical denitrification system. Process. Biochem. 2013, 48, 306–311. [Google Scholar] [CrossRef]
- Khater, D.Z.; El-Khatib, K.; Hassan, H.M. Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J. Genet. Eng. Biotechnol. 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Carson, J.K.; Campbell, L.; Rooney, D.; Clipson, N.; Gleeson, D.B. Minerals in soil select distinct bacterial communities in their microhabitats. FEMS Microbiol. Ecol. 2009, 67, 381–388. [Google Scholar] [CrossRef]
- Ying, Z.; Yu, Y.; Chen, H.; Zhao, J.; You, J.; Ye, J.; Hu, J.; Zhang, S. Structural Characteristics and Functional Genes of Biofilms in Microbial Electrolysis Cells for Chlorobenzene Abatement. ACS ES T Water 2023, 3, 500–509. [Google Scholar] [CrossRef]
- Silva, J.R.; Santos, D.S.; Santos, U.R.; Eguiluz, K.I.; Salazar-Banda, G.R.; Schneider, J.K.; Krause, L.C.; López, J.A.; Hernández-Macedo, M.L. Electrochemical and/or microbiological treatment of pyrolysis wastewater. Chemosphere 2017, 185, 145–151. [Google Scholar] [CrossRef]
- Venkataraman, A.; Rosenbaum, M.; Arends, J.B.; Halitschke, R.; Angenent, L.T. Quorum sensing regulates electric current generation of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem. Commun. 2010, 12, 459–462. [Google Scholar] [CrossRef]
- Freguia, S.; Tsujimura, S.; Kano, K. Electron transfer pathways in microbial oxygen biocathodes. Electrochimica Acta 2010, 55, 813–818. [Google Scholar] [CrossRef]
- Leibeling, S.; Schmidt, F.; Jehmlich, N.; von Bergen, M.; Müller, R.H.; Harms, H. Declining capacity of starving Delftia acidovorans MC1 to degrade phenoxypropionate herbicides correlates with oxidative modification of the initial enzyme. Environ. Sci. Technol. 2010, 44, 3793–3799. [Google Scholar] [CrossRef]
- Zhang, L.-L.; He, D.; Chen, J.-M.; Liu, Y. Biodegradation of 2-chloroaniline, 3-chloroaniline, and 4-chloroaniline by a novel strain Delftia tsuruhatensis H1. J. Hazard. Mater. 2010, 179, 875–882. [Google Scholar] [CrossRef]
- Wu, W.; Huang, H.; Ling, Z.; Yu, Z.; Jiang, Y.; Liu, P.; Li, X. Genome sequencing reveals mechanisms for heavy metal resistance and polycyclic aromatic hydrocarbon degradation in Delftia lacustris strain LZ-C. Ecotoxicology 2016, 25, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tie, B.; Li, Y.; Lei, M.; Wei, X.; Liu, X.; Du, H. Inoculation of soil with cadmium-resistant bacterium Delftia sp. B9 reduces cadmium accumulation in rice (Oryza sativa L.) grains. Ecotoxicol. Environ. Saf. 2018, 163, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Hiraishi, A. Diaphorobacter nitroreducens gen. nov., sp. nov., a poly (3-hydroxybutyrate)-degrading denitrifying bacterium isolated from activated sludge. J. Gen. Appl. Microbiol. 2002, 48, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyuzhnaya, M.; Bowerman, S.; Lara, J.C.; Lidstrom, M.E.; Chistoserdova, L. Methylotenera mobilis gen. nov., sp. nov., an obligately methylamine-utilizing bacterium within the family Methylophilaceae. Int. J. Syst. Evol. Microbiol. 2006, 56, 2819–2823. [Google Scholar] [CrossRef] [Green Version]
- Jangir, Y.; French, S.; Momper, L.M.; Moser, D.P.; Amend, J.P.; El-Naggar, M.Y. Isolation and Characterization of Electrochemically Active Subsurface Delftia and Azonexus Species. Front. Microbiol. 2016, 7, 756. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Huang, L.; Sun, Y. Recovery of flakey cobalt from aqueous Co(II) with simultaneous hydrogen production in microbial electrolysis cells. Int. J. Hydrogen Energy 2014, 39, 654–663. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Zheng, Y.; Zhu, B.; Wu, X.; Zhao, F. Extracellular electron transfer of Methylophilus methylotrophs. Process. Biochem. 2020, 94, 313–318. [Google Scholar] [CrossRef]
- Harris, D.; Ummadi, J.G.; Koley, D. Scanning Electrochemical Microscopy: A Tool to Study the Biomediated Calcification Process by Sporosarcina Pasteurii Biofilm. In Electrochemical Society Meeting Abstracts 229; The Electrochemical Society, Inc.: San Diego, CA, USA, 2016; p. 1667. [Google Scholar]
- Madigan, M.T.; Martinko, J.M. Brock Mikrobiologie, 11th ed.; Pearson Studium: München, Germany, 2006. [Google Scholar]
- Diba, F.; Khan, Z.H.; Uddin, S.Z.; Istiaq, A.; Shuvo, S.R.; Alam, A.S.M.R.U.; Hossain, M.A.; Sultana, M. Bioaccumulation and detoxification of trivalent arsenic by Achromobacter xylosoxidans BHW-15 and electrochemical detection of its transformation efficiency. Sci. Rep. 2021, 11, 21312. [Google Scholar] [CrossRef] [PubMed]
- Kohzuma, T.; Shidara, S.; Suzuki, S. Direct Electrochemistry of Nitrite Reductase from Achromobacter cycloclastes IAM 1013. Bull. Chem. Soc. Jpn. 1994, 67, 138–143. [Google Scholar] [CrossRef]
- Nicholson, A.C.; Gulvik, C.A.; Whitney, A.M.; Humrighouse, B.W.; Bell, M.E.; Holmes, B.; Steigerwalt, A.G.; Villarma, A.; Sheth, M.; Batra, D.; et al. Division of the genus Chryseobacterium: Observation of discontinuities in amino acid identity values, a possible consequence of major extinction events, guides transfer of nine species to the genus Epilithonimonas, eleven species to the genus Kaistella, and three species to the genus Halpernia gen. nov., with description of Kaistella daneshvariae sp. nov. and Epilithonimonas vandammei sp. nov. derived from clinical specimens. Int. J. Syst. Evol. Microbiol. 2020, 70, 4432. [Google Scholar] [CrossRef]
- Hantsis-Zacharov, E.; Senderovich, Y.; Halpern, M. Chryseobacterium bovis sp. nov., isolated from raw cow’s milk. Int. J. Syst. Evol. Microbiol. 2008, 58, 1024–1028. [Google Scholar] [CrossRef]
- Su, W.; Zhang, L.; Li, D.; Zhan, G.; Qian, J.; Tao, Y. Dissimilatory nitrate reduction by Pseudomonas alcaliphila with an electrode as the sole electron donor. Biotechnol. Bioeng. 2012, 109, 2904–2910. [Google Scholar] [CrossRef]
- Raffalt, A.C.; Schmidt, L.; Christensen, H.E.; Chi, Q.; Ulstrup, J. Electron transfer patterns of the di-heme protein cytochrome c4 from Pseudomonas stutzeri. J. Inorg. Biochem. 2009, 103, 717–722. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Genet. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Chen, F.; Ye, Y.; Fan, B.; Lv, M.; Liang, B.; Liu, W.; Cheng, H.-Y.; Chen, Y.; Liu, Y.; Wang, Y.; et al. Simultaneous removal of tetrachloroethylene and nitrate with a novel sulfur-packed biocathode system: The synergy between bioelectrocatalytic dechlorination and sulfur autotrophic denitrification. Chem. Eng. J. 2022, 439, 135793. [Google Scholar] [CrossRef]
- Bond, D.R.; Strycharz-Glaven, S.M.; Tender, L.M.; Torres, C.I. On Electron Transport through Geobacter Biofilms. Chemsuschem 2012, 5, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Chignell, J.F.; De Long, S.K.; Reardon, K.F. Meta-proteomic analysis of protein expression distinctive to electricity-generating biofilm communities in air-cathode microbial fuel cells. Biotechnol. Biofuels 2018, 11, 121. [Google Scholar] [CrossRef] [PubMed]






| Name | Ingredient and Composition |
|---|---|
| AM1 | 0.1 g/L NH4NO3, 0.5 g/L K2HPO4, 0.2 g/L MgSO4·7H2O, 0.01 g/L FeSO4·7H2O, and 20 g/L agar |
| AM2 | 0.1 g/L NH4NO3, 0.5 g/L K2HPO4, 0.2 g/L MgSO4·7H2O, 0.01 g/L FeSO4·7H2O, 2g/L glucose, and 20 g/L agar |
| CMM1 | 0.1 g/L K2HPO4, 0.1 g/L (NH4)2SO4, 1 g/L MgSO4·7H2O, 0.02 g/L CaCl2·7H2O 1% methanol, 1 mL/L trace element solution (5 g/L NaEDTA, 2 g/L FeSO4·7H2O, 0.1 g/L ZnSO4·7H2O, 0.03 g/L MnCl2·4H2O, 0.2 g/L CoCl2·6H2O, 0.1 g/L CuCl2·5H2O, 0.02 g/L NiCl2·4H2O, 0.03 g/L Na2MoO4), and 16 g/L Gelrite |
| LB | 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, and 15 g/L agar |
| TSA | 15 g/L tryptone, 5 g/L soya peptone, 5 g/L NaCl, and 15 g/L agar |
| A | ref. A | B | ref. B | ||||
|---|---|---|---|---|---|---|---|
| Delftia | 137 | Terrimonas | 586 | Paenisporosarcina | 5370 | Salipaludibacillus | 280 |
| Diaphorobacter | 10 | Alphaproteobacteria uncultured | 163 | Achromobacter | 1340 | Fontimonas | 95 |
| Methylophilaceae OM43 clade | 6 | Chitinophagaceae Edaphobaculum | 162 | Planococcaceae Uncultured | 356 | Solimonadaceae uncultured | 15 |
| Delftia | 137 | Chitinophagaceae uncultured | 107 | Sporosarcina | 162 | Nitrosarchaeum | 13 |
| Panacagrimonas | 72 | Bacillaceae uncultured | 125 | Marmoricola | 10 | ||
| Pedobacter | 45 | Nitrosomonadaceae uncultured | 46 | Xanthomonadaceae | 9 | ||
| Desulfovibrio | 25 | Morganellaceae Candidatus Hamiltonella | 11 | Heimdallarchaeia | 8 | ||
| Chitinophagaceae Taibaiella | 21 | Colwelliaceae uncultured | 3 | Thermoanaerobaculaceae Subgroup 10 | 6 | ||
| Gemmatimonadota BD2-11 terrestrial group | 16 | Paracoccus | 5 | ||||
| Bdellovibrio | 14 | Gammaproteobacteria uncultured | 5 | ||||
| Oxalobacteraceae Undibacterium | 12 | Planctomycetales uncultured | 5 | ||||
| Gammaproteobacteria JTB23 | 11 | Candidatus Nitrosotenuis | 4 | ||||
| Rickettsiales Candidatus Jidaibacter | 9 | Clostridium sensu stricto 13 | 3 | ||||
| Aquicella | 8 | Ruminiclostridium | 3 | ||||
| Rickettsiaceae uncultured | 8 | Xanthobacteraceae uncultured | 3 | ||||
| Candidatus Paracaedibacter | 8 | Holosporaceae uncultured | 3 | ||||
| Defluviicoccales uncultured | 7 | Anaerolineae RBG-13-54-9 | 3 | ||||
| Candidatus Accumulibacter | 6 | Anaerolineae SBR1031 | 3 | ||||
| Bradyrhizobium | 6 | ||||||
| Cytophaga | 6 | ||||||
| Iamiaceae | 6 | ||||||
| Methyloligellaceae uncultured | 6 | ||||||
| Sphingobium | 5 | ||||||
| Candidatus Obscuribacter | 5 | ||||||
| Falsarthrobacter | 5 | ||||||
| Fimbriimonas | 5 | ||||||
| Procabacter | 5 | ||||||
| Rurimicrobium | 5 | ||||||
| Rhizobiales Incertae Sedis uncultured | 5 | ||||||
| Actinomarinales uncultured | 5 | ||||||
| Acidobacteriae Subgroup 2 | 5 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Ehrhardt, L.; Günther, P.M.; Köhler, J.M.; Cao, J. Current to Biomass: Media Optimization and Strain Selection from Cathode-Associated Microbial Communities in a Two-Chamber Electro-Cultivation Reactor. Environments 2023, 10, 97. https://doi.org/10.3390/environments10060097
Xie T, Ehrhardt L, Günther PM, Köhler JM, Cao J. Current to Biomass: Media Optimization and Strain Selection from Cathode-Associated Microbial Communities in a Two-Chamber Electro-Cultivation Reactor. Environments. 2023; 10(6):97. https://doi.org/10.3390/environments10060097
Chicago/Turabian StyleXie, Ting, Linda Ehrhardt, Peter Mike Günther, Johann Michael Köhler, and Jialan Cao. 2023. "Current to Biomass: Media Optimization and Strain Selection from Cathode-Associated Microbial Communities in a Two-Chamber Electro-Cultivation Reactor" Environments 10, no. 6: 97. https://doi.org/10.3390/environments10060097
APA StyleXie, T., Ehrhardt, L., Günther, P. M., Köhler, J. M., & Cao, J. (2023). Current to Biomass: Media Optimization and Strain Selection from Cathode-Associated Microbial Communities in a Two-Chamber Electro-Cultivation Reactor. Environments, 10(6), 97. https://doi.org/10.3390/environments10060097









