Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation and Hydrothermal Carbonization
2.2. Product Characterization
2.3. Statistical Analysis
3. Results and Discussion
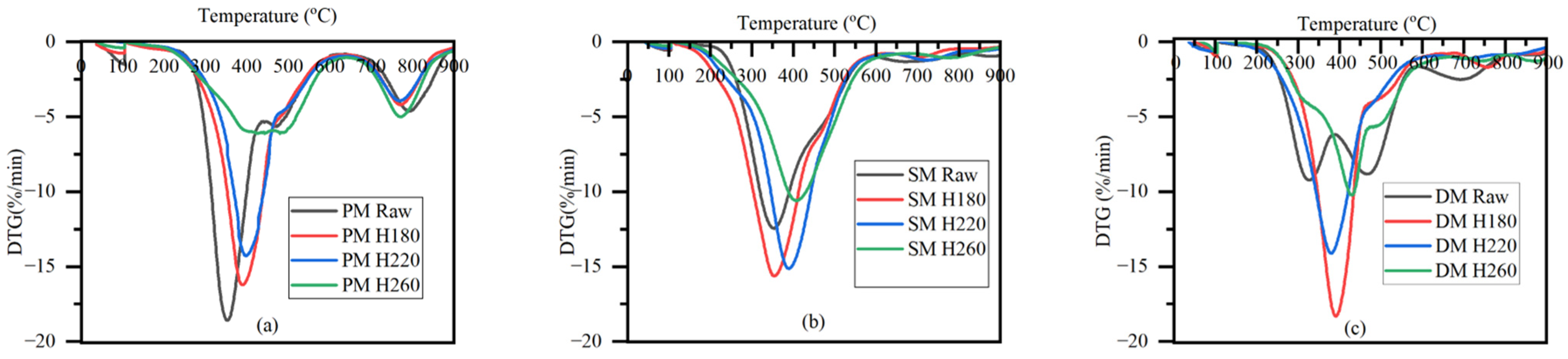
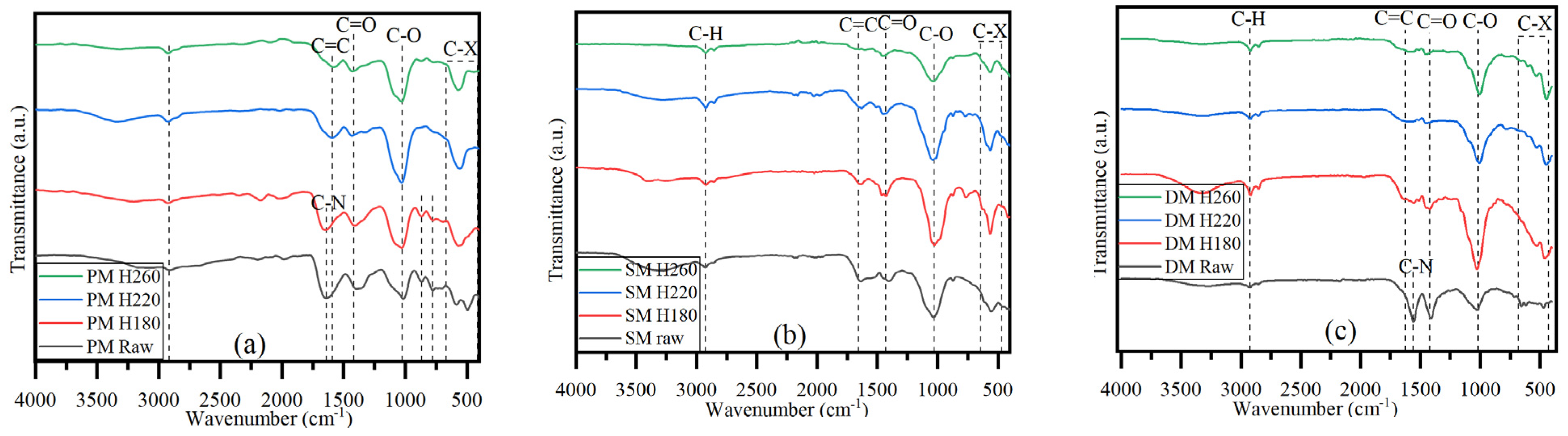
3.1. Effect of HTC Temperature on Thermophysical Properties of Hydrochars and Process Liquids
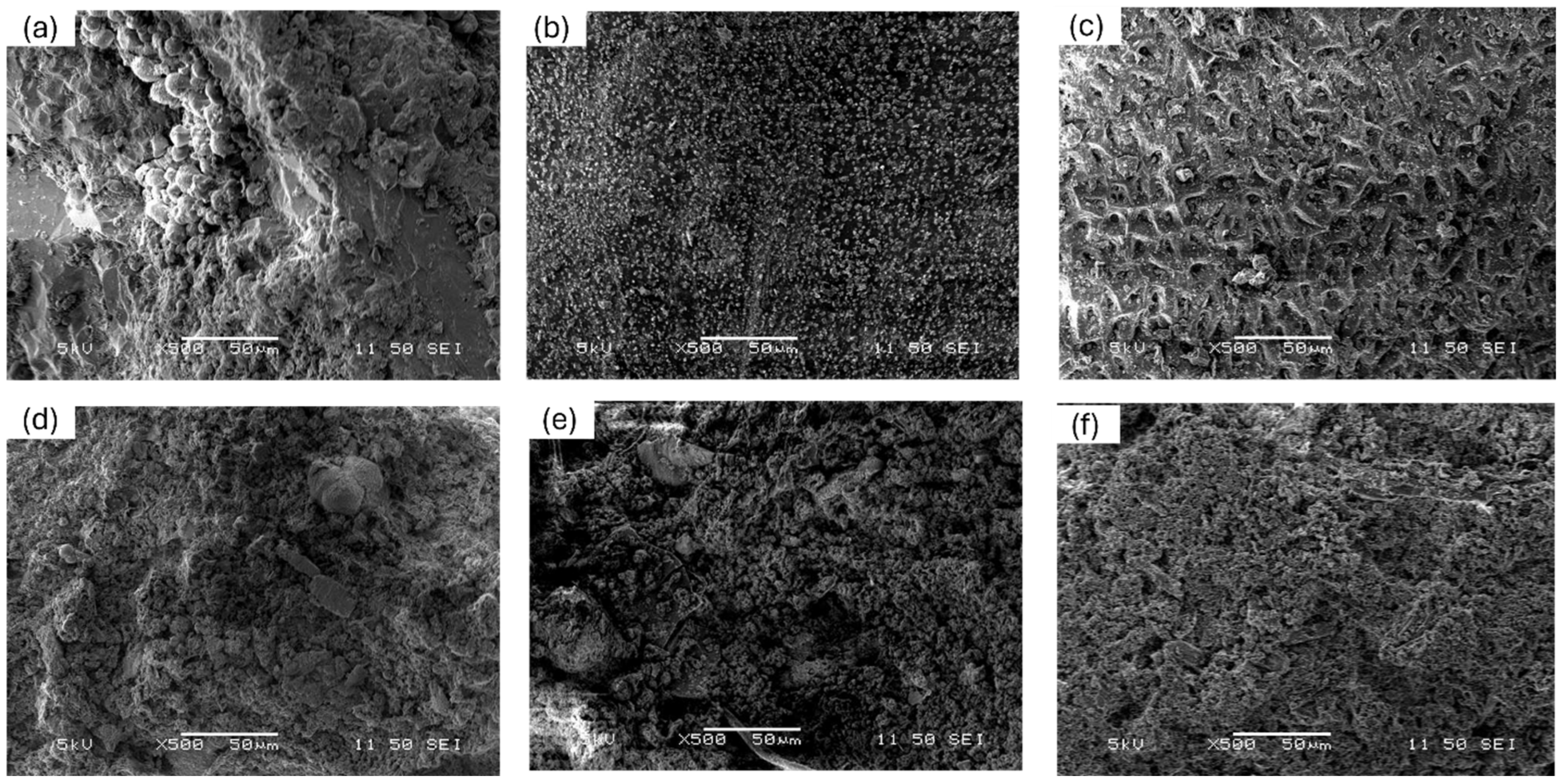
3.2. Surface Morphology Alteration with HTC Temperature for Various Manures
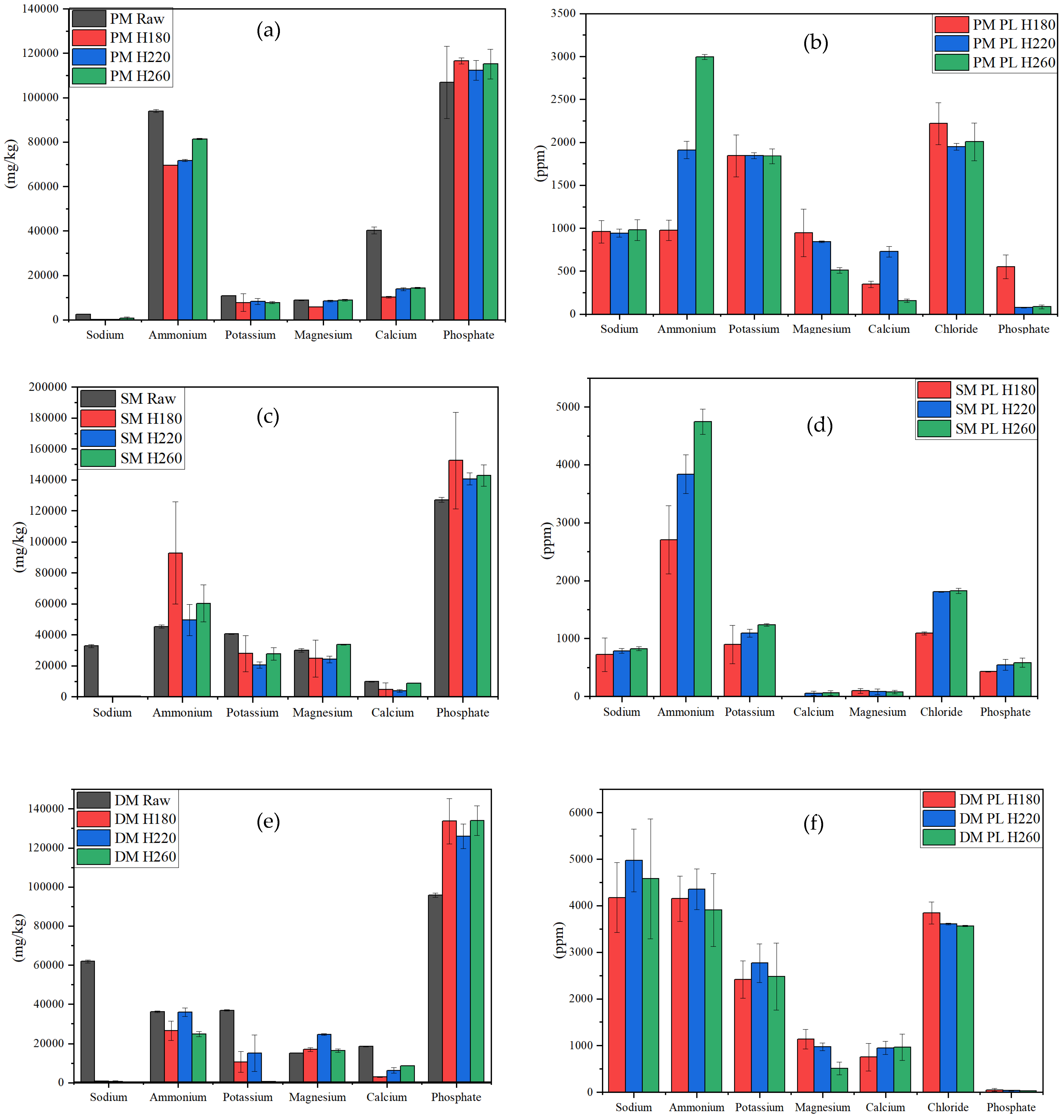
3.3. Ion Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagliari, P.; Wilson, M.; He, Z. Animal Manure Production and Utilization: Impact of Modern Concentrated Animal Feeding Operations. Anim. Manure Prod. Charact. Environ. Concerns Manag. 2020, 67, 1–14. [Google Scholar]
- Qi, J.; Yang, H.; Wang, X.; Zhu, H.; Wang, Z.; Zhao, C.; Li, B.; Liu, Z. State-of-the-art on animal manure pollution control and resource utilization. J. Environ. Chem. Eng. 2023, 11, 110462. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Kumar, R.R.; Park, B.J.; Cho, J.Y. Application and environmental risks of livestock manure. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 497–503. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A review of karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.M.; et al. Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. J. Clean. Prod. 2021, 278, 124019. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Gholizadeh, M.; Hu, X.; Yuan, X.; Sarkar, B.; Vithanage, M.; Masek, O.; Ok, Y.S. Co-hydrothermal carbonization of swine and chicken manure: Influence of cross-interaction on hydrochar and liquid characteristics. Sci. Total Environ. 2021, 786, 147381. [Google Scholar] [CrossRef]
- Khalaf, N.; Shi, W.; Fenton, O.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonization (HTC) of dairy waste: Effect of temperature and initial acidity on the composition and quality of solid and liquid products. Open Res. Eur. 2022, 2, 83. [Google Scholar] [CrossRef]
- Shettigondahalli Ekanthalu, V.; Morscheck, G.; Narra, S.; Nelles, M. Hydrothermal Carbonization—A Sustainable Approach to Deal with the Challenges in Sewage Sludge Management. In Urban Mining and Sustainable Waste Management; Ghosh, S.K., Ed.; Springer: Singapore, 2020; pp. 293–302. [Google Scholar]
- Motavaf, B.; Dean, R.A.; Nicolas, J.; Savage, P.E. Hydrothermal carbonization of simulated food waste for recovery of fatty acids and nutrients. Bioresour. Technol. 2021, 341, 125872. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag. 2019, 88, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Eneji, A.E.; Hussain, Z.; Ashraf, M. Chemical characterization of fresh and composted livestock manures. J. Soil Sci. Plant Nutr. 2013, 13, 115–121. [Google Scholar] [CrossRef]
- Toufiq Reza, M.; Freitas, A.; Yang, X.; Hiibel, S.; Lin, H.; Coronella, C.J. Hydrothermal carbonization (HTC) of cow manure: Carbon and nitrogen distributions in HTC products. Environ. Prog. Sustain. Energy 2016, 35, 1002–1011. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Speciation of Nutrients in Hydrochar Produced from Hydrothermal Carbonization of Poultry Litter under Different Treatment Conditions. ACS Sustain. Chem. Eng. 2018, 6, 11265–11272. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Pan, Z.-Q.; Xiao, X.-F.; Huang, H.-J.; Lai, F.-Y.; Wang, J.-X.; Chen, S.-W. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrolysis 2019, 144, 104692. [Google Scholar] [CrossRef]
- Mariuzza, D.; Lin, J.-C.; Volpe, M.; Fiori, L.; Ceylan, S.; Goldfarb, J.L. Impact of Co-Hydrothermal carbonization of animal and agricultural waste on hydrochars’ soil amendment and solid fuel properties. Biomass Bioenergy 2022, 157, 106329. [Google Scholar] [CrossRef]
- Belete, Y.Z.; Mau, V.; Yahav Spitzer, R.; Posmanik, R.; Jassby, D.; Iddya, A.; Kassem, N.; Tester, J.W.; Gross, A. Hydrothermal carbonization of anaerobic digestate and manure from a dairy farm on energy recovery and the fate of nutrients. Bioresour. Technol. 2021, 333, 125164. [Google Scholar] [CrossRef]
- Hejna, M.; Swiechowski, K.; Rasaq, W.A.; Bialowiec, A. Study on the Effect of Hydrothermal Carbonization Parameters on Fuel Properties of Chicken Manure Hydrochar. Materials 2022, 15, 5564. [Google Scholar] [CrossRef] [PubMed]
- Kantarli, I.C.; Kabadayi, A.; Ucar, S.; Yanik, J. Conversion of poultry wastes into energy feedstocks. Waste Manag. 2016, 56, 530–539. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; Rubia, M.A.d.l. Energy valorization of cow manure by hydrothermal carbonization and anaerobic digestion. Renew. Energy 2020, 160, 623–632. [Google Scholar] [CrossRef]
- Lang, Q.; Liu, Z.; Li, Y.; Xu, J.; Li, J.; Liu, B.; Sun, Q. Combustion characteristics, kinetic and thermodynamic analyses of hydrochars derived from hydrothermal carbonization of cattle manure. J. Environ. Chem. Eng. 2022, 10, 106938. [Google Scholar] [CrossRef]
- Song, C.; Yuan, W.; Shan, S.; Ma, Q.; Zhang, H.; Wang, X.; Niazi, N.K.; Wang, H. Changes of nutrients and potentially toxic elements during hydrothermal carbonization of pig manure. Chemosphere 2020, 243, 125331. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, S.; Wang, J.; Wang, Y.; Fang, X.; Huang, H. Speciation of Main Nutrients (N/P/K) in Hydrochars Produced from the Hydrothermal Carbonization of Swine Manure under Different Reaction Temperatures. Materials 2021, 14, 4114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, G.; Zhang, G.; Cao, T.; Wang, K.; Zhang, X.; Han, L. Recent advances in hydrothermal liquefaction of manure wastes into value-added products. Energy Convers. Manag. 2023, 292, 117392. [Google Scholar] [CrossRef]
- CEN/TS 15414-1:2010; Solid recovered fuels - Determination of moisture content using the oven dry method. Part 1: Determination of total moisture by a reference method. European Committee for Standardization: Brussels, Belgium, 2010.
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Z.; Meng, S.; Liu, S.; Zhao, Z.; Wang, C.; Yin, Q. Effect of lignocellulosic components on the hydrothermal carbonization reaction pathway and product properties of protein. Energy 2022, 259, 125063. [Google Scholar] [CrossRef]
- Sapkota Amy, R.; Lefferts Lisa, Y.; McKenzie, S.; Walker, P. What Do We Feed to Food-Production Animals? A Review of Animal Feed Ingredients and Their Potential Impacts on Human Health. Environ. Health Perspect. 2007, 115, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Xu, J.; Flora, J.R.; Hoque, S.; Berge, N.D. Quantifying the sensitivity of feedstock properties and process conditions on hydrochar yield, carbon content, and energy content. Bioresour. Technol. 2018, 262, 284–293. [Google Scholar] [CrossRef]
- Kieseler, S.; Neubauer, Y.; Zobel, N. Ultimate and proximate correlations for estimating the higher heating value of hydrothermal solids. Energy Fuels 2013, 27, 908–918. [Google Scholar] [CrossRef]
- Sutton, A.; Applegate, T.; Hankins, S.; Hill, B.; Sholly, D.; Allee, G.; Greene, W.; Kohn, R.; Meyer, D.; Powers, W. Manipulation of Animal Diets to Affect Manure Production, Composition and Odors: State of the Science; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Trabue, S.L.; Kerr, B.J.; Scoggin, K.D. Swine diets impact manure characteristics and gas emissions: Part II sulfur source. Sci. Total Environ. 2019, 689, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ravber, M. Hydrothermal Degradation of Fats, Carbohydrates and Proteins in Sunflower Seeds after Treatment with Subcritical Water. Chem. Biochem. Eng. Q. 2015, 29, 351–355. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, L.; Yao, Z.; Jia, J.; Wang, Z.; Liu, Z. Behaviors and interactions during hydrothermal carbonization of protein, cellulose and lignin. Chem. Eng. J. 2023, 476, 146373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, N.; Wu, Y.; Wu, K.; Wu, X.; Liu, J.; Yang, M. Distributions of organic compounds to the products from hydrothermal liquefaction of microalgae. Environ. Prog. Sustain. Energy 2017, 36, 259–268. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Peng, C.; Li, S.; Wang, B.; Liu, X.; Li, C. Effect of temperature on the sulfur fate during hydrothermal carbonization of sewage sludge. Environ. Pollut. 2020, 260, 114067. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Iwabuchi, K.; Itoh, T. Improvement of the fuel properties of dairy manure by increasing the biomass-to-water ratio in hydrothermal carbonization. PLoS ONE 2022, 17, e0269935. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, S.; Gurel, B.; Varol, M.; Gurbuz, H.; Kurtulus, K. Investigation on thermal degradation kinetics and mechanisms of chicken manure, lignite, and their blends by TGA. Environ. Sci. Pollut. Res. Int. 2021, 28, 63894–63904. [Google Scholar] [CrossRef]
- Aich, S.; Behera, D.; Nandi, B.K.; Bhattacharya, S. Relationship between proximate analysis parameters and combustion behaviour of high ash Indian coal. Int. J. Coal Sci. Technol. 2020, 7, 766–777. [Google Scholar] [CrossRef]
- Sharara, M.; Sadaka, S. Thermogravimetric Analysis of Swine Manure Solids Obtained from Farrowing, and Growing-Finishing Farms. J. Sustain. Bioenergy Syst. 2014, 04, 75–86. [Google Scholar] [CrossRef]
- Subramanian, A.; Prabhakar, V.; Rodriguez-Saona, L. Analytical Methods: Infrared Spectroscopy in Dairy Analysis. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Bekiaris, G.; Bruun, S.; Peltre, C.; Houot, S.; Jensen, L.S. FTIR–PAS: A powerful tool for characterising the chemical composition and predicting the labile C fraction of various organic waste products. Waste Manag. 2015, 39, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Ghiri, M.; Ghasemi-Fasaei, R.; Farrokhnejad, E. Factors Affecting Micronutrient Availability in Calcareous Soils of Southern Iran. Arid Land Res. Manag. 2013, 27, 203–215. [Google Scholar] [CrossRef]
- Chen, X.; Fan, X.; Gao, K.; Cheng, Y.; Zhang, K.; Liu, L.; Fang, L.; Park, J.-H.; Chen, X.; Xiao, R. Impacts of EDTA on the fate of nutrients and heavy metals during the hydrothermal carbonization of poultry manure. J. Environ. Chem. Eng. 2023, 11, 110061. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, L.; Chi, M.; Xu, X.; Tai, L.; Chen, Z.; Yu, K.; Liu, Z. Co-hydrothermal carbonization of lignocellulose biomass and polyvinyl chloride: The migration and transformation of chlorine. Chem. Eng. J. 2022, 446, 137155. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, N.; Li, J.; Cui, X. Fate of sodium and chlorine during the co-hydrothermal carbonization of high-alkali coal and polyvinyl chloride. Fuel Process. Technol. 2020, 199, 106277. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, G.; Shang, Y.; Zhao, P.; Cui, X.; Guo, Q. Chlorine migration during hydrothermal carbonization of recycled paper wastes and fuel performance of hydrochar. Process Saf. Environ. Prot. 2022, 158, 495–502. [Google Scholar] [CrossRef]
- Lange, R.; Staaland, H.; Mostad, A. The effect of salinity and temperature on solubility of oxygen and respiratory rate in oxygen-dependent marine invertebrates. J. Exp. Mar. Biol. Ecol. 1972, 9, 217–229. [Google Scholar] [CrossRef]
- Liu, J.-J.; Diao, Z.-H.; Xu, X.-R.; Xie, Q. Effects of dissolved oxygen, salinity, nitrogen and phosphorus on the release of heavy metals from coastal sediments. Sci. Total Environ. 2019, 666, 894–901. [Google Scholar] [CrossRef]
- Dai, L.; Tan, F.; Wu, B.; He, M.; Wang, W.; Tang, X.; Hu, Q.; Zhang, M. Immobilization of phosphorus in cow manure during hydrothermal carbonization. J. Environ. Manag. 2015, 157, 49–53. [Google Scholar] [CrossRef]
| Ultimate Analysis (Elemental Composition) | Process Liquid Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Name | Yield a (%) | C (%) | H (%) | N (%) | S (%) | O b (%) | Ash (%) | pH | Cond. c (mS/cm) | DO d (mg/L) |
| PM Raw | 35.97 ± 0.76 | 4.42 ± 0.16 | 6.17 ± 0.42 | 0.97 ± 0.04 | 17.2 ± 3.75 | 40.67 ± 0.06 | ||||
| PM H180 | 32.12 ± 2.00 | 2.02 | 4.07 ± 0.39 | 3.57 ± 0.87 | 0.68 ± 0.05 | 9.15 ± 3.96 | 45.85 ± 2.42 | 5.61 ± 0.34 | 11.65 ± 0.05 | 9.16 ± 2.16 |
| PM H220 | 29.12 ± 0.93 | 38.63 ± 0.52 | 4.27 ± 0.09 | 3.21 ± 0.12 | 1.24 ± 0.06 | 5.32 ± 5.23 | 46.98 ± 3.76 | 7.11 ± 0.11 | 7.11 ± 0.11 | 8.66 ± 0.74 |
| PM H260 | 28.08 ± 0.42 | 38.98 ± 0.36 | 3.71 ± 0.05 | 3.13 ± 0.17 | 1.33 ± 0.06 | 4.95 ± 1.03 | 48.25 ± 0.09 | 7.63 ± 0.03 | 7.62 ± 0.03 | 7.64 ± 0.61 |
| SM Raw | 34.97 ± 1.23 | 4.41 ± 0.23 | 3.69 ± 0.19 | 1.86 ± 0.07 | 14.4 ± 2.52 | 40.67 ± 0.06 | ||||
| SM H180 | 65.90 ± 2.44 | 39.05 ± 0.68 | 5.56 ± 0.06 | 3.56 ± 0.43 | 1.66 ± 0.04 | 18.49 ± 3.89 | 31.68 ± 1.54 | 7.95 ± 0.19 | 8.04 ± 0.06 | 6.02 ± 0.58 |
| SM H220 | 54.07 ± 0.77 | 39.13 ± 0.41 | 5.08 ± 0.17 | 3.18 ± 0.08 | 1.78 ± 0.07 | 16.42 ± 3.46 | 34.41 ± 1.54 | 7.58 ± 0.18 | 7.63 ± 0.25 | 5.04 ± 0.72 |
| SM H260 | 53.02 ± 1.94 | 40.05 ± 0.57 | 4.43 ± 0.41 | 3.02 ± 0.05 | 1.67 ± 0.09 | 11.56 ± 3.93 | 42.21 ± 1.66 | 7.57 ± 0.37 | 7.81 ± 0.14 | 4.60 ± 0.27 |
| DM Raw | 36.3 ± 0.33 | 5.78 ± 0.44 | 2.64 ± 0.06 | 0.98 ± 0.03 | 12.8 ± 1.40 | 41.5 ± 0.13 | ||||
| DM H180 | 46.08 ± 1.44 | 39.52 ± 1.99 | 5.04 ± 0.22 | 2.13 ± 0.06 | 1.25 ± 0.14 | 21.02 ± 6.01 | 35.04 ± 1.84 | 6.51 ± 0.11 | 6.54 ± 0.16 | 6.93 ± 1.17 |
| DM H220 | 33.59 ± 0.45 | 37.51 ± 0.59 | 4.17 ± 0.36 | 2.31 ± 0.09 | 1.24 ± 0.03 | 12.2 ± 3.48 | 42.57 ± 1.39 | 5.89 ± 0.04 | 5.89 ± 0.04 | 5.63 ± 2.39 |
| DM H260 | 28.52 ± 1.56 | 36.26 ± 1.31 | 3.99 ± 0.08 | 2.42 ± 0.05 | 1.19 ± 0.06 | 3.77 ± 4.16 | 48.37 ± 1.44 | 5.77 ± 0.08 | 5.77 ± 0.08 | 4.93 ± 0.29 |
| Sample Name | Na (%) | Mg (%) | P (%) | S (%) | Cl (%) | K (%) | Ca (%) | Mn (%) | Fe (%) | Zn (%) | Cu (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM Raw | 1.57 ±0.17 | 1.59 ± 0.14 | 6.07 ± 0.05 | 6.6 ± 0.07 | 2.43 ± 0.11 | 7.25 ± 0.75 | 9.57 ± 0.04 | ND * | ND * | ND * | ND * |
| PM H180 | ND * | 0.95 ± 0.11 | 10.95 ± 0.06 | 0.65 ± 0.73 | ND * | 0.21 ± 0.53 | 24.06 ± 0.03 | 0.13 ± 0.21 | 0.22 ± 0.12 | 0.11 ± 0.09 | ND * |
| PM H220 | ND * | 2.54 ± 0.11 | 4.89 ± 0.06 | 0.9 ± 0.09 | ND * | 0.22 ± 0.15 | 10.3 ± 0.03 | 0.3 ± 0.24 | 0.34 ± 0.12 | 0.44 ± 0.05 | ND * |
| PM H260 | ND * | 1.25 ± 0.10 | 12.94 ± 0.06 | 0.46 ± 0.08 | ND * | 0.34 ± 0.09 | 30.22 ± 0.03 | 0.63 ± 0.07 | 0.69 ± 0.06 | 0.48 ± 0.05 | ND * |
| SM Raw | 10.38 ± 0.9 | 5.56 ± 0.08 | 7.58 ± 0.05 | 2.51 ± 0.05 | 2.81 ± 0.05 | 7.97 ± 0.06 | 3.68 ± 0.04 | ND * | ND * | ND * | ND * |
| SM H180 | 1.53 ± 0.12 | 9.01 ± 0.09 | 10.91 ± 0.06 | 3.5 ± 0.06 | 1.06 ± 0.04 | 3.05 ± 0.04 | 5.71 ± 0.03 | 0.21 ± 0.19 | 0.77 ± 0.05 | 0.68 ± 0.03 | 0.33 ± 0.11 |
| SM H220 | 1.2 ± 0.15 | 5.25 ± 0.09 | 5.73 ± 0.06 | 3.9 ± 0.06 | 0.07 ± 0.05 | 2.81 ± 0.04 | 3.44 ± 0.04 | 0.27 ± 0.16 | 0.75 ± 0.08 | 0.53 ± 0.29 | 0.62 ± 0.39 |
| SM H260 | 0.17 ± 0.34 | 8.54 ± 0.09 | 12.23 ± 0.06 | 2.5 ± 0.07 | ND * | 0.49 ± 0.07 | 2.61 ± 0.03 | 0.67 ± 0.06 | 1.7 ± 0.03 | 1.35 ± 0.04 | 1.04 ± 0.05 |
| DM Raw | 8.24 ± 0.09 | 8.23 ± 0.08 | 2.54 ± 0.05 | 7.35 ± 0.04 | 0.71 ± 0.11 | 4.15 ± 0.04 | 10.32 ± 0.03 | ND * | ND * | ND * | ND * |
| DM H180 | 2.57 ± 0.12 | 2.47 ± 0.10 | 2.72 ± 0.07 | 1.92 ± 0.06 | 0.35 ± 0.13 | 1.47 ± 0.05 | 6.95 ± 0.03 | ND * | 2.19 ± 0.03 | ND * | ND * |
| DM H220 | ND * | 4.28 ± 0.09 | 3.9 ± 0.07 | 2.24 ± 0.06 | ND * | 0.98 ±0.05 | 9.53 ± 0.03 | ND * | 3.06 ± 0.03 | 0.67 ± 0.04 | ND * |
| DM H260 | ND * | 5.94 ± 0.09 | 4.84 ± 0.07 | 2.11 ± 0.07 | ND * | 0.82 ± 0.06 | 8.67 ± 0.03 | ND * | 2.96 ± 0.02 | 0.46 ± 0.04 | 0.53 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devnath, B.; Khanal, S.; Shah, A.; Reza, T. Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure. Environments 2024, 11, 150. https://doi.org/10.3390/environments11070150
Devnath B, Khanal S, Shah A, Reza T. Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure. Environments. 2024; 11(7):150. https://doi.org/10.3390/environments11070150
Chicago/Turabian StyleDevnath, Bilash, Sami Khanal, Ajay Shah, and Toufiq Reza. 2024. "Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure" Environments 11, no. 7: 150. https://doi.org/10.3390/environments11070150
APA StyleDevnath, B., Khanal, S., Shah, A., & Reza, T. (2024). Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure. Environments, 11(7), 150. https://doi.org/10.3390/environments11070150







