Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
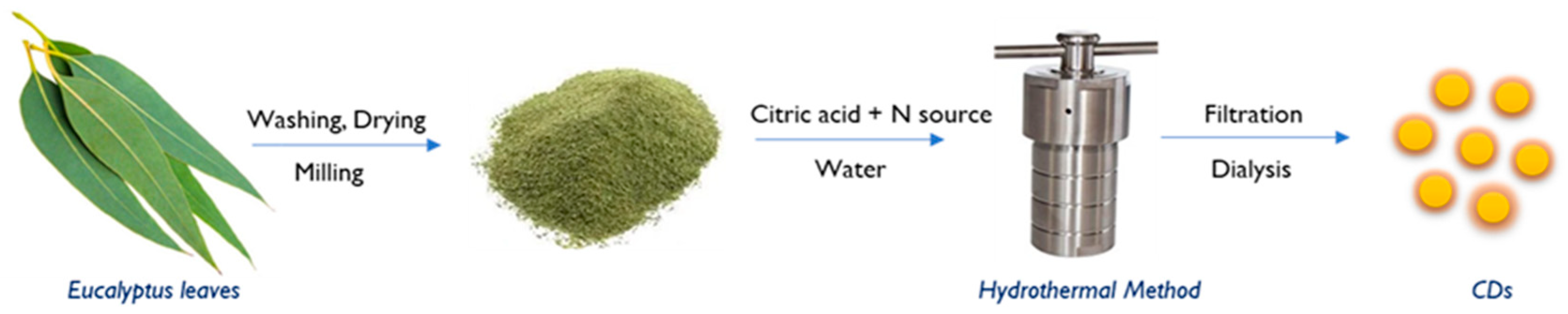
2.3. Preparation of CDs
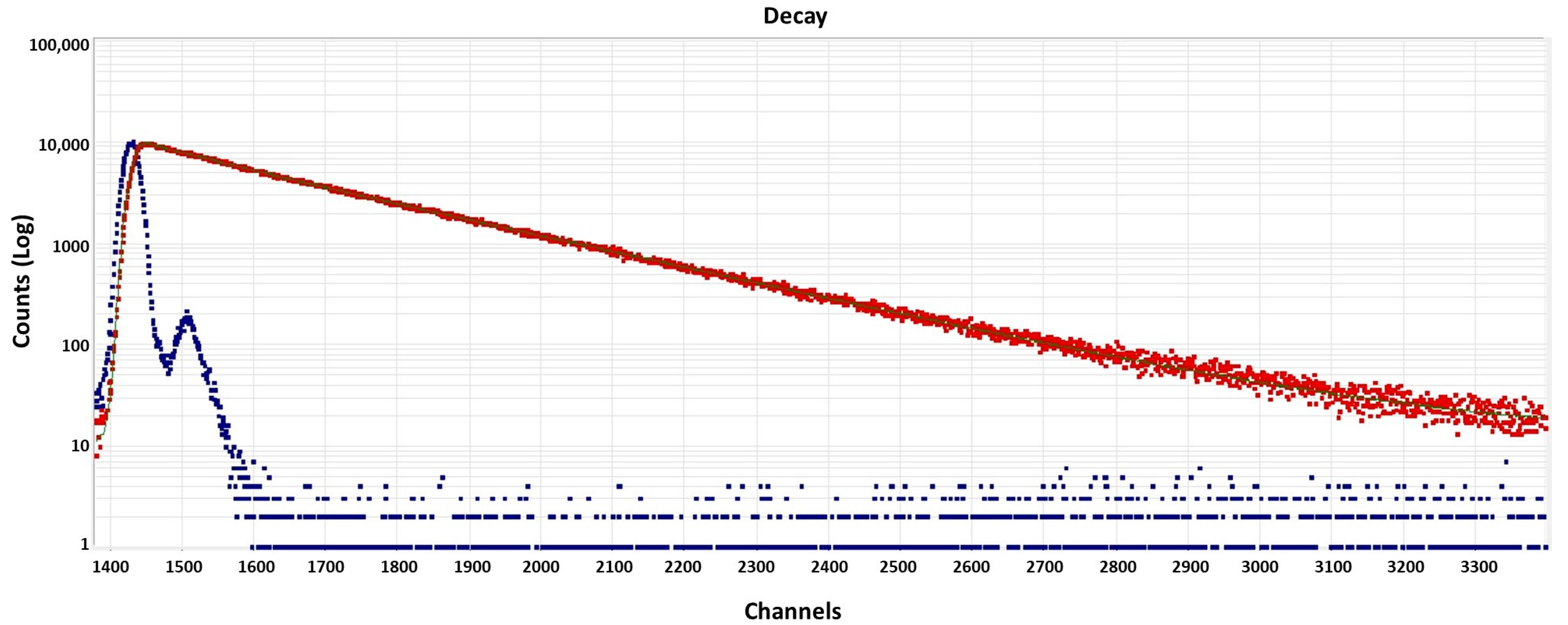
2.4. Quantum Yield Measurements
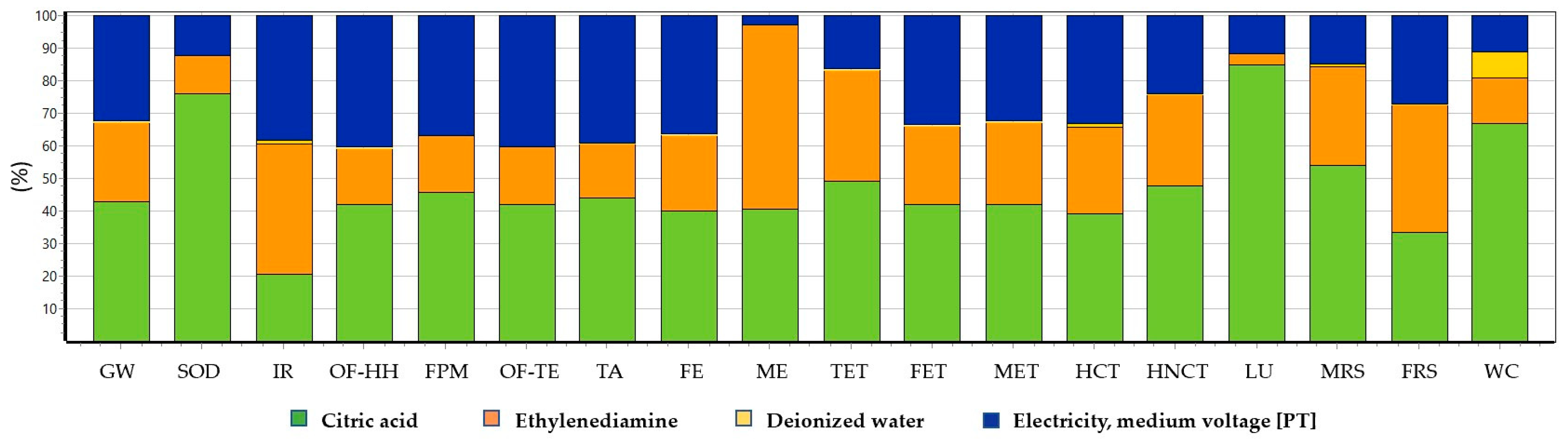
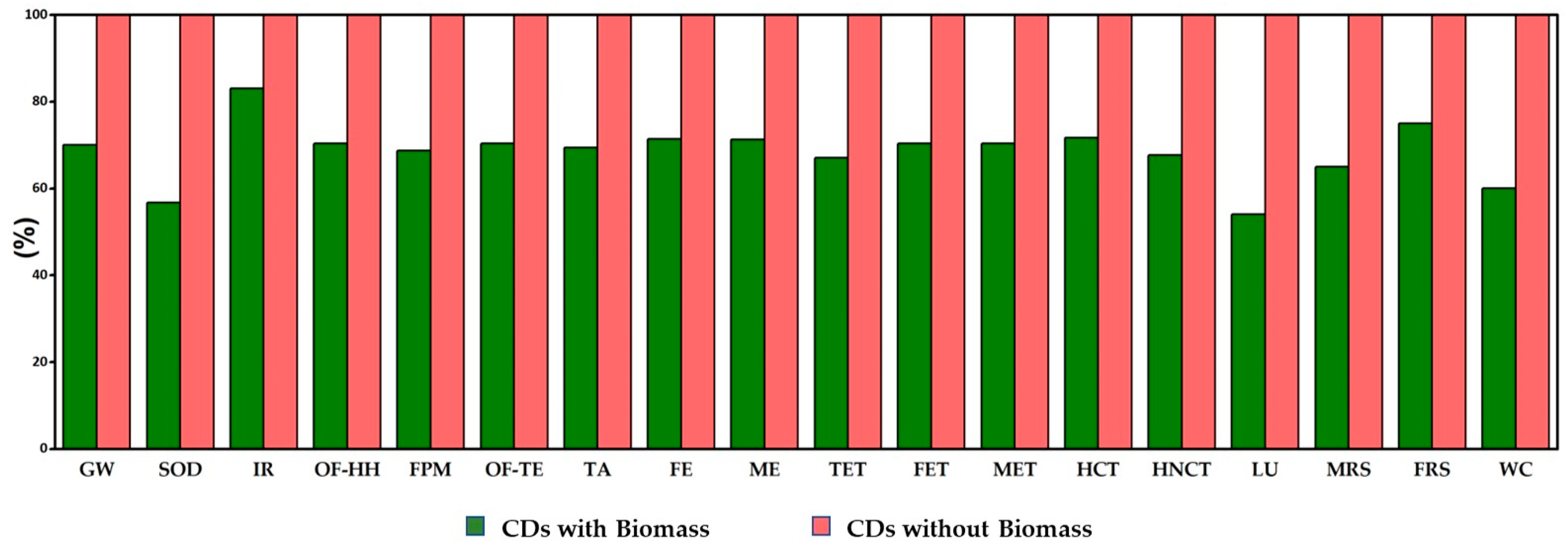
2.5. Environmental Impact Assessment
3. Results and Discussion
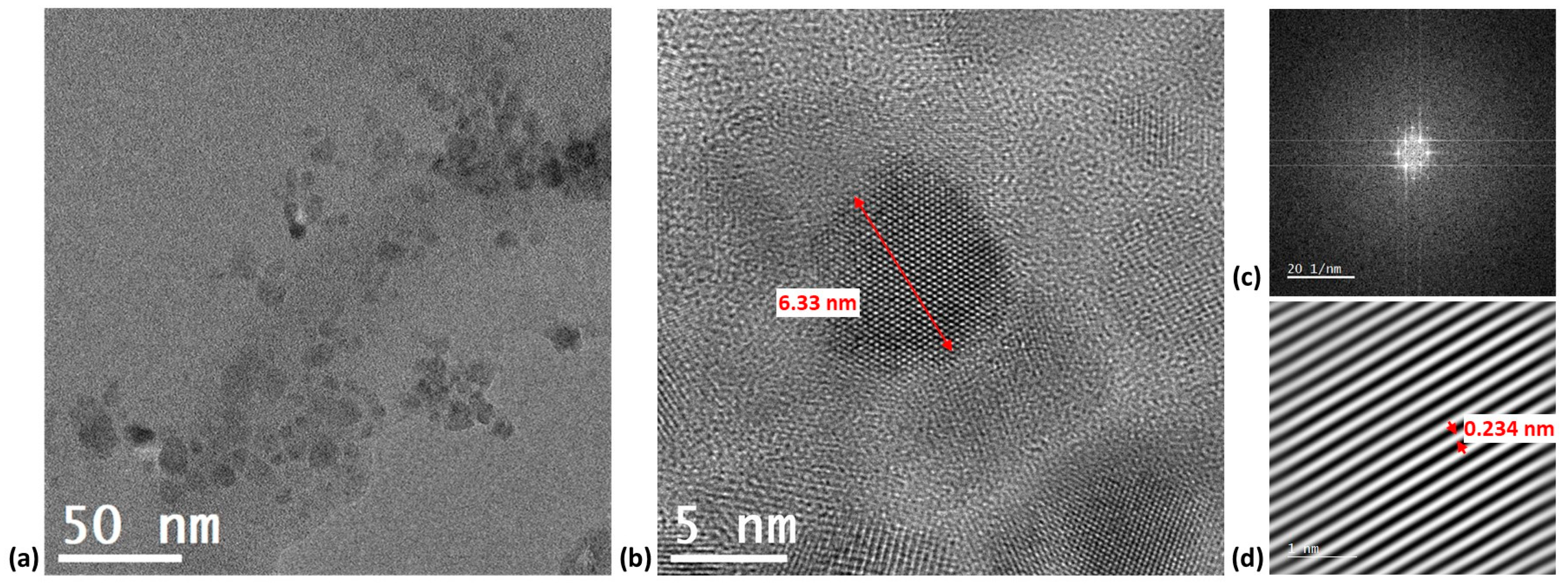
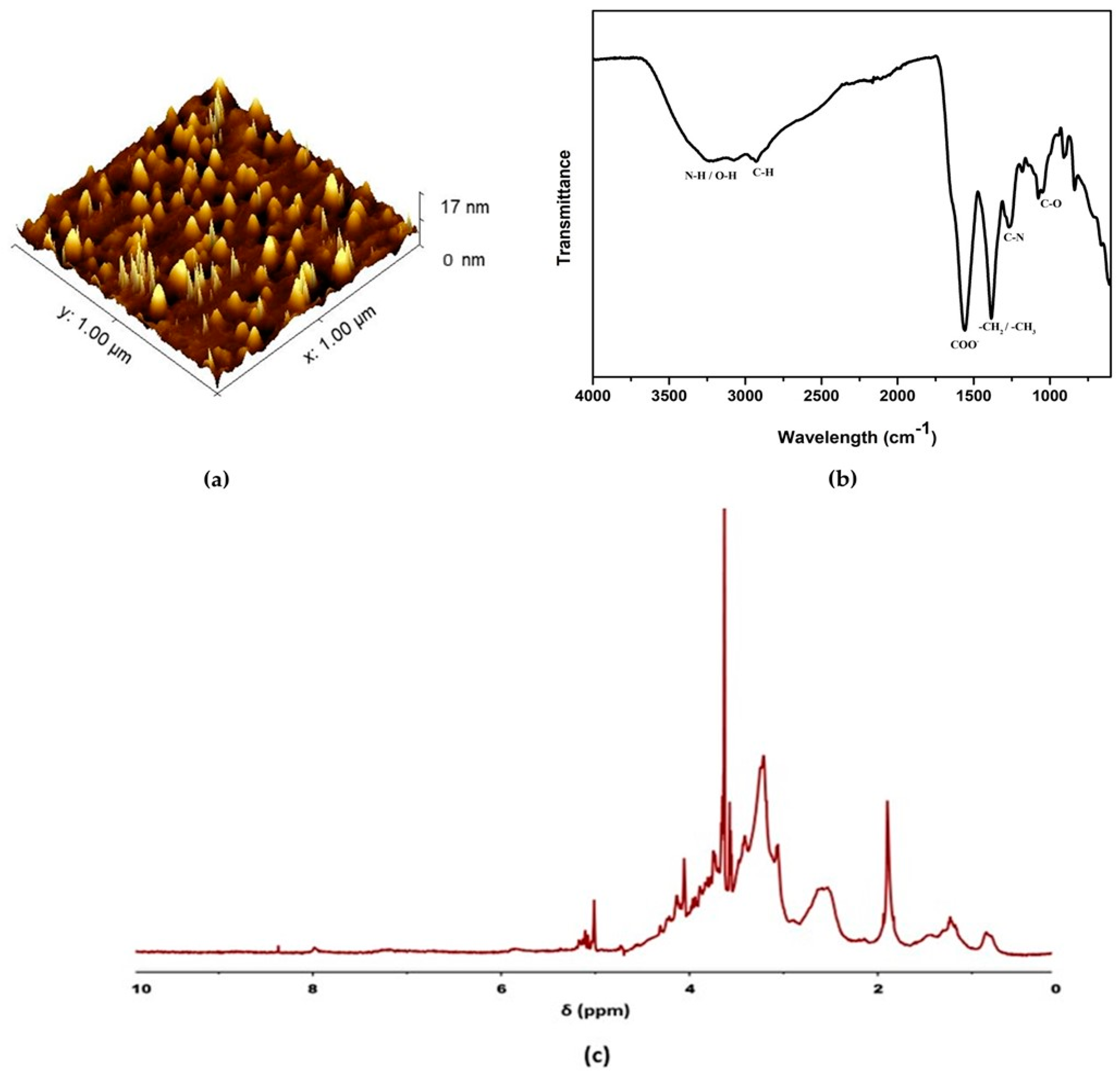
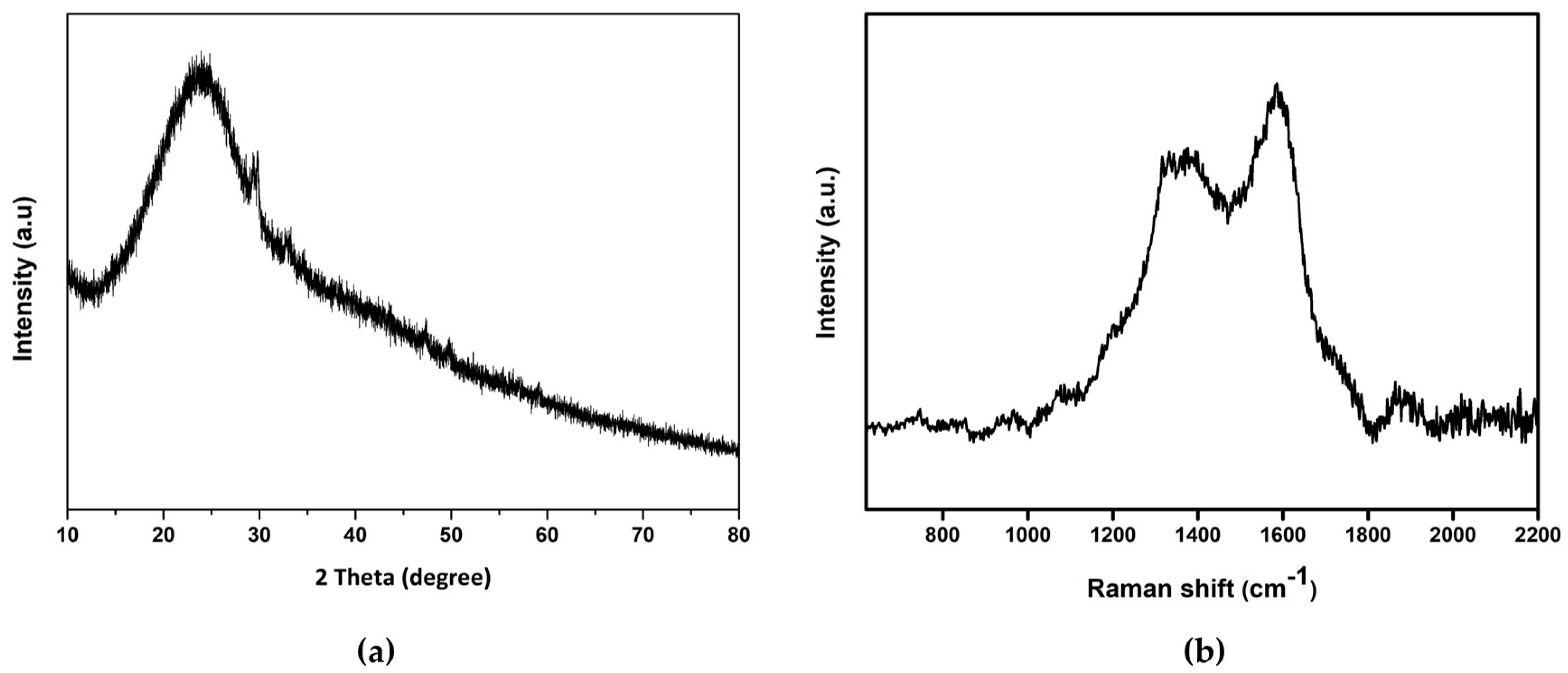
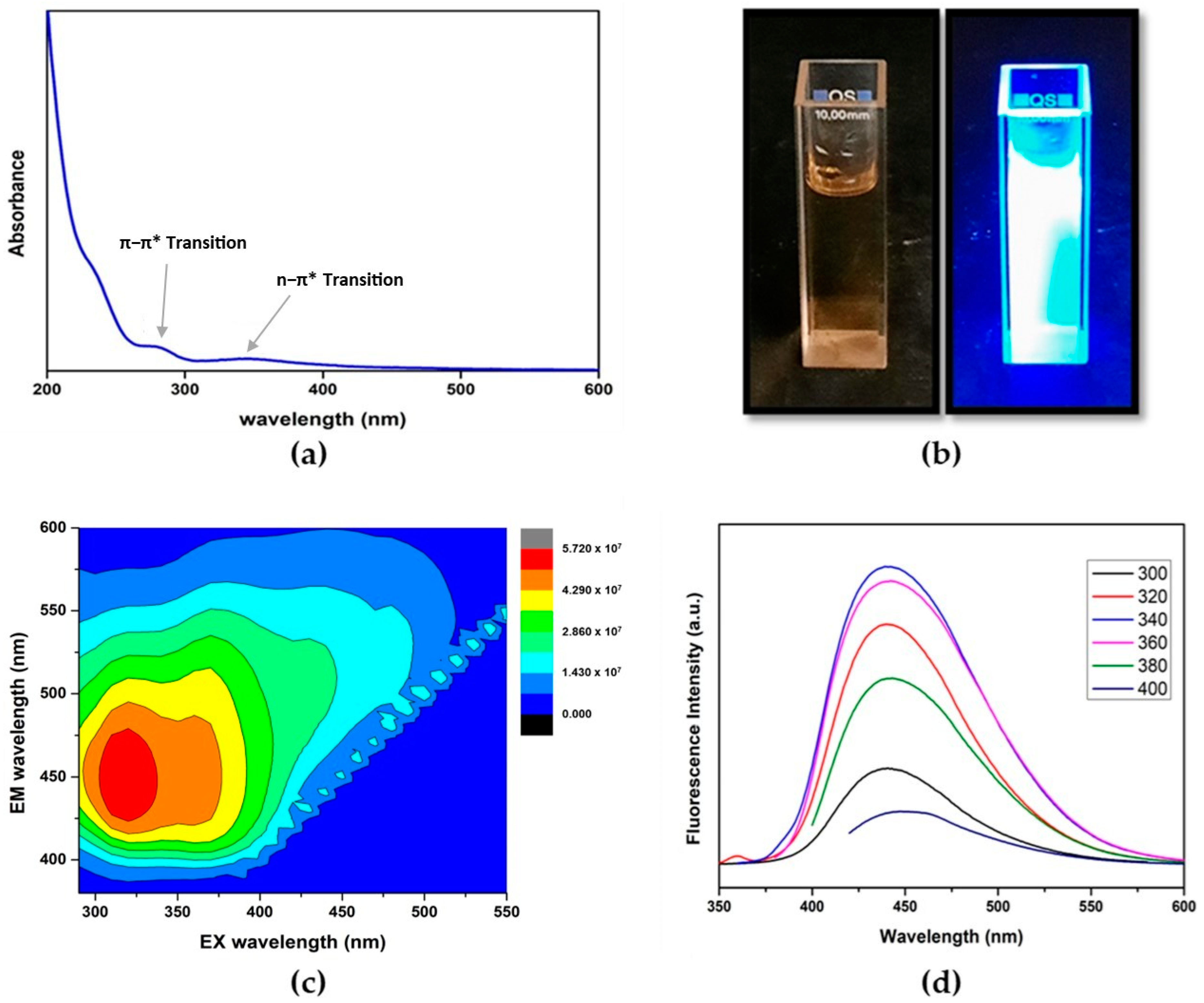
3.1. Surface Morphology
3.2. Optical Properties
3.3. Life Cycle Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.E.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser Ablated Carbon Nanodots for Light Emission. Nanoscale Res. Lett. 2016, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2009, 46, 8812–8814. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon Dots Prepared by Hydrothermal Treatment of Dopamine as an Effective Fluorescent Sensing Platform for the Label-Free Detection of Iron(III) Ions and Dopamine. Chem. A Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011, 21, 2445–2450. [Google Scholar] [CrossRef]
- Lu, Z.; Su, T.; Feng, Y.; Jiang, S.; Zhou, C.; Hong, P.; Sun, S.; Li, C. Potential Application of Nitrogen-Doped Carbon Quantum Dots Synthesized by a Solvothermal Method for Detecting Silver Ions in Food Packaging. Int. J. Environ. Res. Public Health 2019, 16, 2518. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Yang, R. Solid pyrolysis synthesis of excitation-independent emission carbon dots and its application to isoniazid detection. J. Nanopart. Res. 2019, 21, 59. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, Synthesis, and Applications of Carbon Dots: A Review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 126, 114417. [Google Scholar] [CrossRef]
- John, T.S.; Yadav, P.K.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence 2020, 35, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, D.S.; Kasap, H.; Reisner, E. Photocatalytic hydrogen generation coupled to pollutant utilisation using carbon dots produced from biomass. Green Chem. 2020, 22, 2831–2839. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Crista, D.M.A.; El Mragui, A.; Algarra, M.; Esteves Da Silva, J.C.G.; Luque, R.; Pinto Da Silva, L. Turning Spent Coffee Grounds into Sustainable Precursors for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef]
- Hu, Z.; Jiao, X.-Y.; Xu, L. The N,S co-doped carbon dots with excellent luminescent properties from green tea leaf residue and its sensing of gefitinib. Microchem. J. 2019, 154, 104588. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Facile green synthesis of carbon dots from Pyrus pyrifolia fruit for assaying of Al3+ ion via chelation enhanced fluorescence mechanism. J. Mol. Liq. 2018, 264, 9–16. [Google Scholar] [CrossRef]
- Pooja, D.; Singh, L.; Thakur, A.; Kumar, P. Green synthesis of glowing carbon dots from Carica papaya waste pulp and their application as a label-freechemo probe for chromium detection in water. Sens. Actuators B Chem. 2019, 283, 363–372. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Cheng, C.; Xing, M.; Wu, Q. A universal facile synthesis of nitrogen and sulfur co-doped carbon dots from cellulose-based biowaste for fluorescent detection of Fe3+ ions and intracellular bioimaging. Mater. Sci. Eng. C 2019, 99, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Luo, N.; Feng, M.; Peng, X.; Liao, X. Green synthesis of fluorescent N,S-carbon dots from bamboo leaf and the interaction with nitrophenol compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118462. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Niu, Q.; Mou, M.; Wu, Y.; Liu, X.; Yan, Z.; Liao, S. A fluorescence probe based on the nitrogen-doped carbon dots prepared from orange juice for detecting Hg2+ in water. J. Lumin. 2017, 187, 274–280. [Google Scholar] [CrossRef]
- Senol, A.M.; Bozkurt, E. Facile green and one-pot synthesis of seville orange derived carbon dots as a fluorescent sensor for Fe3+ ions. Microchem. J. 2020, 159, 105357. [Google Scholar] [CrossRef]
- Man, Y.; Li, Z.; Kong, W.-L.; Li, W.; Dong, W.; Wang, Y.; Xie, F.; Zhao, D.; Qu, Q.; Zou, W.-S. Starch fermentation wastewater as a precursor to prepare S,N-doped carbon dots for selective Fe(III) detection and carbon microspheres for solution decolorization. Microchem. J. 2020, 159, 105338. [Google Scholar] [CrossRef]
- Jiao, X.-Y.; Li, L.-S.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The synthesis of fluorescent carbon dots from mango peel and their multiple applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Haghani, S.K.; Ensafi, A.A.; Kazemifard, N.; Rezaei, B. A Sensitive and Selective Optical Sensor Based on Molecularly Imprinting Technique Using Green Synthesized Carbon Dots for Determination of Trace Amount of Metronidazole. IEEE Sens. J. 2020, 20, 7. [Google Scholar] [CrossRef]
- Águas, A.; Ferreira, A.; Maia, P.; Fernandes, P.M.; Roxo, L.; Keizer, J.; Silva, J.S.; Rego, F.C.; Moreira, F. Natural establishment of Eucalyptus globulus Labill. in burnt stands in Portugal. For. Ecol. Manag. 2014, 323, 47–56. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea—A molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef] [PubMed]
- Sendão, R.M.S.; Crista, D.M.A.; Afonso, A.C.P.; Martinez de Yuso, M.D.V.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L.P. Insight into the hybrid luminescence showed by carbon dots and molecular fluorophores in solution. Phys. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef] [PubMed]
- Christé, S.; Esteves da Silva, J.C.; Pinto da Silva, L. Evaluation of the Environmental Impact and Efficiency of N-Doping Strategies in the Synthesis of Carbon Dots. Materials 2020, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Ayres, R.U.; de Constance, B.; Cede, F. Life Cycle Analysis: A Critique; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Fernandes, S.; da Silva, J.C.G.E.; da Silva, L.P. Life Cycle Assessment-Based Comparative Study between High-Yield and “Standard” Bottom-Up Procedures for the Fabrication of Carbon Dots. Materials 2022, 15, 3446. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; da Silva, J.C.E.; da Silva, L.P. Comparative life cycle assessment of high-yield synthesis routes for carbon dots. NanoImpact 2021, 23, 100332. [Google Scholar] [CrossRef]
- Sendão, R.; Yuso, M.D.V.M.D.; Algarra, M.; da Silva, J.E.; da Silva, L.P. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, 120080. [Google Scholar] [CrossRef]
- Borghesi, G.; Stefanini, R.; Vignali, G. Life cycle assessment of packaged organic dairy product: A comparison of different methods for the environmental assessment of alternative scenarios. J. Food Eng. 2022, 318, 110902. [Google Scholar] [CrossRef]
- Vega, M.; Llantoy, N.; Chafer, M.; Ushak, S.; Cabeza, L.F. Life cycle assessment of the inclusion of phase change materials in lightweight buildings. J. Energy Storage 2022, 56, 105903. [Google Scholar] [CrossRef]
- Mostafaei, H.; Keshavarz, Z.; Rostampour, M.A.; Mostofinejad, D.; Wu, C. Sustainability Evaluation of a Concrete Gravity Dam: Life Cycle Assessment, Carbon Footprint Analysis, and Life Cycle Costing. Structures 2023, 53, 279–295. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.; Elshout, P.M.; Stam, G.; Verones, F.; Vieira, M.D.; Hollander, A.; Zijp, M.; van Zelm, R. ReCiPe2016 v1.1 A Harmonized Life Cycle Impact Assessment Method at Midpoint and 501 Endpoint Level, Report I: Characterization, RIVM Report 2016-0104a; National Institute for Human Health and the 502 Environment: Debilt, The Netherlands, 2016.
- Hauschild, M.Z.; Huijbregts, M.A.J. Introducing Life Cycle Impact Assessment. In Life Cycle Impact Assessment; Hauschild, M.Z., Huijbregts, M.A.J., Eds.; In LCA Compendium—The Complete World of Life Cycle Assessment; Springer: Dordrecht, The Netherlands, 2015; pp. 1–16. [Google Scholar] [CrossRef]
- Tomskaya, A.; Asanov, I.P.; Yushina, I.; Rakhmanova, M.I.; Smagulova, S. Optical Properties of Tricarboxylic Acid-Derived Carbon Dots. ACS Omega 2022, 7, 44093–44102. [Google Scholar] [CrossRef]
- Long, Y.-M.; Zhou, C.-H.; Zhang, Z.-L.; Tian, Z.-Q.; Lin, Y.; Pang, D.-W. Shifting and non-shifting fluorescence emitted by carbon nanodots. J. Mater. Chem. 2012, 22, 5917–5920. [Google Scholar] [CrossRef]
- Wang, K.; Geng, C.; Wang, F.; Zhao, Y.; Ru, Z. Urea-doped carbon dots as fluorescent switches for the selective detection of iodide ions and their mechanistic study. RSC Adv. 2021, 11, 27645–27652. [Google Scholar] [CrossRef]
- Liu, Y. Green preparation of carbon dots from Momordica charantia L. for rapid and effective sensing of p-aminoazobenzene in environmental samples. Environ. Res. 2021, 198, 111279. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, I.; Bera, M.K. Microwave-Assisted Green Synthesis of Carbon Quantum Dots Derived from Calotropis Gigantea as a Fluorescent Probe for Bioimaging. J. Fluoresc. 2022, 32, 1039–1049. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Gao, X.; Wang, L.; Sun, C.; Zhou, N. Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots. Molecules 2022, 27, 1690. [Google Scholar] [CrossRef]
- Tiwari, A.; Walia, S.; Sharma, S.; Chauhan, S.; Kumar, M.; Gadly, T.; Randhawa, J.K. High quantum yield carbon dots and nitrogen-doped carbon dots as fluorescent probes for spectroscopic dopamine detection in human serum. J. Mater. Chem. B 2023, 11, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Padasalagi, A.B.; Rabinal, M.H.K. Controlled Emission of Carbon Quantum Dots Derived from Waste Silk Sericin. Part. Part. Syst. Charact. 2022, 39, 2200041. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired Carbon Quantum Dots: An Antibiofilm Agents. J. Nanosci. Nanotechnol. 2019, 19, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Özbek, N.; Çekirge, E.; Ocak, M.; Ocak, Ü.T. Highly Blue-fluorescent Carbon Quantum Dots Obtained from Medlar Seed for Hg2+ Determination in Real Water Samples. J. Fluoresc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Niu, A.; Li, J.; Fu, J.-W.; Xu, Q.; Pei, D.-S. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci. Rep. 2016, 6, 37860. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, N.-Y.; Fang, W.-D.; Tan, Q.-G.; Ji, R.; Yang, L.-Y.; Wei, S.; Zhang, X.-W.; Miao, A.-J. Photodegradation of carbon dots cause cytotoxicity. Nat. Commun. 2021, 12, 812. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, P.; Zhang, F.; Feng, X.; Wang, Y.; Yang, Y.; Liu, X. Fluorescent probes for “off–on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 2016, 152, 288–300. [Google Scholar] [CrossRef]
- Emam, A.N. Cyto-toxicity, biocompatibility and cellular response of carbon dots–plasmonic based nano-hybrids for bioimaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Wang, Y.; Li, H.; Yin, J.; Li, M.; Wang, L.; Sun, H.; Chen, L. Large-scale direct pyrolysis synthesis of excitation-independent carbon dots and analysis of ferric (III) ion sensing mechanism. Appl. Surf. Sci. 2020, 538, 148151. [Google Scholar] [CrossRef]
- Mishra, K.; Barai, M.; Ghosh, S. Roles of Impurity and Sample Heterogeneity in Intriguing Photoluminescence Properties of Zero-Dimensional (0D) Carbonaceous Materials. J. Phys. Chem. C 2022, 126, 16905–16918. [Google Scholar] [CrossRef]
- Xu, Q.; Xiao, F.; Xu, H. Green-derived carbon dots: A potent tool for biosensing in food safety. In Critical Reviews in Food Science and Nutrition; Taylor & Francis: Abingdon, UK, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Ahmed, F.; Xu, W.; Hussain, M.M.; Khan, W.U.; Xiong, H. Bioimaging-guided discrimination of normal/cancer cells using Ag+-mediated red fluorescent carbon dots. Chem. Eng. J. 2023, 477, 147300. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; da Silva, J.C.G.E.; da Silva, L.P. Applications of Fluorescent Carbon Dots as Photocatalysts: A Review. Catalysts 2023, 13, 179. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, R.; Liu, X.; Liu, Y.; Zhang, Q.; Cheng, H.; Li, R.; Wang, L.; Wu, X.; Li, B. Carbon Dots as Advanced Drug-Delivery Nanoplatforms for Antiinflammatory, Antibacterial, and Anticancer Applications: A Review. ACS Appl. Nano Mater. 2023, 6, 9071–9084. [Google Scholar] [CrossRef]
- Aftenieva, O.; Brunner, J.; Adnan, M.; Sarkar, S.; Fery, A.; Vaynzof, Y.; König, T.A.F. Directional Amplified Photoluminescence through Large-Area Perovskite-Based Metasurfaces. ACS Nano 2023, 17, 2399–2410. [Google Scholar] [CrossRef]

| Denomination | Proposed Correspondence in Ecoinvent® 3.5 Database |
|---|---|
| Citric acid Ethylenediamine Electricity Deionized water | Citric acid {GLO}| market for | Cut-off, S Ethylenediamine {RER}| production | Cut-off, S Electricity, medium voltage {PT}| market for | Cut-off, S Water, deionised, from tap water, at user {Europe without Switzerland} | market for water, deionised, from tap water, at user | Cut-off, S |
| Biomass Source | Synthesis Method | Conditions | QY (%) | λex/λem (nm) | Ref. |
|---|---|---|---|---|---|
| Green tea leaf | Pyrolysis, oxidation | 2 h of pyrolyzation at 350 °C, 20 h of oxidation | 14.8% | 320/410 | [19] |
| Pyrus pyrifolia fruit | Hydrothermal | 180 °C, 6 h | 10.8% | 390/471 | [20] |
| Carica papaya waste pulp | Pyrolysis | 200 °C, 15 min | 23.7% | 310/462 | [21] |
| Dwarf banana peel | Hydrothermal | 200 °C, 24 h | 23% | 345/445 | [22] |
| Willow catkin | Combustion | Dried at 80 °C and burned to ashes | 13.3% | 310/370 | [23] |
| Chionanthus retusus fruit extract | Hydrothermal | 180 °C, 6 h | 9% | 340/425 | [24] |
| Bamboo leaf | Calcination | 300 °C, 3 h | 5.18% | 313/419 | [25] |
| Orange juice | Hydrothermal | 200 °C, 11 h | 31.7% | 360/449 | [26] |
| Seville orange | Hydrothermal | 130 °C, 12 h | 13.3% | 330/402 | [27] |
| Starch fermentation wastewater | Hydrothermal | 180 °C, 10 h | 24.5% | 460/518 | [28] |
| Mango peel | Pyrolyzation with oxygenolysis | 300 °C, 6 h | 8.5% | 310/425 | [29] |
| Eucalyptus leaf | Hydrothermal | 180 °C, 24 h | 60.7% | 320/445 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johny, A.; Pinto da Silva, L.; Pereira, C.M.; Esteves da Silva, J.C.G. Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves. Environments 2024, 11, 6. https://doi.org/10.3390/environments11010006
Johny A, Pinto da Silva L, Pereira CM, Esteves da Silva JCG. Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves. Environments. 2024; 11(1):6. https://doi.org/10.3390/environments11010006
Chicago/Turabian StyleJohny, Archana, Luís Pinto da Silva, Carlos M. Pereira, and Joaquim C. G. Esteves da Silva. 2024. "Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves" Environments 11, no. 1: 6. https://doi.org/10.3390/environments11010006
APA StyleJohny, A., Pinto da Silva, L., Pereira, C. M., & Esteves da Silva, J. C. G. (2024). Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves. Environments, 11(1), 6. https://doi.org/10.3390/environments11010006









