Influence of Blending High-Calcium Additive on Environmental Safety of B, F, and Se: A Case Study from Thermodynamic Calculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal fly Ash Sample
2.2. Paper Sludge Ash as Inhibitor
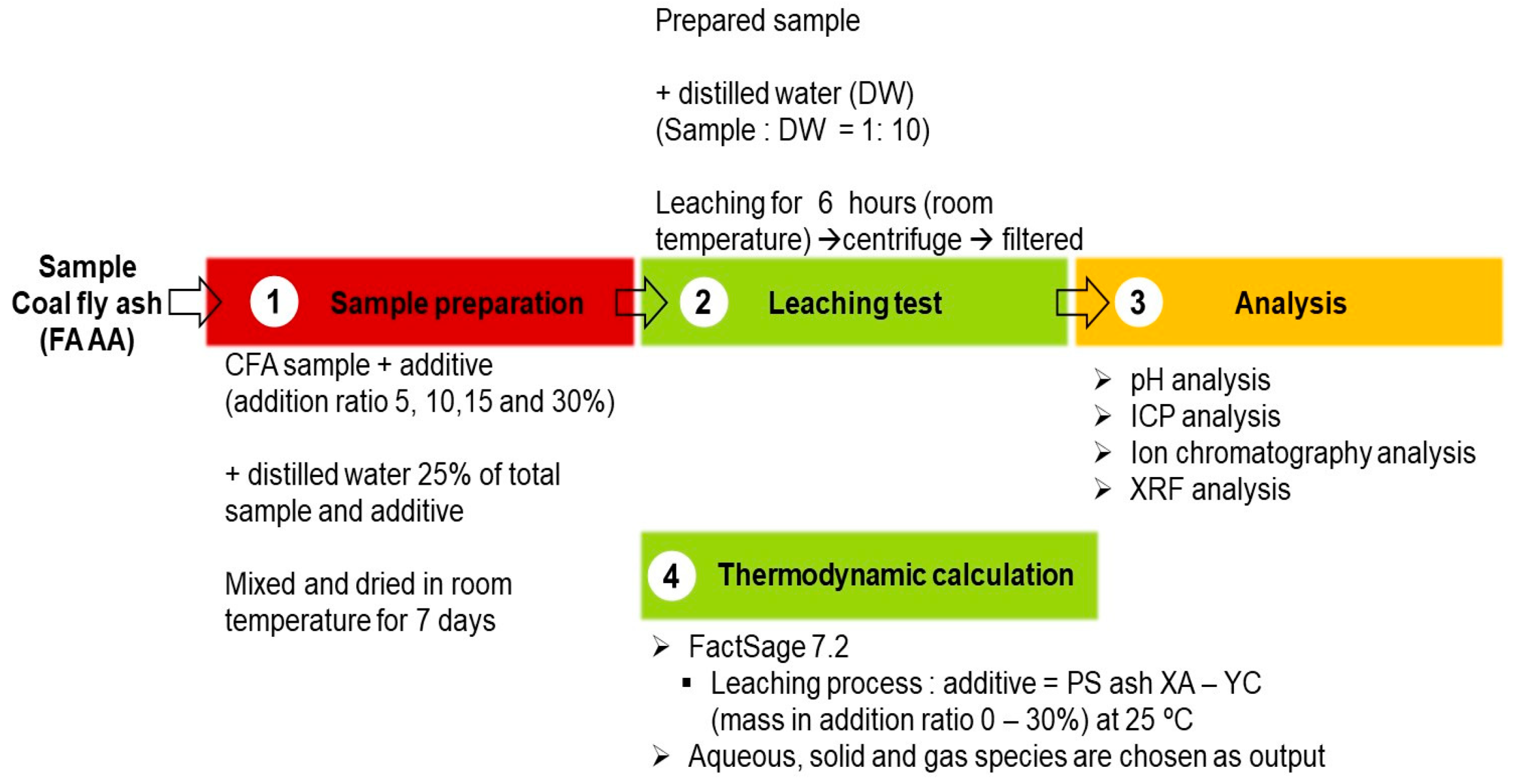
2.3. Leaching Test
2.4. Thermodynamic Calculation Analysis
3. Results and Discussion
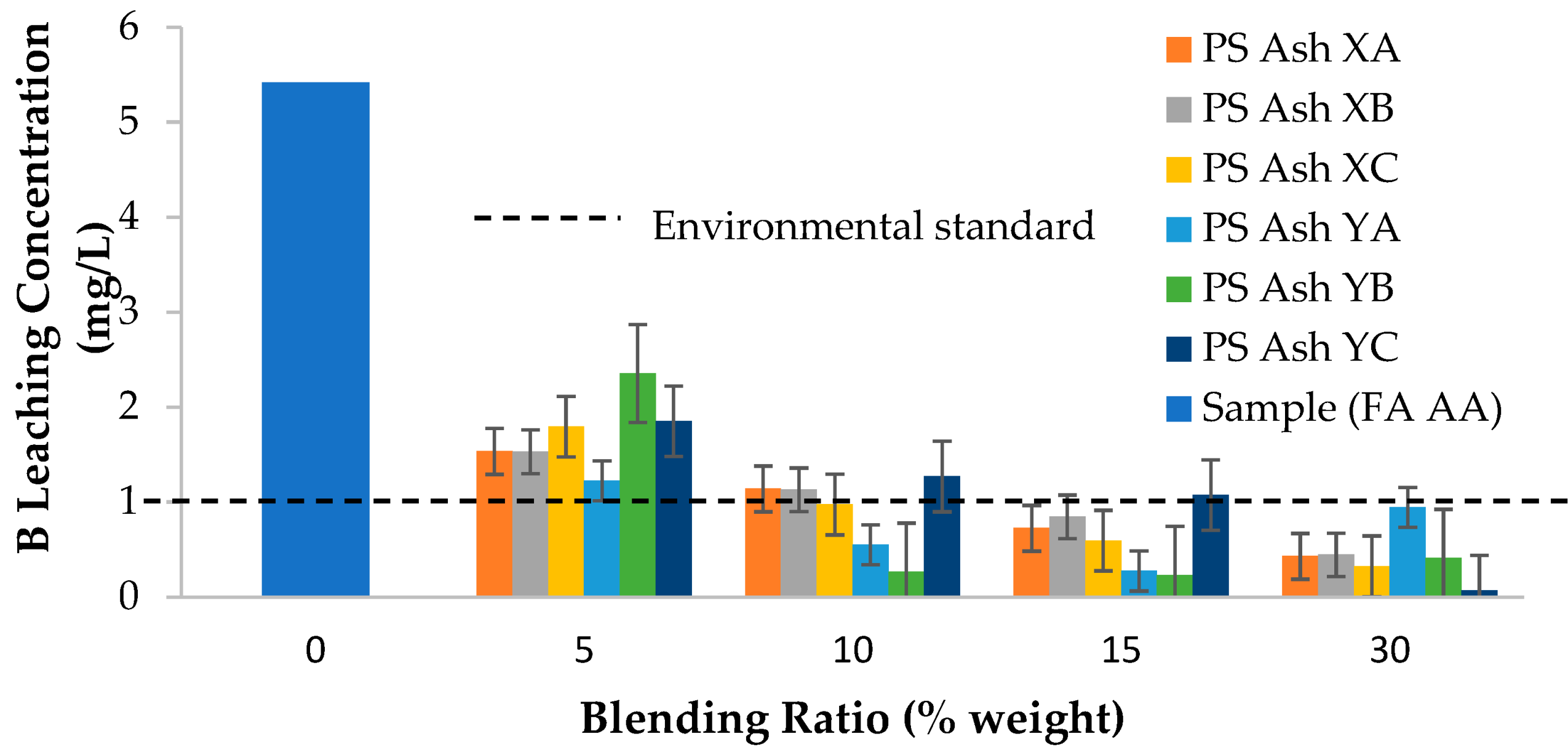
3.1. Leaching Characteristics of Coal Fly Ash and Paper Sludge Ash
3.2. Thermodynamic Calculation: The Transformation and Mechanism of Trace Elements during Leaching Process
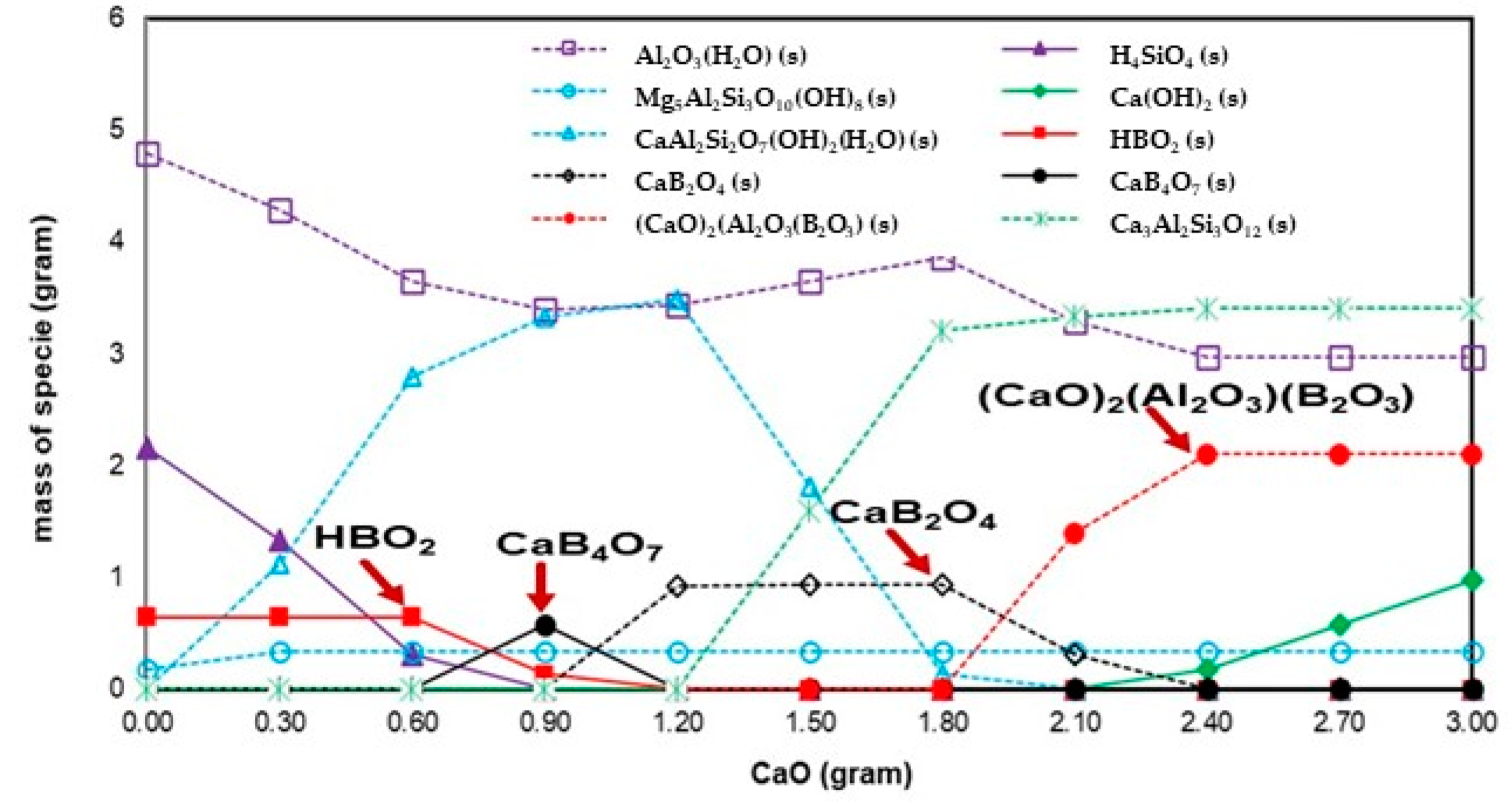
3.2.1. Boron
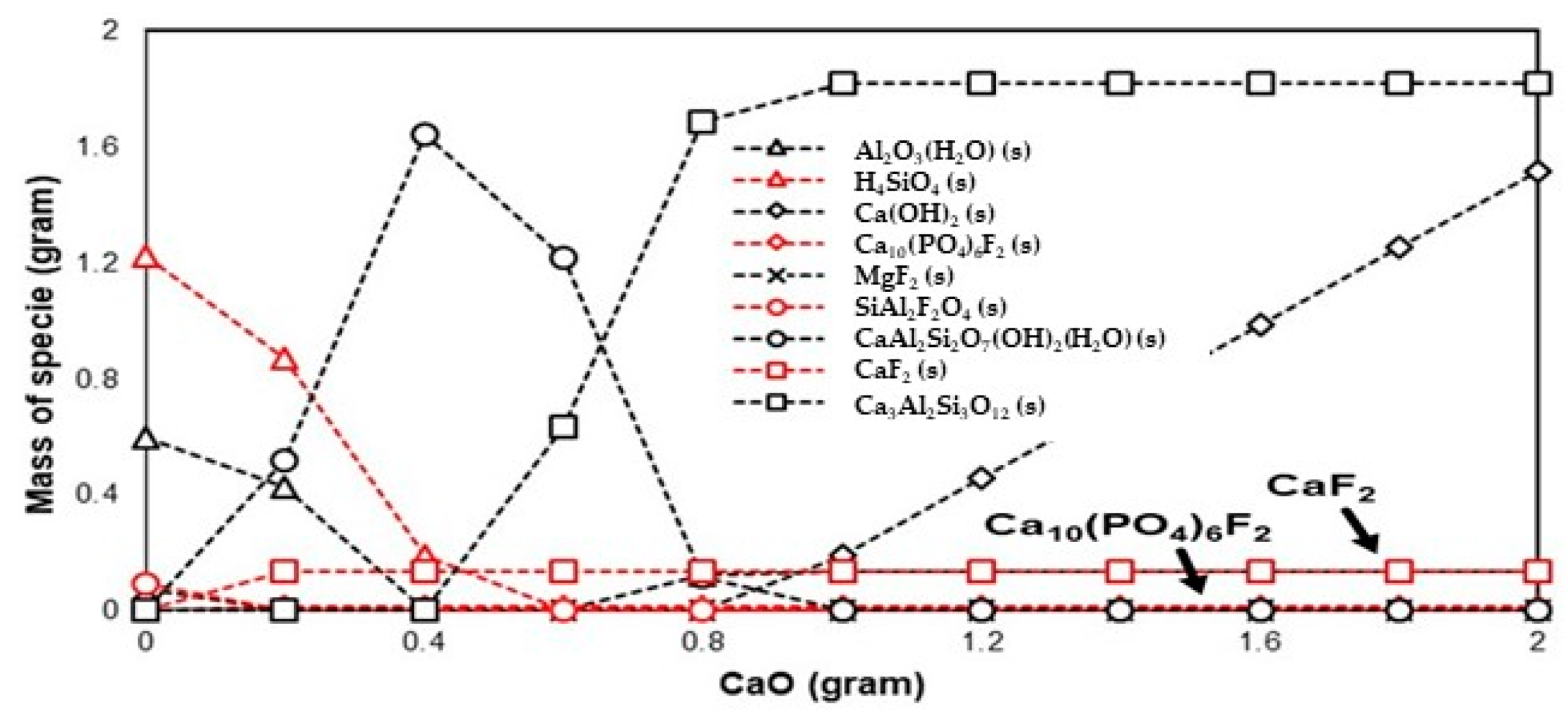
3.2.2. Fluorine
3.2.3. Selenium (Se)
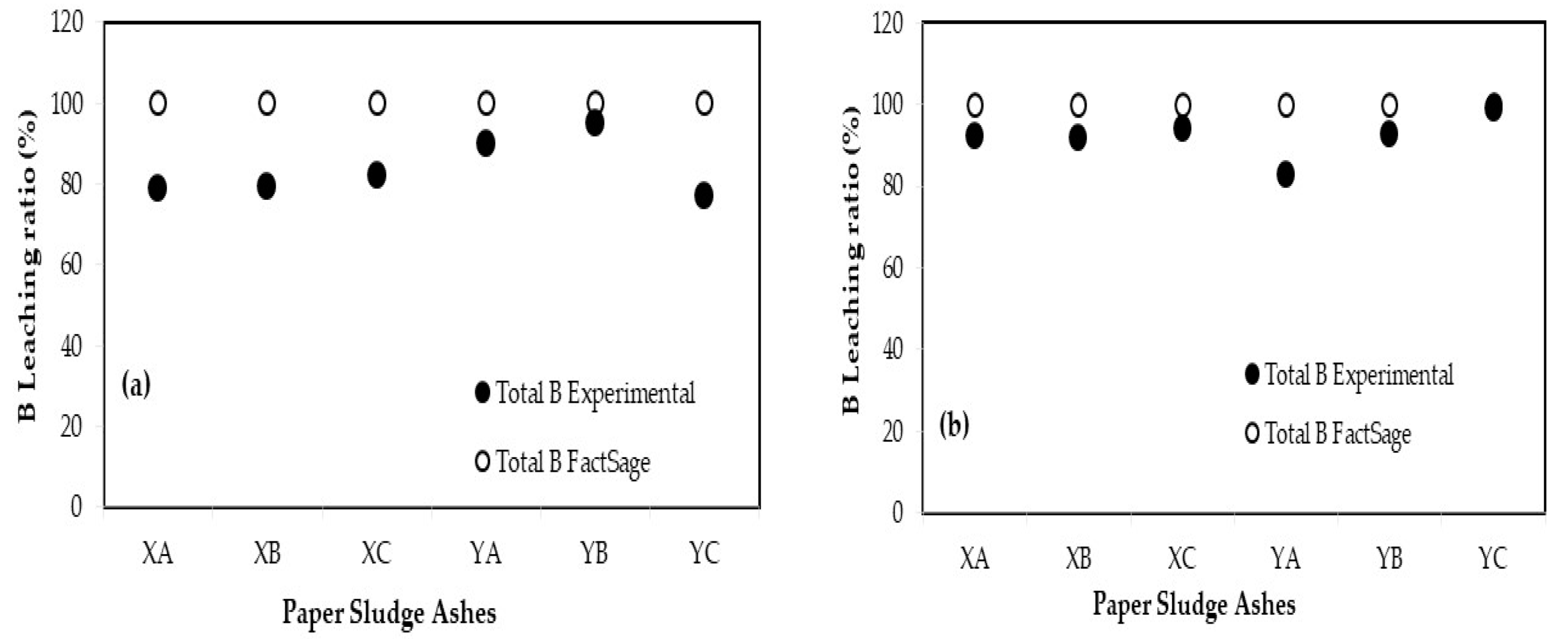
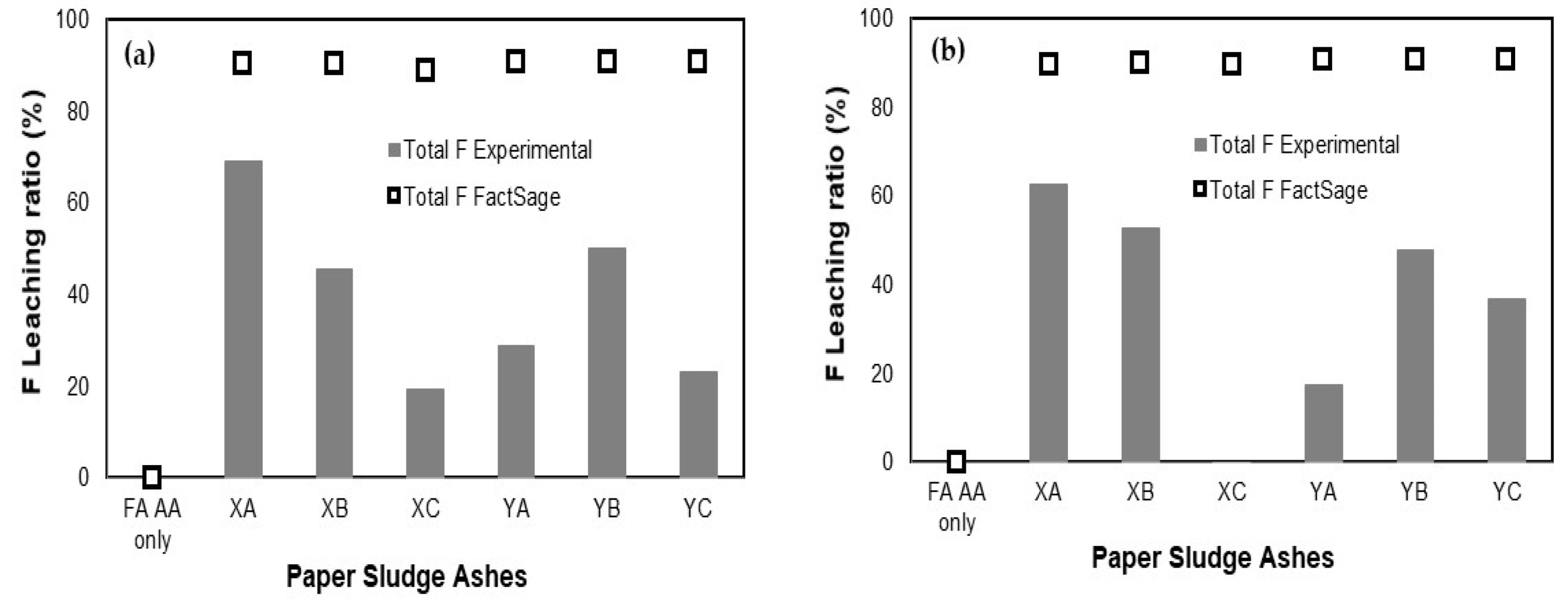
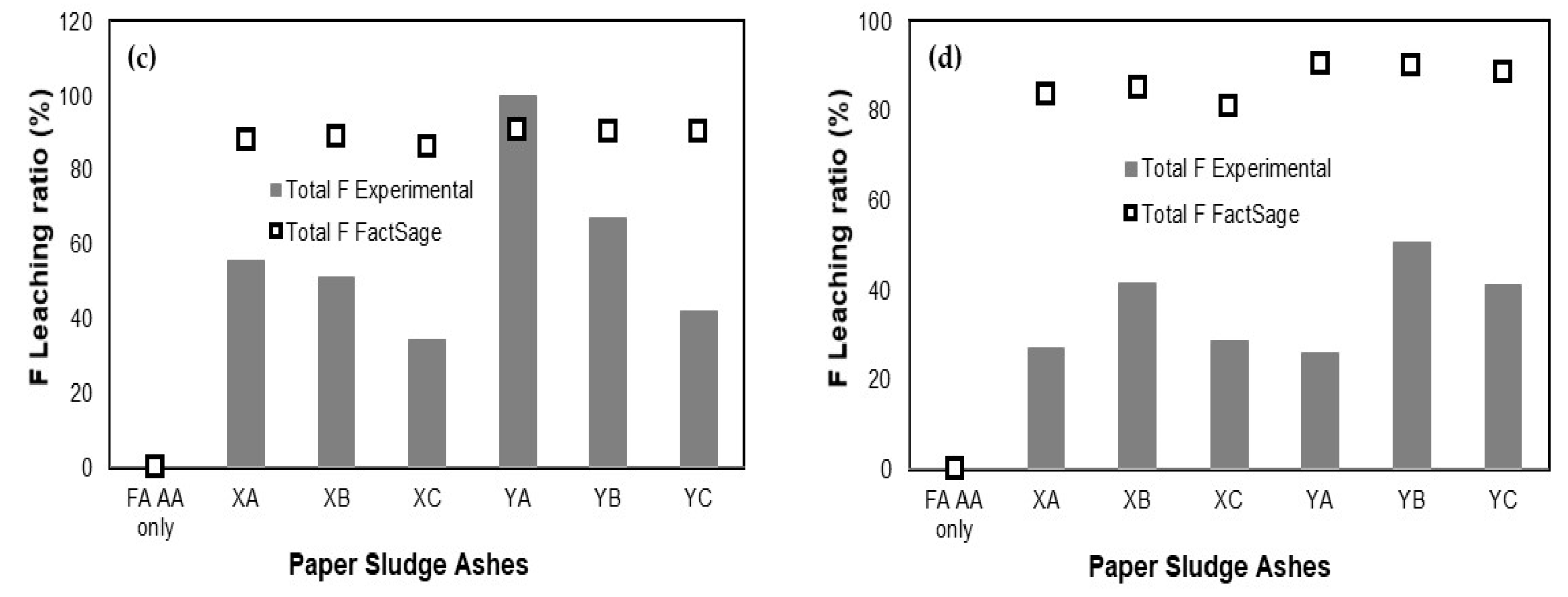
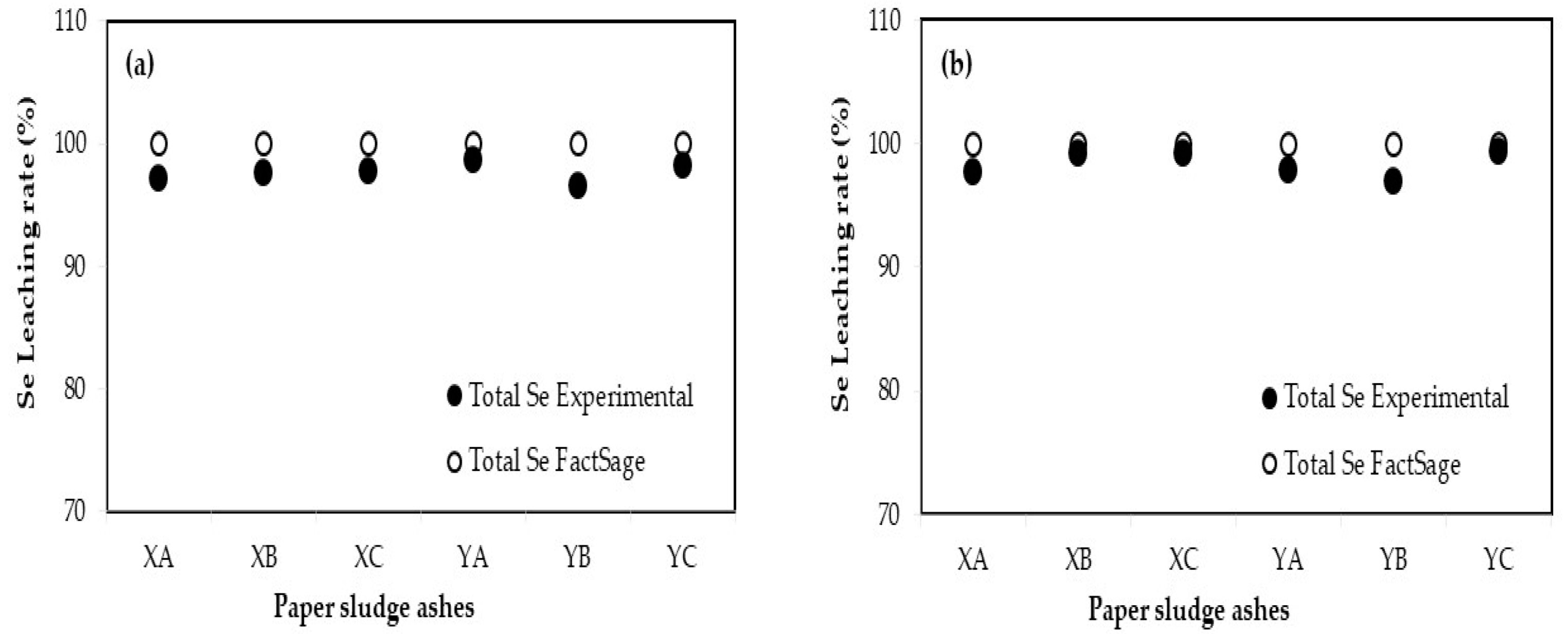
3.3. Comparison of Leaching Ratio between Experimental and FactSage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neupane, G.; Donahoe, R.J.; Bhattacharyya, S.; Dhakal, P. Leaching kinetics of As, Mo, and Se from Acidic Coal Fly Ash Samples. J. Water Resour. Prot. 2017, 9, 890–907. [Google Scholar] [CrossRef]
- Oncioiu, I.; Grecu, E.; Mâşu, S.; Morariu, F.; Popa, M. The effect of fly ash on sunflower growth and human health. Environ. Sci. Pollut. Res. 2018, 25, 35548–35554. [Google Scholar] [CrossRef]
- Phung, H.T.; Lund, L.J.; Page, A.L.; Bradford, G.R. Trace elements in fly ash and their release in water and treated soils 1. J. Environ. Qual. 1979, 8, 171–175. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behaviour of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; de Carvalho, W.; Cabanas, M.; Querol, X.; and Lopez-Soler, A. Mobility of heavy metals from Coal Fly Ash. Environ. Geol. 1994, 44, 264–270. [Google Scholar] [CrossRef]
- Choi, S.K.; Lee, S.; Song, Y.K.; Moon, H.S. Leaching characteristics of selected Korean fly ashes and its implications for the groundwater composition near the ash disposal mound. Fuel 2002, 81, 1083–1090. [Google Scholar] [CrossRef]
- van der Hoek, E.E.; Bonouvrie, P.A.; Comans, R.N. Sorption of As and Se on mineral components of fly ash: Relevance for leaching processes. Appl. Geochem. 1994, 9, 403–412. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sakakibara, K.; Wang, L.; Suto, K.; Inoue, C. Immobilization of B, F, Cr, and As in alkaline coal fly ash through an aging process with water. Env. Monit Assess 2014, 186, 6757–6770. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Nakamura, K.; Ogawa, Y.; Inoue, C. Leaching of As and Se from coal fly ash: Fundamental study for coal ash recycling. Environ. Monit. Assess. 2021, 193, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, S.; Finkelman, R.B.; Graham, I.T.; French, D.; Hower, J.C.; Li, X. Leaching characteristic of alkaline coal combustion by products: A case study from a coal-fired power plant, Hebei Province, China. Fuel 2019, 255, 115710. [Google Scholar] [CrossRef]
- Guo, B.; Nakama, S.; Tian, Q.; Pahlevi, N.D.; Hu, Z.; Sasaki, K. Suppression processes of anionic pollutants released from fly ash by various Ca additives. J. Hazard. Mater. 2019, 371, 474–483. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Y.; Xue, X.; Ru, H.; Huang, X.; Yang, H. Leaching of fluorine and rare earths from bastnaesite calcined with alumium hydroxide and the recovery of fluorine as cryolite. RSC Adv. 2017, 7, 14043. [Google Scholar]
- Szostek, R.; Ciecko, Z. Effect of soil contamination with fluorine on the yield and content of nitrogen froms in the biomass crops. Environ. Sci Pollut Res 2017, 24, 8588–8601. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Sakakibara, K.; Seki, T.; Inoue, C. Immobilization of boron and arsenic in alkaline coal fly ash through an aging process with water and elucidation of the immobilization mechanism. Water Air Soil Pollut. 2018, 229, 229–359. [Google Scholar] [CrossRef]
- Neupane, G.; Donahoe, R.J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests. Fuel 2013, 104, 758–770. [Google Scholar] [CrossRef]
- Baba, A.; Guldal, G.; Sengunalp, F. Leaching characteristics of fly ash from fluidized bed combustion thermal power plant: Case study: Can (Canakkale-Turkey). Fuel Process. Technol. 2010, 91, 1073–1080. [Google Scholar] [CrossRef]
- Hanum, F.F.; Desfitri, E.R.; Hayakawa, Y.; Kambara, S. Preliminary Study on Additives for Controlling As, Se, B, and F Leaching from Coal Fly Ash. Minerals 2018, 8, 493. [Google Scholar] [CrossRef]
- Hartuti, S.; Hanum, F.F.; Takeyama, A.; Kambara, S. Effect of additives on Arsenic, Boron and Selenium leaching from coal fly ash. Minerals 2017, 7, 99. [Google Scholar] [CrossRef]
- Desfitri, E.R.; Sutopo, U.M.; Hayakawa, Y.; Kambara, S. Effect of additive material on controlling chromium (Cr) leaching from coal fly ash. Minerals 2020, 10, 563. [Google Scholar] [CrossRef]
- Liu, J.; Xie, C.; Xie, W.; Zhang, X.; Chang, K.; Sun, J.; Kuo, J.; Xie, W.; Liu, C.; Sun, J.; et al. Arsenic Partitioning Behavior during Sludge Co-combustion: Thermodynamic Equilibrium Simulation. Waste Biomass Valorization 2019, 10, 2297–2307. [Google Scholar] [CrossRef]
- Sutopo, U.M.; Desfitri, E.R.; Hayakawa, Y.; Kambara, S. A role of mineral oxides on trace elements behavior during pulverized coal combustion. Minerals 2021, 11, 1270. [Google Scholar] [CrossRef]
- Sutopo, U.M.; Desfitri, E.R.; Hanum, F.F.; Hayakawa, Y.; Kambara, S. An experimental and thermodynamic analysis on the leaching process of arsenic (As) from coal fly ash. J. Jpn. Inst. Energy 2021, 100, 102–109. [Google Scholar] [CrossRef]
- Jiao, F.; Ninomiya, Y.; Zhang, L.; Yamada, N.; Sato, A.; Dong, Z. Effect of coal blending on the leaching characteristics of arsenic in fly ash fluidized bed coal combustion. Fuel Process. Technol. 2013, 106, 769–775. [Google Scholar] [CrossRef]
- Ministry of the Environment. Test Method of Metals Contained in Industrial Waste. 1973. Available online: https://www.env.go.jp/hourei/add/k048.pdf (accessed on 11 January 2024).
- Ministry of the Environment. Japan Regulation and Environmental Law: Environmental Quality Standards for Water Pollution. 1994. Available online: https://www.env.go.jp/content/900454947.pdf (accessed on 11 January 2024).
- Hayashi, S.; Takahashi, T. Chemical state of boron in coal fly ash investigated by focused-ion-beam time-of-flight secondary ion mass spectrometry (FIB-TOF-SIMS) and satellite-transition magic angle spinning Nuclear magnetic resonance (STMAS NMR). Chemosphere 2010, 80, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Luo, Z.; Zhang, J.; Zhao, Y. Modes of occurence of fluorine by extraction and SEM method in a coal-fired power plant from Inner Mongolia China. Minerals 2015, 5, 863–869. [Google Scholar] [CrossRef]
- Xie, P.; Guo, W.; Yan, X.; Zheng, X. Fluorine in Lopingian superhigh-organic-sulfur coals from the Lalang Coal Mine, Guangxie, Southern China. Fuel 2017, 208, 483–490. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Tang, Y.; Tang, Y.; Shi, H.; Ladwig, K. Leaching characteristics of arsenic and selenium from coal fly ash: Role of calcium. Energy Fuel 2009, 23, 2959–2966. [Google Scholar] [CrossRef]
- Roy, B.; Choo, L.W.; Battacharya, S. Prediction of distribution of trace elements under Oxy-fuel combustion condition using Victorian brown coals. Fuel 2013, 114, 135–142. [Google Scholar] [CrossRef]



| Chemical Composition | Coal Fly Ash | Additives | ||||||
|---|---|---|---|---|---|---|---|---|
| PS Ash XA | PS Ash XB | PS Ash XC | PS Ash YA | PS Ash YB | PS Ash YC | |||
| SiO2 | % | 59.38 | 30.26 | 29.19 | 32.95 | 24.47 | 28.21 | 31.06 |
| Al2O3 | 20.45 | 15.89 | 15.59 | 16.52 | 14.10 | 15.61 | 16.88 | |
| TiO2 | 0.62 | 0.74 | 0.76 | 0.78 | 0.31 | 0.46 | 0.76 | |
| Fe2O3 | 15.65 | 0.73 | 0.51 | 0.66 | 15.76 | 1.15 | 0.61 | |
| CaO | 1.13 | 46.78 | 48.86 | 42.77 | 42.03 | 51.63 | 47.46 | |
| MgO | 0.61 | 2.46 | 2.38 | 2.55 | 2.12 | 2.19 | 2.12 | |
| Na2O | 0.47 | 0.12 | 0.08 | 0.24 | 0.38 | 0.04 | 0.12 | |
| K2O | 1.06 | 0.54 | 0.50 | 0.59 | 0.10 | 0.13 | 0.15 | |
| P2O5 | 0.08 | 0.50 | 0.43 | 0.45 | 0.31 | 0.23 | 0.15 | |
| MnO | 0.07 | 0.06 | 0.05 | 0.04 | 0.08 | 0.04 | 0.04 | |
| V2O5 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | |
| SO3 | 0.49 | 1.91 | 1.64 | 2.43 | 0.33 | 0.30 | 0.64 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Trace Elements | ||||||||
| B | mg/kg | 160.00 | 100.00 | 91.00 | 110.00 | 32.00 | 40.00 | 95.00 |
| F | 10.00 | 240.00 | 280.00 | 350.00 | 180.00 | 170.00 | 210.00 | |
| Se | 3.87 | 0.60 | 0.70 | 1.20 | 0.20 | 0.40 | 0.70 | |
| Sample and Additives | Leaching Concentration | pH | |||
|---|---|---|---|---|---|
| B (mg/L) | F (mg/L) | Se (µg/L) | Ca (mg/L) | ||
| FA AA | 5.42 | 1.31 | 37.75 | 181 | 10.04 |
| PS ash XA | <LOD | 2.30 | 4.45 | 828 | 12.32 |
| PS ash XB | <LOD | 2.25 | 4.60 | 949.5 | 12.34 |
| PS ash XC | <LOD | 9.06 | <LOD | 677 | 12.24 |
| PS ash YA | 0.02 | 0.40 | 5.70 | 986 | 12.51 |
| PS ash YB | 0.04 | 0.47 | <LOD | 1010 | 12.49 |
| PS ash YC | 0.16 | 0.65 | <LOD | 1045 | 12.46 |
| Japanese environmental limit [25] | 1.00 | 0.80 | 10.00 | - | - |
| Trace Elements | Function of PS Ash | Function of Blending Ratio | ||||
|---|---|---|---|---|---|---|
| Fa Statistic | p-Value | F Critical | Fa Statistic | p-Value | F Critical | |
| B | 0.01 | 1.00 | 2.62 | 261.33 | 5.33 × 10−20 | 2.76 |
| F | 1.33 | 0.29 | 2.62 | 3.28 | 0.027 | 2.76 |
| Se | 0.06 | 1.00 | 2.62 | 107.04 | 2.39 × 10−15 | 2.76 |
| Component Interaction | Speciation Forms | ||
|---|---|---|---|
| B | F | Se | |
| TEs + H2O | HBO2(s) | SiAl2F2O4(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + SiO2 | HBO2(s) and H4SiO4(s) | SiAl2F2O4(s) and H4SiO4(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + Al2O3 | HBO2(s) | SiAl2F2O4(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + TiO2 | HBO2(s) | SiAl2F2O4(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + Fe2O3 | HBO2(s) | SiAl2F2O4(s) and Fe2O3(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + CaO | HBO2(s), CaB2O4(s), CaB4O7(s), and (CaO)2(Al2O3)(B2O3)(s) | Ca(OH)2(s) and CaF2(s) | SeO32−(aq), CaSeO4(H2O)2, and Se(s) |
| TEs + H2O + MgO | HBO2(s) and Mg2B2O5(s) | Mg(OH)2(s) and MgF2(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + Na2O | HBO2(s), NaBO2(s), and Na2B4O7(s) | Na3AlF6(s) and NaAlSiO4(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + K2O | HBO2(s), KBO2(s), and K2B4O7(s) | KAl3Si3(OH)2(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + P2O5 | HBO2(s) | H4SiO4(s) and AlF3(s) | SeO32−(aq) and Se(s) |
| TEs + H2O + SO3 | HBO2(s) | H4SiO4(s) and AlF3(s) | SeO32−(aq) and Se(s) |
| Additives | Blending Ratio (%) | Leaching Ratio (%) a | Relative Error (%) b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | FactSage | |||||||||
| B | F | Se | B | F | Se | B | F | Se | ||
| XA | 10 | 78.97 | 62.58 | 97.17 | 100 | 89.44 | 100 | 23.51 | 35.33 | 2.87 |
| 30 | 92.07 | 27.14 | 97.71 | 100 | 83.75 | 100 | 8.26 | 102.10 | 2.31 | |
| XB | 10 | 79.15 | 52.81 | 97.66 | 100 | 89.62 | 100 | 23.27 | 51.69 | 2.37 |
| 30 | 91.79 | 41.38 | 99.18 | 100 | 85.14 | 100 | 8.56 | 69.17 | 0.83 | |
| XC | 10 | 82.01 | −1.06 | 97.75 | 100 | 89.31 | 100 | 19.77 | 204.82 | 2.28 |
| 30 | 94.00 | 28.54 | 99.14 | 100 | 81.04 | 100 | 6.18 | 95.83 | 0.86 | |
| YA | 10 | 89.89 | 17.28 | 98.68 | 100 | 90.70 | 100 | 10.65 | 136.00 | 1.33 |
| 30 | 82.63 | 25.91 | 97.77 | 100 | 90.38 | 100 | 19.02 | 110.89 | 2.26 | |
| YB | 10 | 95.15 | 47.61 | 96.61 | 100 | 90.65 | 100 | 4.97 | 62.26 | 3.45 |
| 30 | 92.49 | 50.47 | 96.94 | 100 | 90.19 | 100 | 7.80 | 56.48 | 3.11 | |
| YC | 10 | 77.24 | 36.56 | 98.13 | 100 | 90.57 | 100 | 25.68 | 84.97 | 1.89 |
| 30 | 98.75 | 41.29 | 99.28 | 100 | 88.74 | 100 | 1.26 | 72.98 | 0.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutopo, U.M.; Desfitri, E.R.; Hayakawa, Y.; Kambara, S. Influence of Blending High-Calcium Additive on Environmental Safety of B, F, and Se: A Case Study from Thermodynamic Calculation. Environments 2024, 11, 32. https://doi.org/10.3390/environments11020032
Sutopo UM, Desfitri ER, Hayakawa Y, Kambara S. Influence of Blending High-Calcium Additive on Environmental Safety of B, F, and Se: A Case Study from Thermodynamic Calculation. Environments. 2024; 11(2):32. https://doi.org/10.3390/environments11020032
Chicago/Turabian StyleSutopo, Ulung Muhammad, Erda Rahmilaila Desfitri, Yukio Hayakawa, and Shinji Kambara. 2024. "Influence of Blending High-Calcium Additive on Environmental Safety of B, F, and Se: A Case Study from Thermodynamic Calculation" Environments 11, no. 2: 32. https://doi.org/10.3390/environments11020032
APA StyleSutopo, U. M., Desfitri, E. R., Hayakawa, Y., & Kambara, S. (2024). Influence of Blending High-Calcium Additive on Environmental Safety of B, F, and Se: A Case Study from Thermodynamic Calculation. Environments, 11(2), 32. https://doi.org/10.3390/environments11020032







