Differential Cytotoxicity, Inflammatory Responses, and Aging Effects of Human Skin Cells in Response to Fine Dust Exposure
Abstract
1. Introduction
2. Experimental Methods
2.1. Preparation and Treatment of Fine Dusts
2.2. Cell Culture of Human Skin Cells
2.3. Cell Viability Assay
2.4. Measurement of Inflammatory Cytokine IL-8
2.5. Analysis of Cell Behavior in Response to Fine Dust
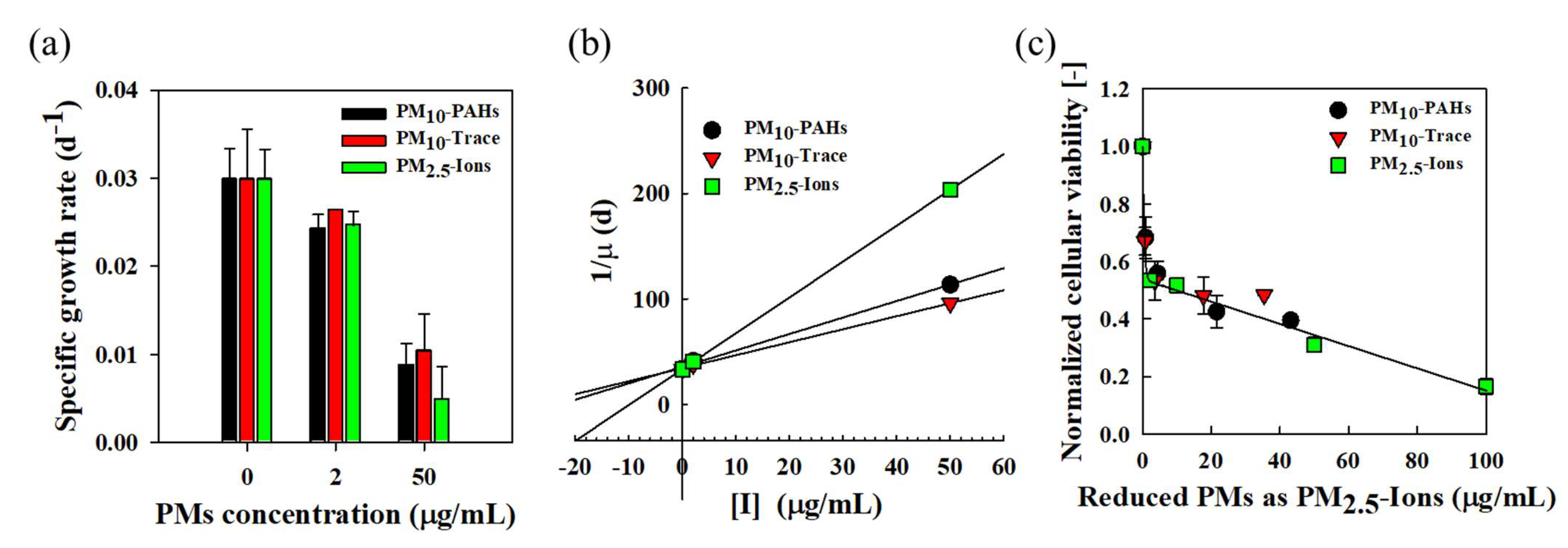
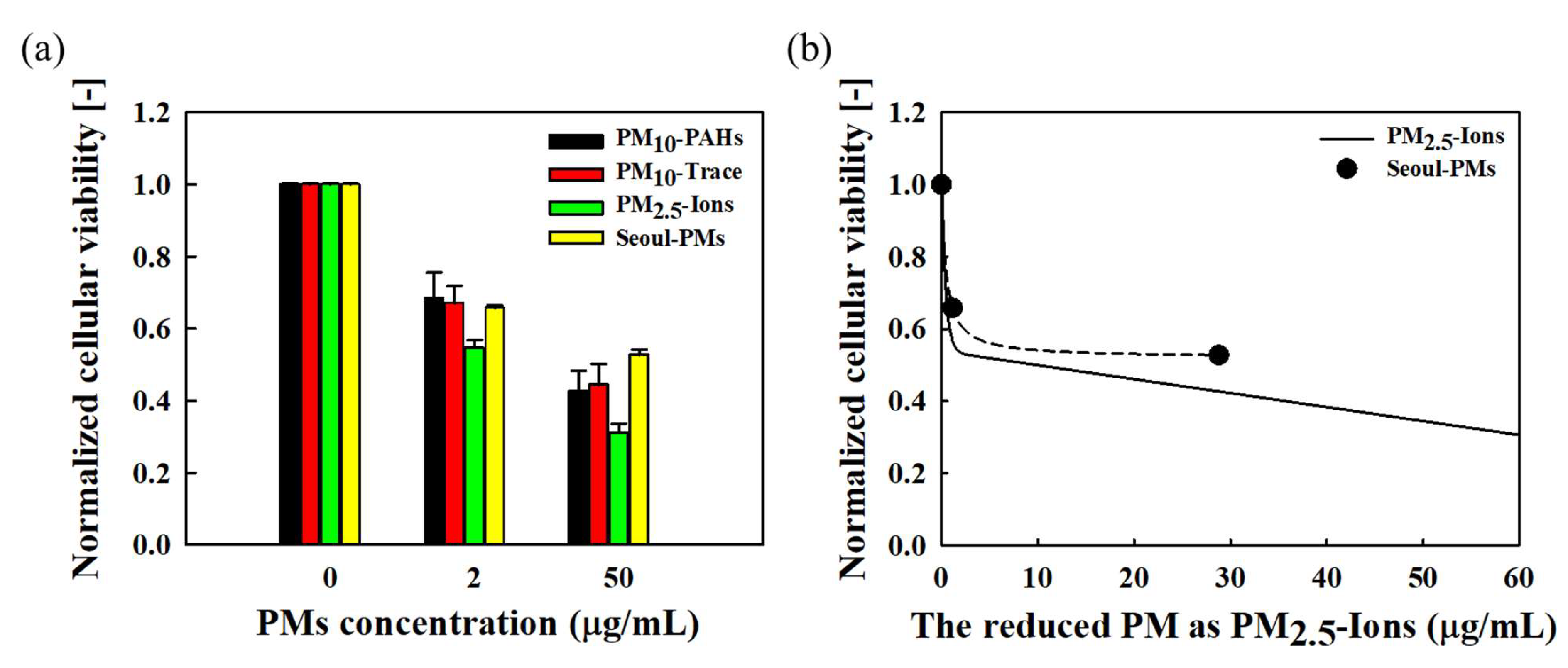
2.6. Analysis of Cytotoxicity and Growth Inhibition of Mixed Fine Dust
2.7. Statistical Analysis
3. Results and Discussion
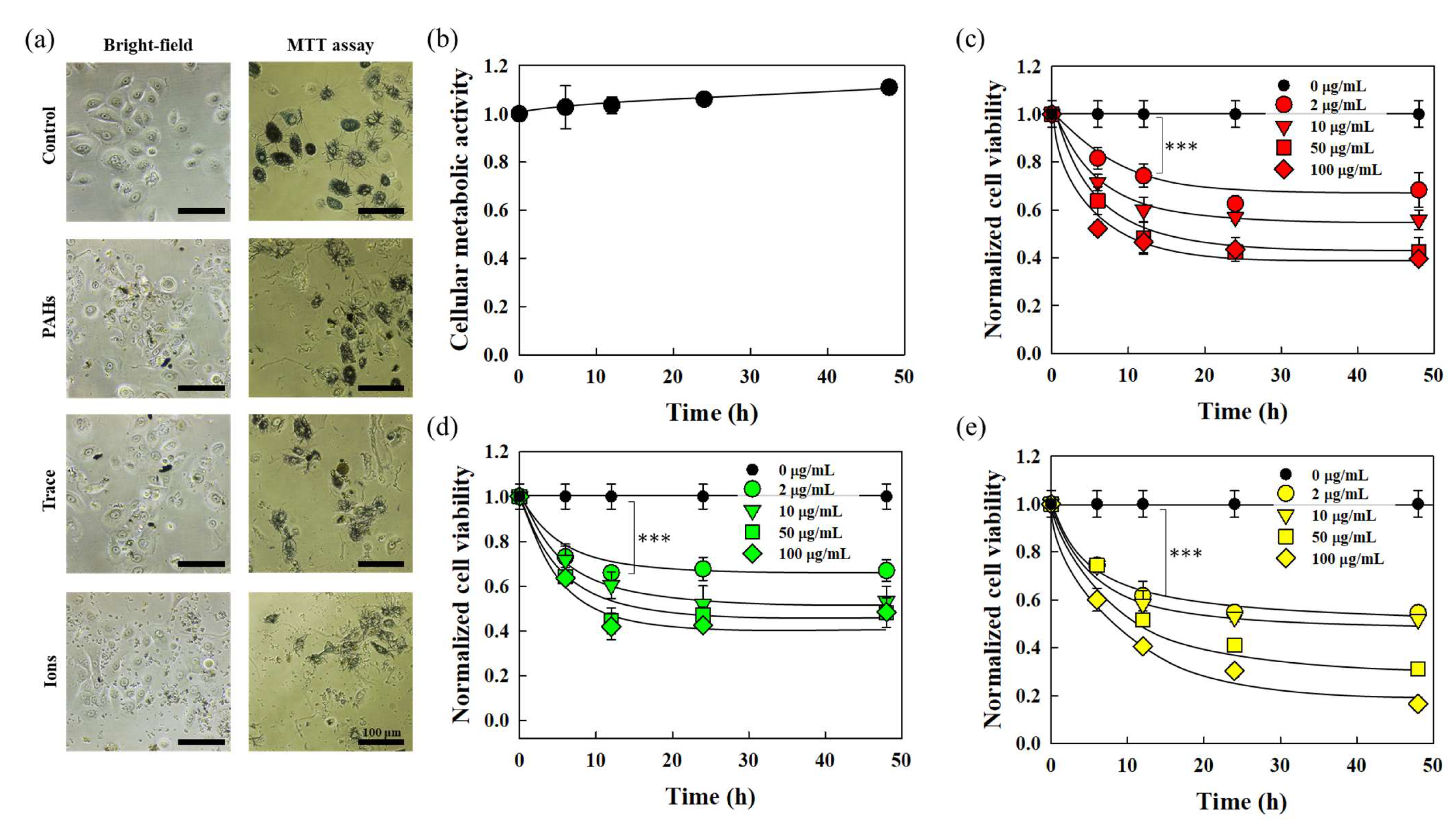
3.1. Cytotoxicity of Skin Cells in Response to Fine Dust
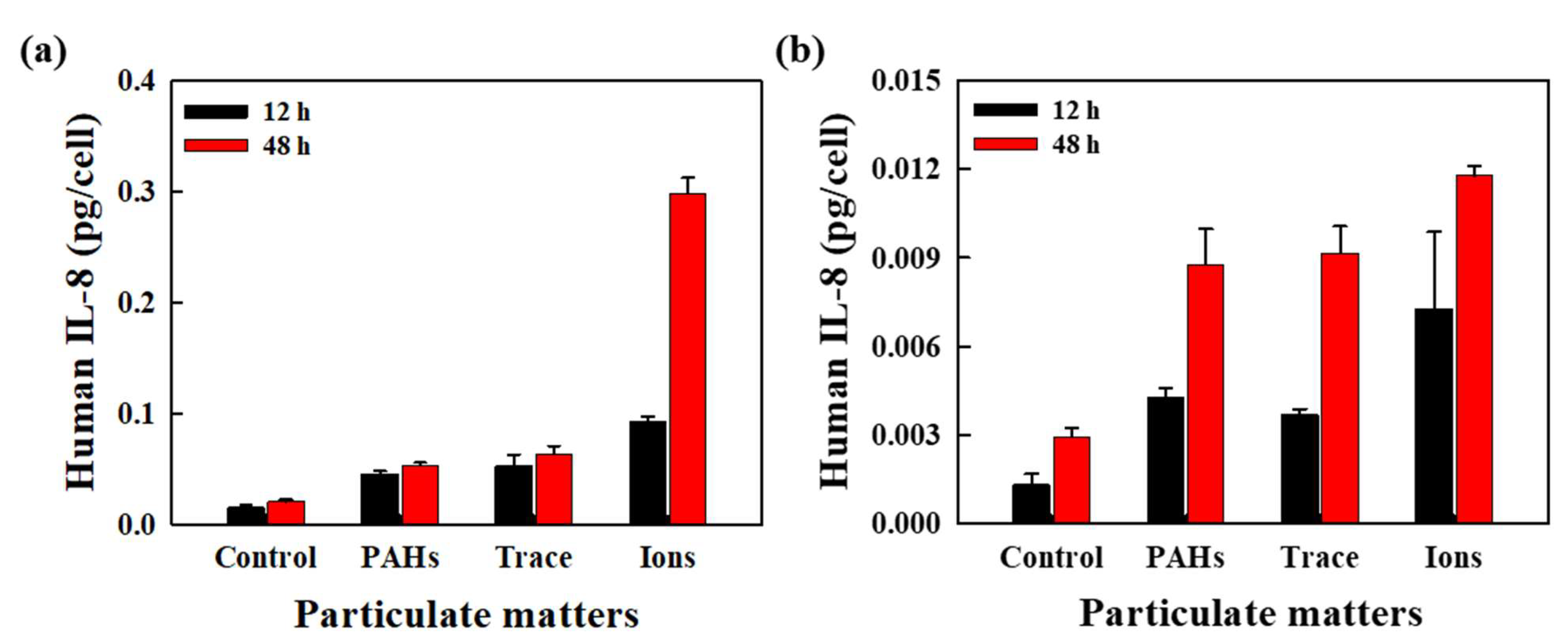
3.2. Evaluation of Inflammatory Cytokine Expression Induced by Fine Dust
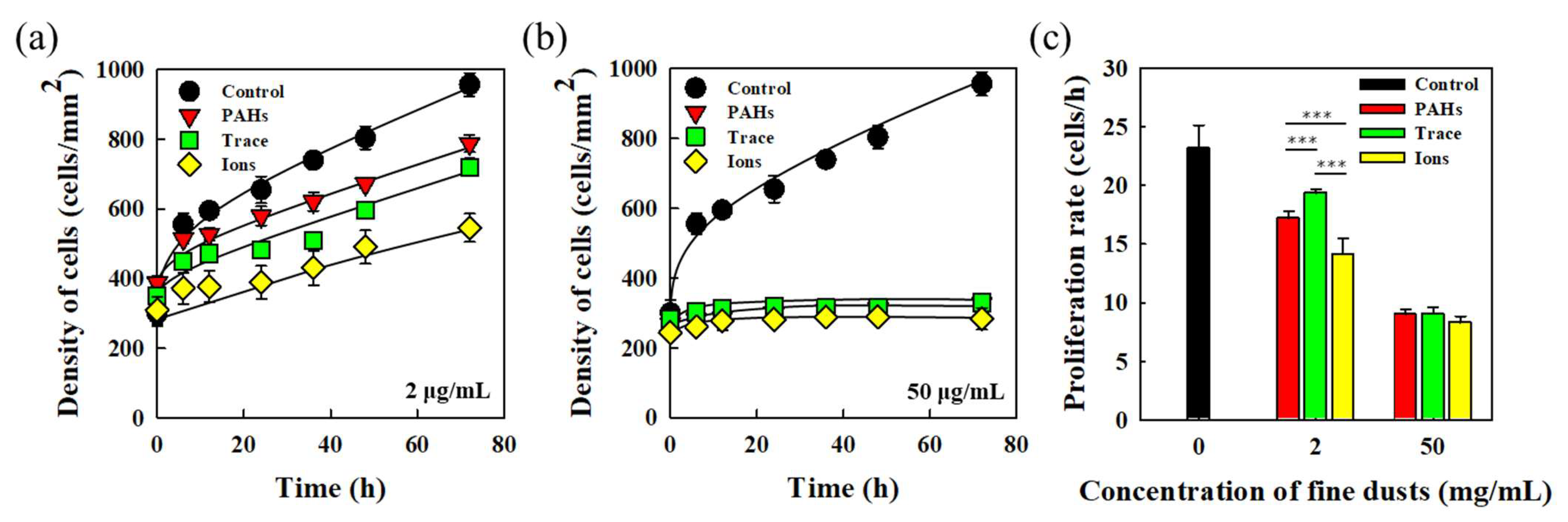
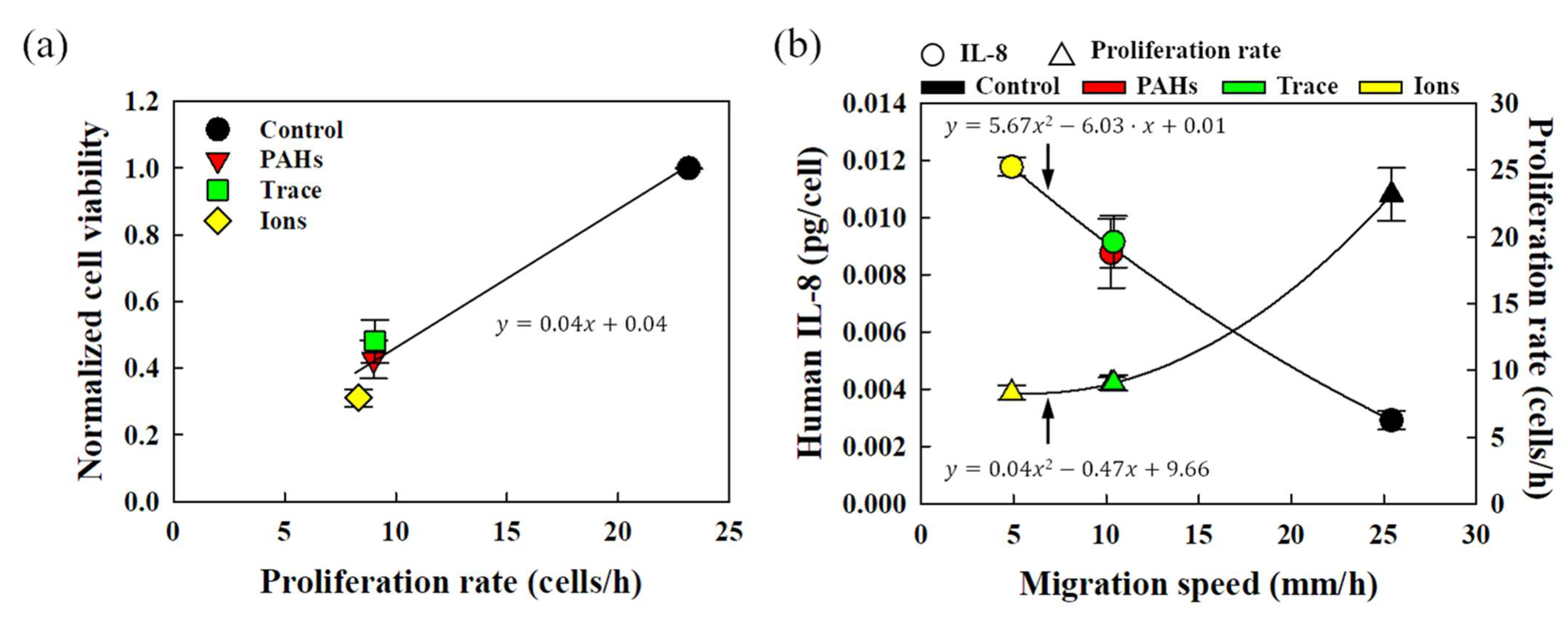
3.3. Proliferative Characteristics of HEKn in Response to Fine Dust
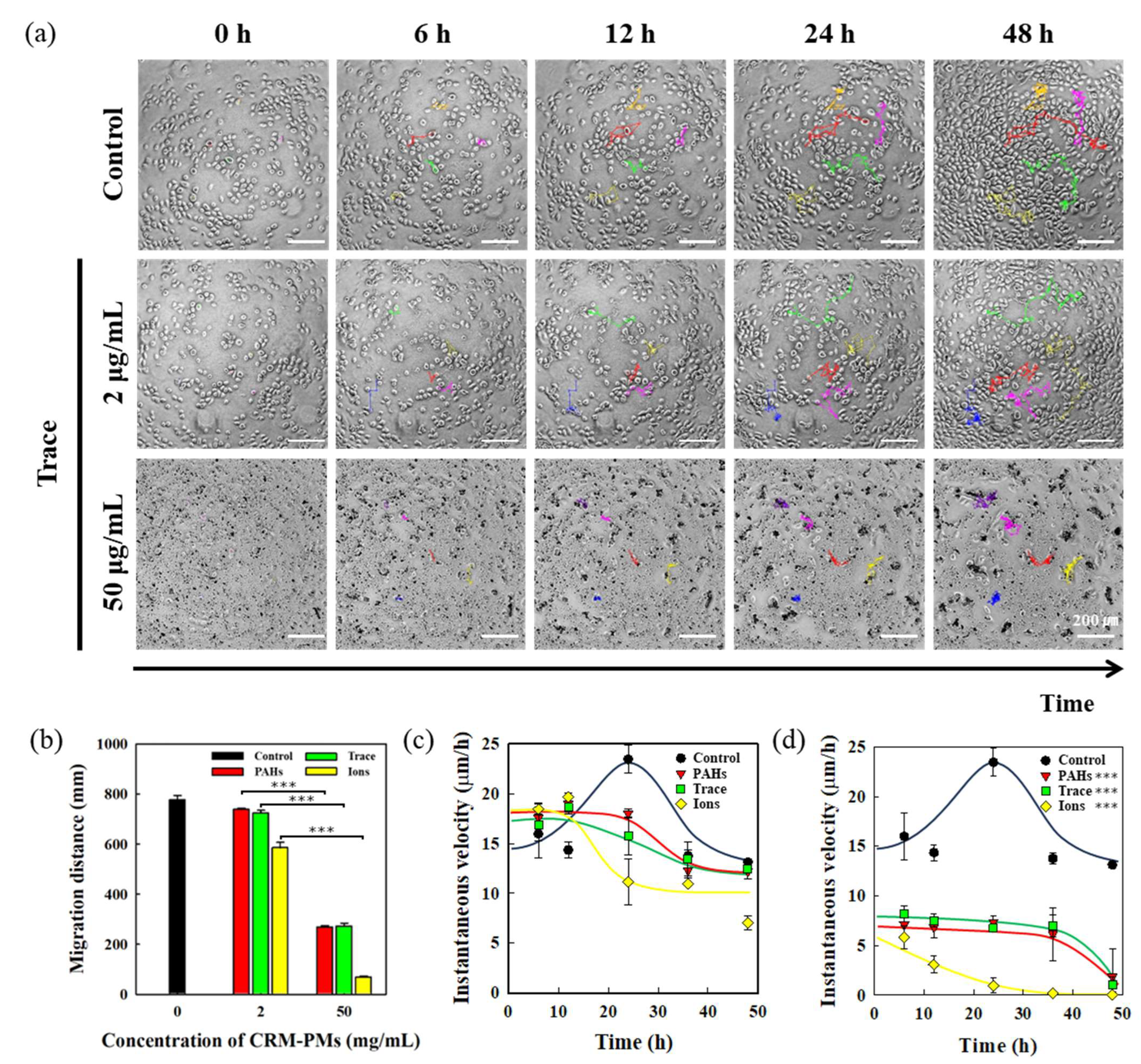
3.4. Analysis of HEKn Migration in Response to Fine Dust
3.5. Correlation Analysis of HEKn Metabolic and Behavioral Characteristics in Response to Fine Dust
3.6. Cytotoxicity and Growth Inhibition of Mixed Fine Dust
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaddick, G.; Thomas, M.L.; Mudu, P.; Ruggeri, G.; Gumy, S. Half the world’s population are exposed to increasing air pollution. npj Clim. Atmos. Sci. 2020, 3, f23. [Google Scholar] [CrossRef]
- Fitoussi, R.; Faure, M.-O.; Beauchef, G.; Achard, S. Human skin responses to environmental pollutants: A review of current scientific models. Environ. Pollut. 2022, 306, 119316. [Google Scholar] [CrossRef] [PubMed]
- Hamra Ghassan, B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet Jonathan, M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor Particulate Matter Exposure and Lung Cancer: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett Richard, T.; Turner Michelle, C.; Cohen, A.; Krewski, D.; Jerrett, M.; Gapstur Susan, M.; Thun Michael, J. Lung Cancer and Cardiovascular Disease Mortality Associated with Ambient Air Pollution and Cigarette Smoke: Shape of the Exposure–Response Relationships. Environ. Health Perspect. 2011, 119, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Yang, A.Y.; Huang, M.T.; Liu, Y.; Lee, J.H.; Khor, T.O.; Su, Z.Y.; Shu, L.; Lu, Y.; Conney, A.H.; et al. Nrf2 null enhances UVB-induced skin inflammation and extracellular matrix damages. Cell Biosci. 2014, 4, 39. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights [Review]. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne Particulate Matter and Human Health: Toxicological Assessment and Importance of Size and Composition of Particles for Oxidative Damage and Carcinogenic Mechanisms. J. Environ. Sci. Health Part C 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Jindal, S.; Chaudhary, Y.; Aggarwal, K.K. Toxicity of polyaromatic hydrocarbons and their biodegradation in the environment. In Green Chemistry Approaches to Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2024; pp. 43–66. [Google Scholar]
- Sombiri, S.; Balhara, N.; Attri, D.; Kharb, I.; Giri, A. An overview on occurrence of polycyclic aromatic hydrocarbons in food chain with special emphasis on human health ailments. Discov. Environ. 2024, 2, 87. [Google Scholar] [CrossRef]
- Coxon, T.; Goldstein, L.; Odhiambo, B.K. Analysis of spatial distribution of trace metals, PCB, and PAH and their potential impact on human health in Virginian Counties and independent cities, USA. Environ. Geochem. Health 2019, 41, 783–801. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.-J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- gon Ryou, H.; Heo, J.; Kim, S.Y. Source apportionment of PM10 and PM2.5 air pollution, and possible impacts of study characteristics in South Korea. Environ. Pollut. 2018, 240, 963–972. [Google Scholar] [CrossRef]
- Cho, K.-S.; Ryu, H.-W.; Choi, H.-M. Toxicity evaluation of complex metal mixtures using reduced metal concentrations: Application to iron oxidation by Acidithiobacillus ferrooxidans. J. Microbiol. Biotechnol. 2008, 18, 1298–1307. [Google Scholar] [PubMed]
- Akhtar, U.S.; McWhinney, R.D.; Rastogi, N.; Abbatt JP, D.; Evans, G.J.; Scott, J.A. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal. Toxicol. 2010, 22 (Suppl. 2), 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, L.; Zhang, Y.; Hu, H.; Shi, Y.; Liang, S.; Zhao, T.; Fu, Y.; Duan, J.; Sun, Z. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in human cardiomyocytes. Ecotoxicol. Environ. Saf. 2018, 161, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Marmolino, D.; Manto, M. Pregabalin antagonizes copper-induced toxicity in the brain: In vitro and in vivo studies. Neurosignals 2011, 18, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.T.; Soukup, J.; Harder, S.; Becker, S. Mitochondrial oxidant production by a pollutant dust and NO-mediated apoptosis in human alveolar macrophage. Am. J. Physiol. Cell Physiol. 2003, 284, C24–C32. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Cooper, K.L.; Huestis, J.; Xu, H.; Burchiel, S.W.; Hudson, L.G.; Liu, K.J. S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite. Oncotarget 2016, 7, 80482. [Google Scholar] [CrossRef]
- Kim, H.B.; Choi, M.G.; Chung, B.Y.; Um, J.Y.; Kim Jin, C.; Park, C.W.; Kim, H.O. Particulate matter 2.5 induces the skin barrier dysfunction and cutaneous inflammation via AhR- and T helper 17 cell-related genes in human skin tissue as identified via transcriptome analysis. Exp. Dermatol. 2023, 32, 547–554. [Google Scholar] [CrossRef]
- Coquette, A.; Berna, N.; Vandenbosch, A.; Rosdy, M.; De Wever, B.; Poumay, Y. Analysis of interleukin-1α (IL-1α) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol. Vitr. 2003, 17, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Hetland, R.B.; Cassee, F.R.; Refsnes, M.; Schwarze, P.E.; Låg, M.; Boere AJ, F.; Dybing, E. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol. Vitr. 2004, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Yuhong, H.; Thomas, C.; Sandrah, P.E.; Tingyu, Y.; Xinci, C.; Mario, V.; Nathan, P.; Fred, L.; Deborah, L.; Nathana, L.; et al. Joint effects of traffic-related air pollution and hypertensive disorders of pregnancy on maternal postpartum depressive and anxiety symptoms. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef]
- Kermani, M.; Rahmatinia, T.; Oskoei, V.; Norzaee, S.; Shahsavani, A.; Farzadkia, M.; Kazemi, M.H. Potential cytotoxicity of trace elements and polycyclic aromatic hydrocarbons bounded to particulate matter: A review on in vitro studies on human lung epithelial cells. Environ. Sci. Pollut. Res. 2021, 28, 55888–55904. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.-H.; Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix—A review. Bioact. Mater. 2022, 15, 145–159. [Google Scholar] [CrossRef]
- Weaver, D.F. Amyloid beta is an early responder cytokine and immunopeptide of the innate immune system. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12100. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Xiang, X.; Li, S.; Wang, M.; Liang, Z.; Ren, J. Integrated evaluation the antioxidant activity of epicatechin from cell dynamics. Biotechnol. Prog. 2023, 39, e3328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, J.; Fan, S.; Wu, P.; Cong, W.; Lin, Y.; Lan, S.; Song, S.; Shao, B.; Dai, W. Nanoparticulates reduce tumor cell migration through affinity interactions with extracellular migrasomes and retraction fibers. Nanoscale Horiz. 2022, 7, 779–789. [Google Scholar] [CrossRef]
- Prudovsky, I. Cellular mechanisms of FGF-stimulated tissue repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ellwein, L.B. Cell Proliferation in Carcinogenesis. Science 1990, 249, 1007–1011. [Google Scholar] [CrossRef]
- Campos, A.; Clemente-Blanco, A. Cell cycle and DNA repair regulation in the damage response: Protein phosphatases take over the reins. Int. J. Mol. Sci. 2020, 21, 446. [Google Scholar] [CrossRef]
- Heydarnezhad Asl, M.; Pasban Khelejani, F.; Bahojb Mahdavi, S.Z.; Emrahi, L.; Jebelli, A.; Mokhtarzadeh, A. The various regulatory functions of long noncoding RNAs in apoptosis, cell cycle, and cellular senescence. J. Cell. Biochem. 2022, 123, 995–1024. [Google Scholar] [CrossRef]
- Wei, H.; Yuan, W.; Yu, H.; Geng, H. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in rat alveolar macrophages. Environ. Sci. Pollut. Res. 2021, 28, 25819–25829. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Y.; Asweto, C.O.; Feng, L.; Yang, X.; Zhang, Y.; Sun, Z. Fine particle matters induce DNA damage and G2/M cell cycle arrest in human bronchial epithelial BEAS-2B cells. Environ. Sci. Pollut. Res. 2017, 24, 25071–25081. [Google Scholar] [CrossRef]


| CRM-PMs | KI,i (μg/mL) | αi [-] | Composition of Representative Seoul-PMs (%) [14] | Concentration of Simulated Seoul-PMs (μg/mL) | Reduced Concentration of the Seoul-PMs as PM2.5-Ions (μg/mL) | ||
|---|---|---|---|---|---|---|---|
| Low PMs | High PMs | Low PMs | High PMs | ||||
| PM10-PAHs | 22.9 | 2.32 | 22.6 | 0.45 | 11.30 | 0.20 | 4.87 |
| PM10-Trace | 27.9 | 2.82 | 45.6 | 0.91 | 22.80 | 0.32 | 8.06 |
| PM2.5-Ions | 9.9 | 1.00 | 31.7 | 0.64 | 15.85 | 0.63 | 15.85 |
| Total | 100.0 | 2.00 | 50.00 | 1.15 | 28.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.E.; Lim, J.W.; Jeong, J.H.; Ryu, H.W. Differential Cytotoxicity, Inflammatory Responses, and Aging Effects of Human Skin Cells in Response to Fine Dust Exposure. Environments 2024, 11, 259. https://doi.org/10.3390/environments11110259
Kim TE, Lim JW, Jeong JH, Ryu HW. Differential Cytotoxicity, Inflammatory Responses, and Aging Effects of Human Skin Cells in Response to Fine Dust Exposure. Environments. 2024; 11(11):259. https://doi.org/10.3390/environments11110259
Chicago/Turabian StyleKim, Tae Eun, Jun Woo Lim, Jae Hyun Jeong, and Hee Wook Ryu. 2024. "Differential Cytotoxicity, Inflammatory Responses, and Aging Effects of Human Skin Cells in Response to Fine Dust Exposure" Environments 11, no. 11: 259. https://doi.org/10.3390/environments11110259
APA StyleKim, T. E., Lim, J. W., Jeong, J. H., & Ryu, H. W. (2024). Differential Cytotoxicity, Inflammatory Responses, and Aging Effects of Human Skin Cells in Response to Fine Dust Exposure. Environments, 11(11), 259. https://doi.org/10.3390/environments11110259







