Possible Impacts of Elevated CO2 and Temperature on Growth and Development of Grain Legumes
Abstract
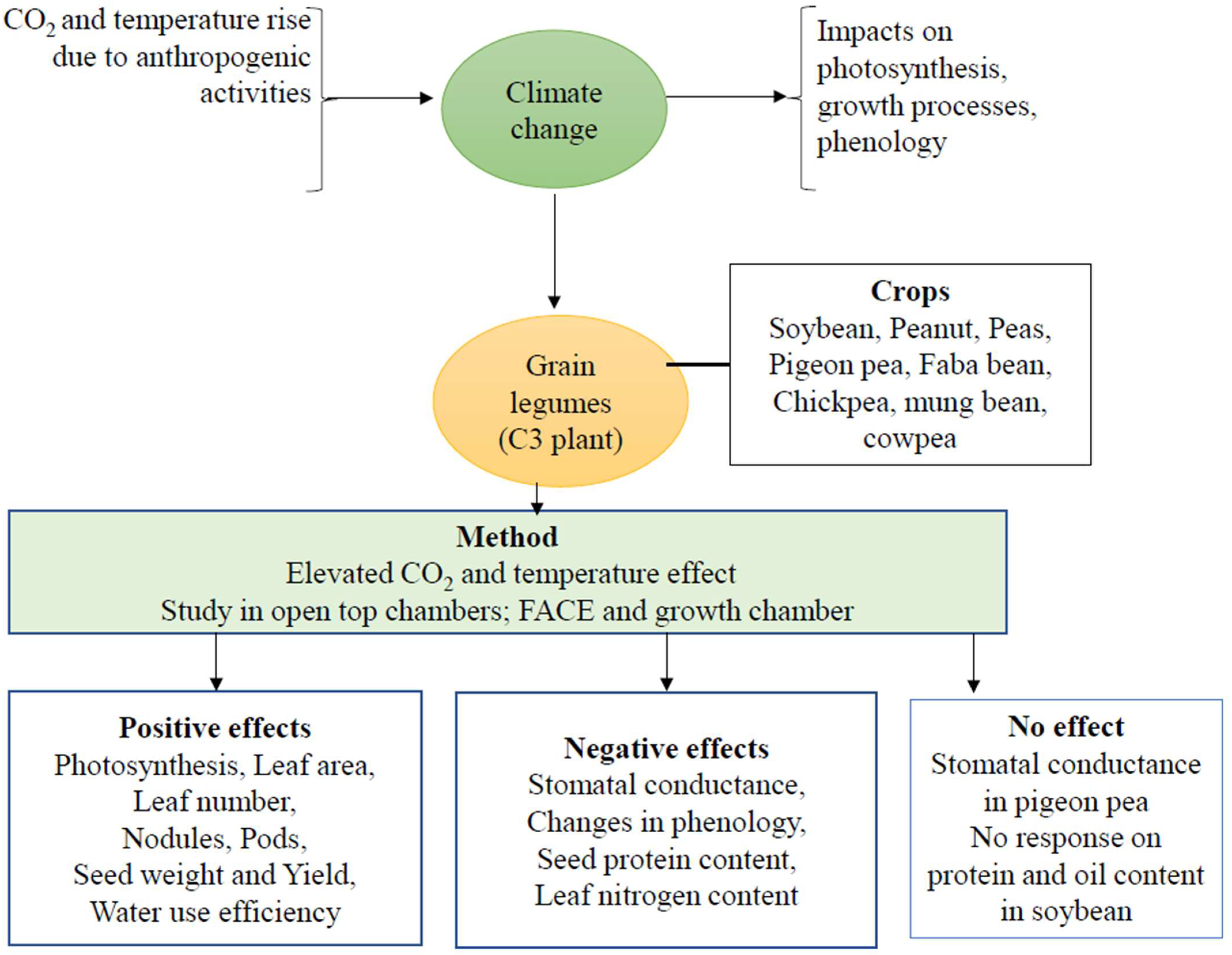
:Highlights
- This review presents a summary of the literature on the effects of elevated CO2 and temperature on grain legumes
- Fertilization effect of elevated CO2 has a positive effect on photosynthesis and biomass growth
- Grain nutrition content of grain legumes can decrease
- Pollinators’ visits to flowering plants can be higher, enhancing pollination
Abstract
1. Introduction
2. Mechanisms of Plant Responses to Elevated [CO2] and Temperature Stresses
3. Elevated [CO2] and Temperature Effects on Crop Growth and Development
3.1. Crop Phenology
3.2. Photosynthesis
3.3. Growth and Productivity
| Crop | Method | [CO2] Level | Temperature Level and Response | Elevated CO2 Response on Yield | Elevated CO2 Response on Photosynthesis | Reference |
|---|---|---|---|---|---|---|
| Peanut (Arachis hypogaea L.) | Canopy Evapotranspiration And Assimilation Chamber | AC: 400 ppm EC: 650 ppm | None | Increased pod yield by 39% under water stress. | Increased 41% Rubisco efficiency, reduced 16% leaf N. | [14] |
| Peanut (Arachis hypogaea L.) | Open Top Chambers (OTC) | AC: 380 ppm EC: 550 ppm EC: 700 ppm | None | Decreased kernel yield by 32% at 550 ppm and 28% at 700 ppm. Pod yield unaffected. | - | [72] |
| Peanut (Arachis hypogaea L.) | OTC | AC: 375 ppm EC: 548 ppm EC: 730 ppm | None | Increased pod number by 16%. | Increased net photosynthesis by 23%. Reduced stomatal conductance by 42%. | [68] |
| Mung bean (Vigna radiata L.) | Free-Air Carbon Dioxide Enrichment (FACE) | AC: 400 ppm EC: 550 ppm | None | Increased BM by 34%, seed yield by 34–50%. | - | [28] |
| Mung bean (Vigna radiata L.) | FACE: Open Air Condition | AC: 400 ppm EC: 550 ± 17 ppm | None | Increased BM 12%, seed yield 14%. | Increased net photosynthesis by 7–19%, Chl and total Chl content by 11–12% | [71] |
| Mung bean (Vigna radiata L.) | Growth Chamber (Model ATC26, Winnipeg, MB). | AC: 400 ppm EC: 700 ppm | Day/night: 6/22 °C, 32/28 °C, | Higher stem and leaf biomass, higher transpiration. | - | [31] |
| Mung bean (Vigna radiata L.) | OTC | EC: 600 ± 50 ppm | None | [CO2] exposure of 0–20 days caused 28–35% higher shoot growth. | Decreases in Chl a by 10% and 18% at 15 and 35 days after germination. | [56] |
| Mung bean (Vigna radiata L.) | OTC | AC: Field experiment EC: 700 ppm | None | Increased seed number and weight by 34.6% and 25%. | It increased photosynthesis by 25–29%, chloride by 30–39%, and carotenoid production by 8–15%. | [98] |
| Mung bean (Vigna radiata L.) | FACE | AC: 411 ± 15 ppm EC: 550 ± 19 ppm | None | Increase in pod BM by 26%, seed yield by 26%, and plant BM by 17%. | - | [99] |
| Pea (Pisum sativum) | Australian Grains Free-Air [CO2] Enrichment (AGFACE) | AC: 400 ppm EC: 550 ppm | None | Increased 33% number of nodules | Reduced stomatal conductance by 44% with lower amino acid. | [19] |
| Pea (Pisum sativum) | OTC | AC: 360 ppm EC: 700 ppm EC: 1000 ppm | None | Increase BM by 40% at 1000 ppm and leghemoglobin by 38%. | No response. | [24] |
| Faba bean (Vicia faba L.) | Australian Grains Free-Air [CO2] Enrichment | AC: 400 ppm EC: 550 ppm | None | Increased seed yield by 58% under irrigated and 23% under drought. | - | [16] |
| Pigeon pea (Cajanus cajan L.) | OTC | AC: 380 ppm EC: 580 ppm | None | Increased yield and pods by 12% and 76% (Pusa-992 genotype). Decrease seed yield by 33% (PS-2009 genotype). | - | [70] |
| Cowpea (Vigna Unguiculata) | Growth Chambers | AC: 370 ppm EC: 550 ppm | day/night: 26/20 °C, 29/23 °C, 32/29 °C | Increased seed number and weight by 21 and 23%. | Decreased pod number and weight by 23 and 24% under 32/29 °C and 29/23 °C. | [20] |
| Soybean (Glycine max (L.) Merr.) | Rhizotron Chambers | AC: 380 ppm EC: 800 ppm | None | Decreased number of leaves by 14–23%, leaf area by 10–12%. Increased number of pods by 55%. | - | [62] |
| Soybean (Glycine max (L.) Merr.) | OTC | AC: 390 ± 30 ppm EC: 550 ± 30 ppm | None | Reduced seed protein by 2–6% in different genotypes. | - | [6] |
| Soybean (Glycine max (L.) Merr.) | OTC | AC: 410 ppm EC: 610 ppm | None | Seed yield with increases of 101% and 65% in high-WUE and low-WUE, respectively. | Increased canopy photosynthesis by 37% (high-WUE genotype) and 76.3% (low-WUE genotype). | [79] |
| Chickpea (Cicer arietinum L.) | FAOCE (Free-Air Ozone [CO2] Enrichment) Chambers | AC: ambient. EC: 550 ± 25 ppm | None | Increased seed yield by 26–31% | Increased net photosynthetic rate by 11–17%. | [57] |
| Black gram (Vigna mungo) | OTC | AC: 365 ppm EC: 550 ppm and 700 ppm | None | Higher BM by 65% at 700 ppm and 39% at 550 ppm. Higher HI by 39% at 550 ppm and 40% at 700 ppm. | - | [100] |
3.4. Grain Nutritional Content
3.5. Soil Moisture or Drought Stress
3.6. Soil Health
3.7. Legume Insect Pests
4. Gaps in Existing Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sreeharsha, R.V.; Mudalkar, S.; Sengupta, D.; Unnikrishnan, D.K.; Reddy, A.R. Mitigation of drought-induced oxidative damage by enhanced carbon assimilation and an efficient antioxidative metabolism under high [CO2] environment in pigeon pea (Cajanus cajan L.). Photosynth. Res. 2019, 139, 425–439. [Google Scholar] [CrossRef] [PubMed]
- IPCC 2023. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; p. 184. [Google Scholar]
- NOAA 2024. National Oceanic and Atmospheric Administration. Carbon Dioxide Now More Than 50% Higher Than Pre-Industrial Levels 2024. Available online: www.noaa.gov (accessed on 15 March 2024).
- Iizumi, T.; Shiogama, H.; Imada, Y.; Hanasaki, N.; Takikawa, H.; Nishimori, M. Crop production losses associated with anthropogenic climate change for 1981–2010 compared with preindustrial levels. Int. J. Climatol. 2018, 38, 5405–5417. [Google Scholar] [CrossRef]
- Karavolias, N.G.; Horner, W.; Abugu, M.N.; Evanega, S.N. Application of gene editing for climate change in agriculture. Front. Sust. Food Syst. 2021, 5, 685801. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Jin, J.; Zhang, Q.; Wang, G.; Liu, C.; Wu, J.; Wang, C.; Liu, X. Impact of elevated [CO2] on seed quality of soybean at the fresh edible and mature stages. Front. Plant Sci. 2018, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Heckathorn, S.A.; Wang, X.; Philpott, S.M. A meta-analysis of plant physiological and growth responses to temperature and elevated [CO2]. Oecologia 2012, 169, 1–3. [Google Scholar] [CrossRef]
- Vaidya, S.; Vanaja, M.; Sathish, P.; Anitha, Y.; Jyothi, L.N. Impact of elevated [CO2] on growth and physiological parameters of groundnut (Arachis hypogaea L.) genotypes. J. Plant Physiol. Pathol. 2014, 3, 1. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Reich, M.; Löw, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated [CO2] and drought: A meta-analysis on crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Davey, P.A.; Bernacchi, C.J.; Dermody, O.C.; Heaton, E.A.; Moore, D.J.; Morgan, P.B.; Naidu, S.L.; Yoora, H.S.; Zhu, X.G.; et al. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob. Chang. Biol. 2002, 8, 695–709. [Google Scholar] [CrossRef]
- FAO 1990. Food & Agriculture Organization of the United Nations (FAO). Global Map of Aridity. Spatial Resolution of 10 Arc Minutes and Temporal Resolution of 1961–1990; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. Available online: https://data.apps.fao.org/map/catalog/static/api/records/221072ae-2090-48a1-be6f-5a88f061431a (accessed on 28 February 2024).
- Qader, S.H.; Dash, J.; Alegana, V.A.; Khwarahm, N.R.; Tatem, A.J.; Atkinson, P.M. The role of earth observation in achieving sustainable agricultural production in arid and semi-arid regions of the world. Remote Sens. 2021, 13, 3382. [Google Scholar] [CrossRef]
- Laza, H.E.; Baker, J.T.; Yates, C.; Mahan, J.R.; Burow, M.D.; Puppala, N.; Gitz, D.C.; Emendack, Y.Y.; Layland, N.; Ritchie, G.L.; et al. Effect of elevated CO2 on peanut performance in a semi-arid production region. Agric. For. Meteorol. 2021, 308–309, 108599. [Google Scholar] [CrossRef]
- Pang, J.; Turner, N.C.; Du, Y.L.; Colmer, T.D.; Siddique, K.H.M. Pattern of water use and seed yield under terminal drought in chickpea genotypes. Front. Plant Sci. 2017, 8, 1375. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Uddin, S.; Tausz-Posch, S.; Fitzgerald, G.J.; Armstrong, R.; Tausz, M. Elevated [CO2] improves yield and N2 fixation but not grain N concentration of faba bean (Vicia faba L.) subjected to terminal drought. Environ. Exp. Bot. 2019, 165, 161–173. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Leakey, A.D.B.; Ort, D.R.; Long, S.P. FACE-ing the facts: Inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol. 2008, 179, 5–9. [Google Scholar] [CrossRef]
- Ratnakumar, P.; Vadez, V. Groundnut (Arachis hypogaea) genotypes tolerant to intermittent drought maintain a high harvest index and have small leaf canopy under stress. Funct. Plant Biol. 2011, 38, 1016–1023. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Fitzgerald, G.J.; Tausz-Posch, S.; Armstrong, R.; Tausz, M. Free air [CO2] enrichment (FACE) improves water use efficiency and moderates drought effect on N2 fixation of Pisum sativum L. Plant Soil 2019, 436, 587–606. [Google Scholar] [CrossRef]
- Angelotti, F.; Barbosa, L.G.; Barros, J.R.A.; dos Santos, C.A.F. Cowpea development under different temperatures and carbon dioxide concentrations. Pesqui. Agropecu. Trop. 2020, 50, e59377. [Google Scholar] [CrossRef]
- Rana, D.S.; Dass, A.; Rajanna, G.A.; Kaur, R. Biotic and abiotic stress management in pulses. Indian J. Agron. 2016, 61, 238–248. [Google Scholar]
- Meena, H.N.; Ajay, B.C.; Rajanna, G.A.; Yadav, R.S.; Jain, N.K.; Meena, M.S. Polythene mulch and potassium application enhances peanut productivity and biochemical traits under sustained salinity stress condition. Agric. Water Manag. 2022, 273, 107903. [Google Scholar] [CrossRef]
- Rajanna, G.A.; Dhindwal, A.S.; Sriharsha, V.P. Performance of clusterbean (Cyamopsis tetragonoloba L.) under variable irrigation schedules and crop-establishment techniques. Indian J. Agron. 2016, 61, 223–230. [Google Scholar] [CrossRef]
- Cabrerizo, P.M.; González, E.M.; Aparicio-Tejo, P.M.; Arrese-Igor, C. Continuous CO2 enrichment leads to increased nodule biomass, carbon availability to nodules and activity of carbon-metabolizing enzymes but does not enhance specific nitrogen fixation in pea. Physiol. Plant. 2001, 113, 33–40. [Google Scholar] [CrossRef]
- Ross, D.J.; Newton, P.C.D.; Tate, K.R. Elevated [CO2] effects on herbage production and soil carbon and nitrogen pools and mineralization in a species-rich, grazed pasture on a seasonally dry sand. Plant Soil 2004, 260, 183–196. [Google Scholar] [CrossRef]
- Gamper, H.; Hartwig, U.A.; Leuchtmann, A. Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 yr of selection under elevated atmospheric [CO2] partial pressure. New Phytol. 2005, 167, 531–554. [Google Scholar] [CrossRef]
- Rogers, A.; Ainsworth, E.A.; Leakey, A.D.B. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 2009, 151, 1009–1016. [Google Scholar] [CrossRef]
- Dey, S.K.; Chakrabarti, B.; Prasanna, R.; Mittal, R.; Singh, S.D.; Pathak, H. Growth and biomass partitioning in mungbean with elevated carbon dioxide, phosphorus levels and cyanobacteria inoculation. J. Agrometeorol. 2016, 18, 7–12. [Google Scholar]
- Hao, X.Y.; Han, X.; Lam, S.K.; Wheeler, T.; Ju, H.; Wang, H.R.; Li, Y.C.; Lin, E.D. Effects of fully open-air [CO2] elevation on leaf ultrastructure, photosynthesis, and yield of two soybean cultivars. Photosynthetica 2012, 50, 362–370. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Phys. 2017, 8, 578. [Google Scholar] [CrossRef]
- Reardon, M.E.; Qaderi, M.M. Individual and interactive effects of temperature, carbon dioxide and abscisic acid on mung bean (Vigna radiata) plants. J. Plant Interact. 2017, 12, 295–303. [Google Scholar] [CrossRef]
- Singh, R.N.; Mukherjee, J.; Sehgal, V.K.; Bhatia, A. Effect of elevated ozone, carbon dioxide and their interaction on growth, biomass and water use efficiency of chickpea (Cicer arietinum L.). J. Agrometeorol. 2017, 19, 301–305. [Google Scholar] [CrossRef]
- Needelman, B.A. What are soils. Natl. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Gopinath, K.A.; Rajanna, G.A.; Venkatesh, G.; Jayalakshmi, M.; Kumari, V.V.; Prabhakar, M.; Rajkumar, B.; Chary, G.R.; Singh, V.K. Influence of crops and different production systems on soil carbon fractions and carbon sequestration in rainfed areas of Semiarid Tropics in India. Sustainability 2022, 14, 4207. [Google Scholar] [CrossRef]
- Rajanna, G.A.; Suman, A.; Venkatesh, P. Mitigating Drought Stress Effects in Arid and Semi-Arid Agro-Ecosystems through Bioirrigation Strategies—A Review. Sustainability 2023, 15, 3542. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- García-Palacios, P.; Crowther, T.W.; Dacal, M.; Hartley, I.P.; Reinsch, S.; Rinnan, R.; Rousk, J.; van den Hoogen, J.; Ye, J.S.; Bradford, M.A. Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nat. Rev. Earth Environ. 2021, 2, 507–517. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Ayyogari, K.; Sidhya, P.; Pandit, M.K. Impact of climate change on vegetable cultivation-a review. Int. J. Agric. Environ. Biotechnol. 2014, 7, 145–155. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Oleson, K.W.; Flanner, M.G.; Thornton, P.E.; Swenson, S.C.; Lawrence, P.J.; Zeng, X.; Yang, Z.L.; Levis, S.; Sakaguchi, K.; et al. Parameterization improvements and functional and structural advances in version 4 of the Community Land Model. J. Adv. Model. Earth Syst. 2011, 3, 27. [Google Scholar]
- Purcell, C.; Batke, S.P.; Yiotis, C.; Caballero, R.; Soh, W.K.; Murray, M.; McElwain, J.C. Increasing stomatal conductance in response to rising atmospheric [CO2]. Ann. Bot. 2018, 121, 1137–1149. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Seed composition, seedling emergence and early seedling vigour of red kidney bean seed produced at elevated temperature and carbon dioxide. J. Agron. Crop Sci. 2009, 195, 148–156. [Google Scholar] [CrossRef]
- Marty, C.; Bassirirad, H. Seed germination and rising atmospheric [CO2] concentration: A meta-analysis of parental and direct effects. New Phytol. 2014, 202, 401–414. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, H.; Wietoska, H.; Heckmann, H.; Godde, D. Changes in D1-protein turnover and recovery of photosystem II activity precede accumulation of chlorophyll in plants after release from mineral stress. Planta 1996, 199, 34–42. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Uni. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 8, 1323–1367. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuča, K.; Jaćevic, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Ul-Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.C.; Pal, M.; Das, M.; Sengupta, U.K. Growth, [CO2] exchange rate and dry matter partitioning in mungbean (Vigna radiata L.) grown under elevated [CO2]. Indian J. Exp. Biol. 2001, 39, 572–577. [Google Scholar] [PubMed]
- Das, M.; Zaidi, P.H.; Pal, M.; Sengupta, U.K. Stage sensitivity of mungbean (Vigna radiata L. Wilczek) to an elevated level of carbon dioxide. J. Agron. Crop Sci. 2002, 188, 219–224. [Google Scholar] [CrossRef]
- Bhatia, A.; Mina, U.; Kumar, V.; Tomer, R.; Kumar, A.; Chakrabarti, B.; Singh, R.N.; Singh, B. Effect of elevated ozone and carbon dioxide interaction on growth, yield, nutrient content and wilt disease severity in chickpea grown in Northern India. Heliyon 2021, 7, e06049. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Thomas, J.M.G. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Glob. Chang. Biol. 2002, 8, 710–721. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hall, A.E.; Madore, M.A. Interactive effects of high-temperature and elevated carbon dioxide concentration on cowpea [Vigna unguiculata (L.) Walp]. Plant Cell Environ. 1993, 16, 835–842. [Google Scholar] [CrossRef]
- Ellis, R.H.; Craufurd, P.Q.; Summerfield, R.J.; Roberts, E.H. Linear relations between carbon dioxide concentration and rate of development towards flowering in sorghum, cowpea and soybean. Ann. Bot. 1995, 75, 193–198. [Google Scholar] [CrossRef]
- Earl, H.J. Stomatal and non-stomatal restrictions to carbon assimilation in soybean (Glycine max) lines differing in water use efficiency. Environ. Exp. Bot. 2002, 48, 237–246. [Google Scholar] [CrossRef]
- Madhu, M.; Hatfield, J. Elevated carbon dioxide and soil moisture on early growth response of soybean. Agric. Sci. 2015, 6, 263–278. [Google Scholar] [CrossRef]
- Junior, C.E.G.; Medeiros, J.F.; Sobrinho, E.J.; Figueiredo, V.B.; Costa, J.P.N.; Santos, W.O. Development and water requirements of cowpea under climate change conditions in the Brazilian semi-arid region. Rev. Bras. Deengenharia Agríc. E Ambient. 2016, 20, 783–788. [Google Scholar] [CrossRef]
- Booker, F.L.; Burkey, K.O.; Pursley, W.A.; Heagle, A.S. Elevated carbon dioxide and ozone effects on peanut: I. gas-exchange, biomass, and leaf chemistry. Crop Sci. 2007, 47, 1475–1487. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated [CO2] effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Zarzycki, J. A short history of RubisCO: The rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Curr. Opin. Biotechnol. 2018, 49, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, R.W.; Bjorkman, O. Physiological effects. In Carbon Dioxide and Plants: The Response of Plants to Rising Levels of Atmospheric Carbon Dioxide; Lemon, E.R., Ed.; Westview Press: Boulder, CO, USA, 1983; pp. 65–105. [Google Scholar]
- Burkey, K.O.; Booker, F.L.; Pursley, W.A.; Heagle, A.S. Elevated carbon dioxide and ozone effects on peanut: II. seed yield and quality. Crop Sci. 2007, 47, 1488–1497. [Google Scholar] [CrossRef]
- Singh, S.K.; Kakani, V.G.; Surabhi, G.K.; Reddy, K.R. Cowpea (Vigna unguiculata [L.] Walp.) genotypes response to multiple abiotic stresses. J. Photochem. Photobiol. B Biol. 2010, 100, 135–146. [Google Scholar] [CrossRef]
- Saha, S.; Sehgala, V.K.; Nagarajana, S.; Pal, M. Impact of elevated atmospheric CO2 on radiation utilization and related plant biophysical properties in pigeon pea (Cajanus cajan L.). Agric. For. Meteorol. 2012, 158–159, 63–70. [Google Scholar] [CrossRef]
- Cao, J.; Han, X.; Seneweera, S.; Li, P.; Zong, Y.-Z.; Dong, Q.; Lin, E.D.; Hao, X.-Y. Leaf photosynthesis and yield components of mung bean under fully open-air elevated [CO2]. J. Integr. Agric. 2015, 14, 977–983. [Google Scholar]
- Bagudam, R.; Kancherla, E.; Abady, S.; Wankhade, A.P.; Deshmukh, D.B.; Vemula, A.; Kadirimangalam, S.R.; Kumar, S.S.; Reddy, S.N.; Pasupuleti, J. Influence of elevated [CO2] on growth, yield, haulm, and kernel quality of groundnut (Arachis hypogaea L.). Acta Physiol. Plant. 2023, 45, 67. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Blaceburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Remote Sens. Environ. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- McLeod, A.R.; Long, S.P. Free-air carbon dioxide enrichment (FACE) in global change research: A review. Adv. Ecol. Res. 1999, 28, 1–56. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Ann. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Bernacchi, C.; Kimball, B.; Quarles, D.; Long, S.; Ort, D. Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol. 2007, 143, 134–144. [Google Scholar] [CrossRef]
- Soba, D.; Shub, T.; Runionc, G.B.; Priorc, S.A.; Fritschid, F.B.; Aranjueloa, I.; Sanz-Saezb, A. Effects of elevated [CO2] on photosynthesis and seed yield parameters in two soybean genotypes with contrasting water use efficiency. Environ. Exp. Bot. 2020, 178, 104154. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, B.B.; Wu, Y.; Zou, Z.R. Interactive effects of drought stresses and elevated [CO2] concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Crop responses to elevated carbon dioxide and temperature. In Climate Change and Crops; Singh, S.N., Ed.; Springer: Berlin, Germany, 2009; pp. 1–18. [Google Scholar]
- Ralston, L.; Subramanian, S.; Matsuno, M.; Yu, O. Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol. 2005, 137, 1375–1388. [Google Scholar] [CrossRef]
- Kretzschmar, F.D.S.; MarinhoAidar, M.P.; Salgado, I.; Braga, M.R. Elevated [CO2] atmosphere enhances production of defense-related flavonoids in soybean elicited by NO and a fungal elicitor. Environ. Exp. Bot. 2009, 65, 319–329. [Google Scholar] [CrossRef]
- O’Neill, B.F.; Zangerl, A.R.; Dermody, O.; Bilgin, D.D.; Casteel, C.L.; Zavala, J.A.; Berenbaum, M.R. Impact of elevated levels of atmospheric [CO2] and herbivory on flavonoids of soybean (Glycine max L.). J. Chem. Ecol. 2010, 36, 35–45. [Google Scholar] [CrossRef]
- Warren, J.M.; Norby, R.J.; Wullschleger, S.D. Elevated CO2 enhances leaf senescence during extreme drought in a temperate forest. Tree Physiol. 2011, 31, 117–130. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Mohanty, P. Elevated temperature stress effects on photosystems: Characterization and evaluation of the nature of heat induced impairments. In Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Singal, G.S., Renger, G., Sopory, S.K., Irrgang, K.D., Govindjee, Eds.; Narosa Publishing House: New Delhi, India, 1999; pp. 617–648. [Google Scholar]
- Mohanty, P.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Mimuro, M.; Carpentier, R.; Allakhverdiev, S.I. Heat stress: Susceptibility, recovery and regulation. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation, Advances in Photosynthesis and Respiration 34; Eaton-Rye, J.J., Tripathy, B.C., Sharkey, T.D., Eds.; Springer Science++Business Media B.V.: Berlin/Heidelberg, Germany, 2012; pp. 251–274. [Google Scholar]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Mariem, B.S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Repková, J.; Brestič, M.; Olšovská, K. Leaf growth under temperature and light control. Plant Soil Environ. 2009, 55, 551–557. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Basraon, N.K.; Chinnappa, C.C.; Reid, D.M. Combined effects of temperature, ultraviolet-B radiation, and watering regime on growth and physiological processes in canola (Brassica napus) seedlings. Int. J. Plant Sci. 2010, 171, 466–481. [Google Scholar] [CrossRef]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems, 3rd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; p. 371. [Google Scholar]
- Inagaki, N. Processing of D1 Protein: A Mysterious Process Carried Out in Thylakoid Lumen. Int. J. Mol. Sci. 2022, 23, 2520. [Google Scholar] [CrossRef] [PubMed]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef]
- McClain, A.M.; Sharkey, T.D. Rapid CO2 changes cause oscillations in photosynthesis that implicate PSI acceptor-side limitations. J. Exp. Bot. 2023, 74, 3163–3173. [Google Scholar] [CrossRef]
- Shanker, A.K.; Gunnapaneni, D.; Bhanu, D.; Vanaja, M.; Lakshmi, N.J.; Yadav, S.K.; Prabhakar, M.; Singh, V.K. Elevated CO2 and water stress in combination in plants: Brothers in arms or partners in crime? Biology 2022, 11, 1330. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Lamichaney, A.; Parihar, A.K.; Hazra, K.K.; Dixit, G.P.; Katiyar, P.K.; Singh, D.; Singh, A.K.; Kumar, N.; Singh, N.P. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L). Front. Plant Sci. 2021, 2, 437. [Google Scholar] [CrossRef]
- Mishra, A.K.; Agrawal, S.B. Cultivar specific response of [CO2] fertilization on two tropical mung bean (Vigna radiata L.) cultivars: ROS generation, antioxidant status, physiology, growth, yield and seed quality. J. Agron. Crop Sci. 2014, 200, 273–289. [Google Scholar] [CrossRef]
- Li, P.; Han, X.; Zong, Y.; Li, H.; Lin, E.; Han, Y.; Hao, X. Effects of free-air [CO2] enrichment (FACE) on the uptake and utilization of N, P and K in Vigna radiata. Agric. Ecosyst. Environ. 2015, 202, 120–125. [Google Scholar] [CrossRef]
- Vanaja, M.; Reddy, P.R.; Lakshmi, N.J.; Maheswari, M.; Vagheera, P.; Ratnakumar, P.; Jyothi, M.; Yadav, S.K.; Venkateswarlu, B. Effect of elevated atmospheric [CO2] concentrations on growth and yield of black gram (Vigna mungo L. Hepper)—A rainfed pulse crop. Plant Soil Environ. 2007, 53, 81–88. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Light quality and quantity regulate aerobic methane emissions from plants. Physiol. Plant. 2017, 159, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Sannagoudar, M.S.; Patil, R.H.; Rajanna, G.A.; Ghosh, A.; Singh, A.K.; Halli, H.M.; Khandibagur, V.; Kumar, S.; Kumar, R.V. Rising temperature coupled with reduced rainfall will adversely affect yield of kharif sorghum genotypes. Curr. Sci. 2023, 124, 921. [Google Scholar]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Nemeskeri, E. Heat tolerance in grain legumes. Bodenkultur 2004, 55, 3–11. [Google Scholar]
- Piramila, B.H.M.; Prabha, A.L.; Nandagopalan, V.; Stanley, A.L. Effect of heat treatment on germination, seedling growth and some biochemical parameters of dry seeds of black gram. Int. J. Pharm. Phytopharm. Res. 2012, 1, 194–202. [Google Scholar]
- Chakraborty, U.; Pradhan, D. High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J. Plant Inter. 2011, 6, 43–52. [Google Scholar]
- Prasad, P.V.V.; Craufurd, P.Q.; Kakani, V.G.; Wheeler, T.R.; Boote, K.J. Influence of temperature during pre and post anthesis stages of floral development on fruit set and pollen germination in groundnut (Arachis hypogaea L.). Aust. J. Plant Physiol. 2001, 28, 233–240. [Google Scholar]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Barghi, S.S.; Mostafaii, H.; Peighami, F.; Zakaria, R.A. Path analysis of yield and its components in lentil under end season heat condition. Int. J. Agric. Res. Rev. 2012, 2, 969–974. [Google Scholar]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Bunce, J.A. Elevated carbon dioxide effects on reproductive phenology and seed yield among soybean cultivars. Crop Sci. 2015, 55, 339–343. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Yang, S.; Jin, J.; Wang, G.; Liu, C.; Herbert, S.J.; Liu, X. Soybean intraspecific genetic variation in response to elevated [CO2]. Arc. Agron. Soil Sci. 2019, 65, 1733–1744. [Google Scholar] [CrossRef]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef]
- Rogers, G.S.; Milham, P.J.; Thibaud, M.C.; Conroy, J.P. Interactions between rising [CO2] concentration and nitrogen supply in cotton. I. Growth and leaf nitrogen concentration. Funct. Plant Biol. 1996, 23, 119–125. [Google Scholar] [CrossRef]
- Köhler, I.H.; Huber, S.C.; Bernacchi, C.J.; Baxter, I.R. Increased temperatures may safeguard the nutritional quality of crops under future elevated CO2 concentrations. Plant J. 2019, 97, 872–886. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A. Responses of nutrient dynamics in barley seedlings to the interaction of salinity and carbon dioxide enrichment. Environ. Exp. Bot. 2014, 99, 86–99. [Google Scholar] [CrossRef]
- Weigel, H.J.; Manderscheid, R. [CO2] enrichment effects on forage and grain nitrogen content of pasture and cereal plants. J. Crop Improv. 2005, 13, 73–89. [Google Scholar] [CrossRef]
- Kennedy, M. As carbon dioxide levels rise, major crops are losing nutrients. June 19, 2018 story on NPR’s Morning Edition. Available online: https://www.npr.org/sections/thesalt/2018/06/19/616098095/as-carbon-dioxide-levels-rise-major-crops-are-losing-nutrients (accessed on 1 March 2024).
- Martel, A.B.; Qaderi, M.M. Does salicylic acid mitigate the adverse effects of temperature and ultraviolet-B radiation on pea (Pisum sativum) plants? Environ. Exp. Bot. 2016, 122, 39–48. [Google Scholar] [CrossRef]
- Peak, D.; Mott, K.A. A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ. 2011, 34, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, J.; Huang, B. Effects of elevated [CO2] concentration on water relations and photosynthetic responses to drought stress and recovery during rewatering in tall fescue. J. Am. Soc. Hortic. Sci. 2015, 140, 19–26. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Leishman, M.R. Competitive interactions between established grasses and woody plant seedlings under elevated [CO2] levels are mediated by soil water availability. Oecologia 2015, 177, 499–506. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Salvatore, M.; Ferrara, A.F.; House, J.; Federici, S.; Rossi, S.; Biancalani, R.; Condor Golec, R.D.; Jacobs, H.; Flammini, A.; et al. The contribution of agriculture, forestry and other land use activities to global warming, 1990–2012. Glob. Chang. Biol. 2015, 21, 2655–2660. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Mendoza, O.; De Neve, S.; Deroo, H.; Sleutel, S. Mineralisation of ryegrass and soil organic matter as affected by ryegrass application doses and changes in soil structure. Biol. Fertil. Soils 2022, 58, 679–691. [Google Scholar] [CrossRef]
- Hu, J.; Liao, X.; Vardanyan, L.G.; Huang, Y.; Inglett, K.S.; Wright, A.L.; Reddy, K.R. Duration and frequency of drainage and flooding events interactively affect soil biogeochemistry and N flux in subtropical peat soils. Sci. Total Environ. 2020, 727, 138740. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Sheng, R.; Wei, W.; Zhu, B.; Zhang, W. Differential contributions of electron donors to denitrification in the flooding-drying process of a paddy soil. Appl. Soil Ecol. 2022, 177, 104527. [Google Scholar] [CrossRef]
- Lipson, D.A.; Wilson, R.F.; Oechel, W.C. Effects of elevated atmospheric CO2 on soil microbial biomass, activity, and diversity in a chaparral ecosystem. Appl. Environ. Microbiol. 2005, 71, 8573–8580. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Janssens, I. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Holland, E.A.; Braswell, B.H.; Sulzman, J.; Lamarque, J.F. Nitrogen deposition onto the United States and Western Europe: Synthesis of observations and models. Ecol. Appl. 2005, 15, 38–57. [Google Scholar] [CrossRef]
- Massad, T.J.; Dyer, L.A. A meta-analysis of the effects of global environmental change on plant-herbivore interactions. Arthropod-Plant Interact. 2010, 4, 181–188. [Google Scholar] [CrossRef]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Tiwari, L.D.; Pal, M.; Sengupta, U.K. CO2-mediated changes in mung bean chemistry: Impact on plant–herbivore interactions. Curr. Sci. 2002, 82, 1148–1151. [Google Scholar]
- Akbar, S.M.; Pavani, T.; Nagaraja, T.; Sharma, H.C. Influence of CO2 and temperature on metabolism and development of Helicoverpa armigera (Noctuidae: Lepidoptera). Environ. Entomol. 2016, 45, 229–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Wan, G.; Liu, B.; Xing, G.; Chen, F. Effects of elevated CO2 on plant chemistry, growth, yield of resistant soybean, and feeding of a target lepidoptera pest, Spodoptera litura (Lepidoptera: Noctuidae). Environ. Ent. 2018, 47, 848–856. [Google Scholar]
- Guo, H.; Sun, Y.; Li, Y.; Tong, B.; Harris, M.; Zhu-Salzman, K.; Ge, F. Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Glob. Chang. Biol. 2013, 19, 3210–3223. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Huang, Z.; Liu, X.; Li, C.; Gao, Y.; Gui, F.; Chang, X.; Chen, F. Effect of elevated CO2 on interactions between the host plant Phaseolus vulgaris and the invasive western flower thrips, Frankliniella occidentalis. J. Pest Sci. 2021, 94, 43–54. [Google Scholar] [CrossRef]
- O’Neill, B.F.; Zangerl, A.R.; DeLucia, E.H.; Casteel, C.; Zavala, J.A.; Berenbaum, M.R. Leaf temperature of soybean grown under elevated CO2 increases Aphis glycines (Hemiptera: Aphididae) population growth. Insect Sci. 2011, 18, 419–425. [Google Scholar] [CrossRef]
- Yan, H.Y.; Guo, H.G.; Sun, Y.C.; Ge, F. Plant phenolics mediated bottom-up effects of elevated CO2 on Acyrthosiphon pisum and its parasitoid Aphidius avenae. Insect Sci. 2020, 27, 70–184. [Google Scholar] [CrossRef]
- Jat, B.L.; Pagaria, P.; Mali, G.; Khan, T. Impact of major environmental conditions on pest population dynamics and plant resistance mechanisms in legumes. Indian J. Plant Prot. 2021, 49, 215–226. [Google Scholar]
- Sreenivas, A.G.; Ashoka, J.; Nadagoud, S.; Kuchnoor, P.H. Effect of elevated CO2 and temperature on biochemistry of groundnut and its effect on development of leaf eating caterpillar, Spodoptera litura Fabricius. Legume Res. 2019, 42, 399–404. [Google Scholar]
- Niziolek, O.K.; Berenbaum, M.R.; DeLucia, E.H. Impact of elevated CO2 and increased temperature on Japanese beetle herbivory. Insect Sci. 2013, 20, 513–523. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Ashman, T.L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Otieno, M.; Peters, M.K.; Duque, L.; Steffan-Dewenter, I. Interactive effects of ozone and carbon dioxide on plant-pollinator interactions and yields in a legume crop. Environ. Adv. 2022, 9, 100285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adireddy, R.G.; Anapalli, S.S.; Reddy, K.N.; Mubvumba, P.; George, J. Possible Impacts of Elevated CO2 and Temperature on Growth and Development of Grain Legumes. Environments 2024, 11, 273. https://doi.org/10.3390/environments11120273
Adireddy RG, Anapalli SS, Reddy KN, Mubvumba P, George J. Possible Impacts of Elevated CO2 and Temperature on Growth and Development of Grain Legumes. Environments. 2024; 11(12):273. https://doi.org/10.3390/environments11120273
Chicago/Turabian StyleAdireddy, Rajanna G., Saseendran S. Anapalli, Krishna N. Reddy, Partson Mubvumba, and Justin George. 2024. "Possible Impacts of Elevated CO2 and Temperature on Growth and Development of Grain Legumes" Environments 11, no. 12: 273. https://doi.org/10.3390/environments11120273
APA StyleAdireddy, R. G., Anapalli, S. S., Reddy, K. N., Mubvumba, P., & George, J. (2024). Possible Impacts of Elevated CO2 and Temperature on Growth and Development of Grain Legumes. Environments, 11(12), 273. https://doi.org/10.3390/environments11120273









