Quantification and Categorization of Macroplastics (Plastic Debris) within a Headwaters Basin in Western North Carolina, USA: Implications to the Potential Impacts of Plastic Pollution on Biota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Characterization of Plastic Debris
2.3. Laboratory Microplastic Production Analysis
2.4. Spatial Land Use and Terrain Analysis
3. Results
3.1. Land Use Characteristics
3.2. Plastic Debris
3.2.1. Spatial and Temporal Trends in the Quantity of Plastic Debris
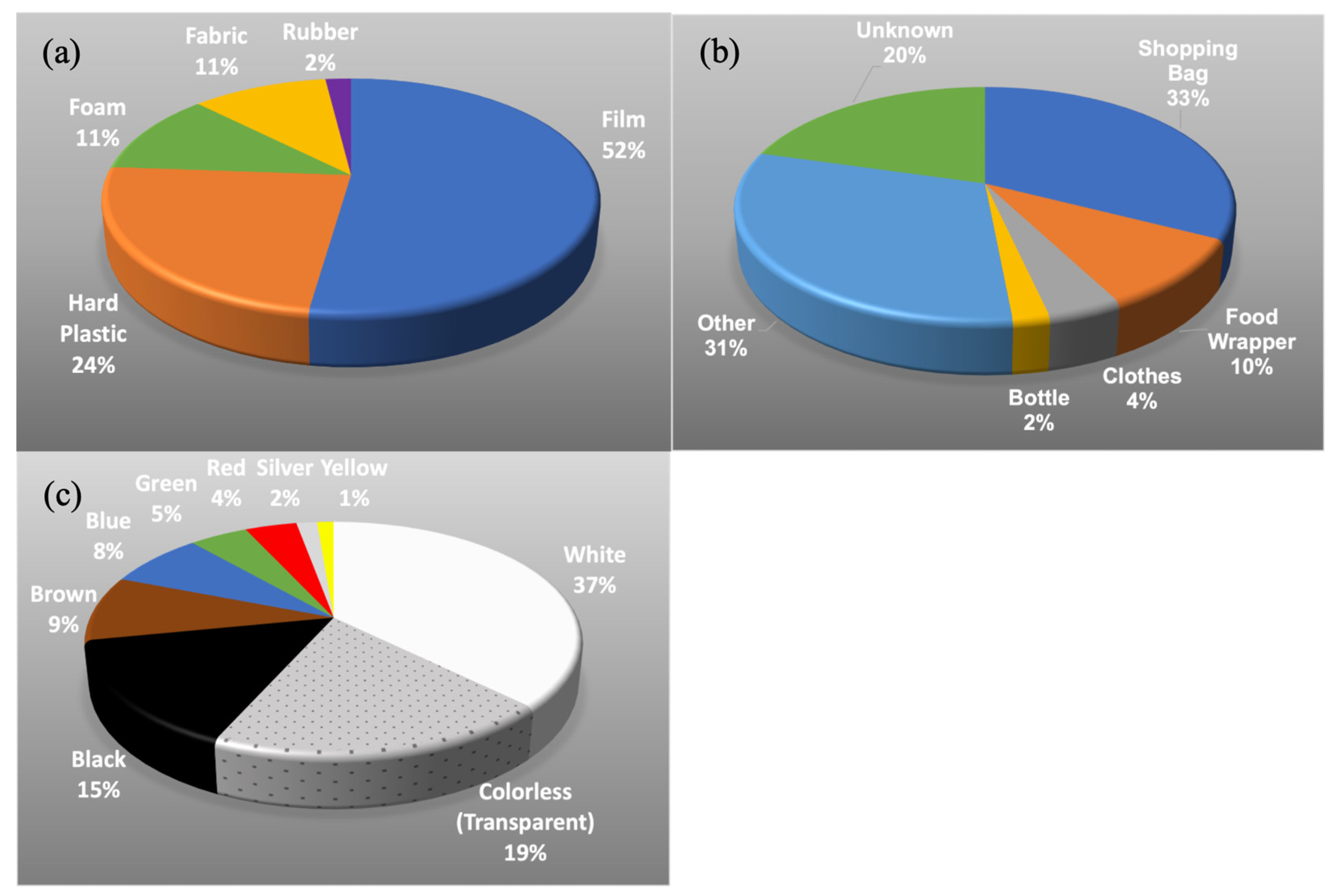
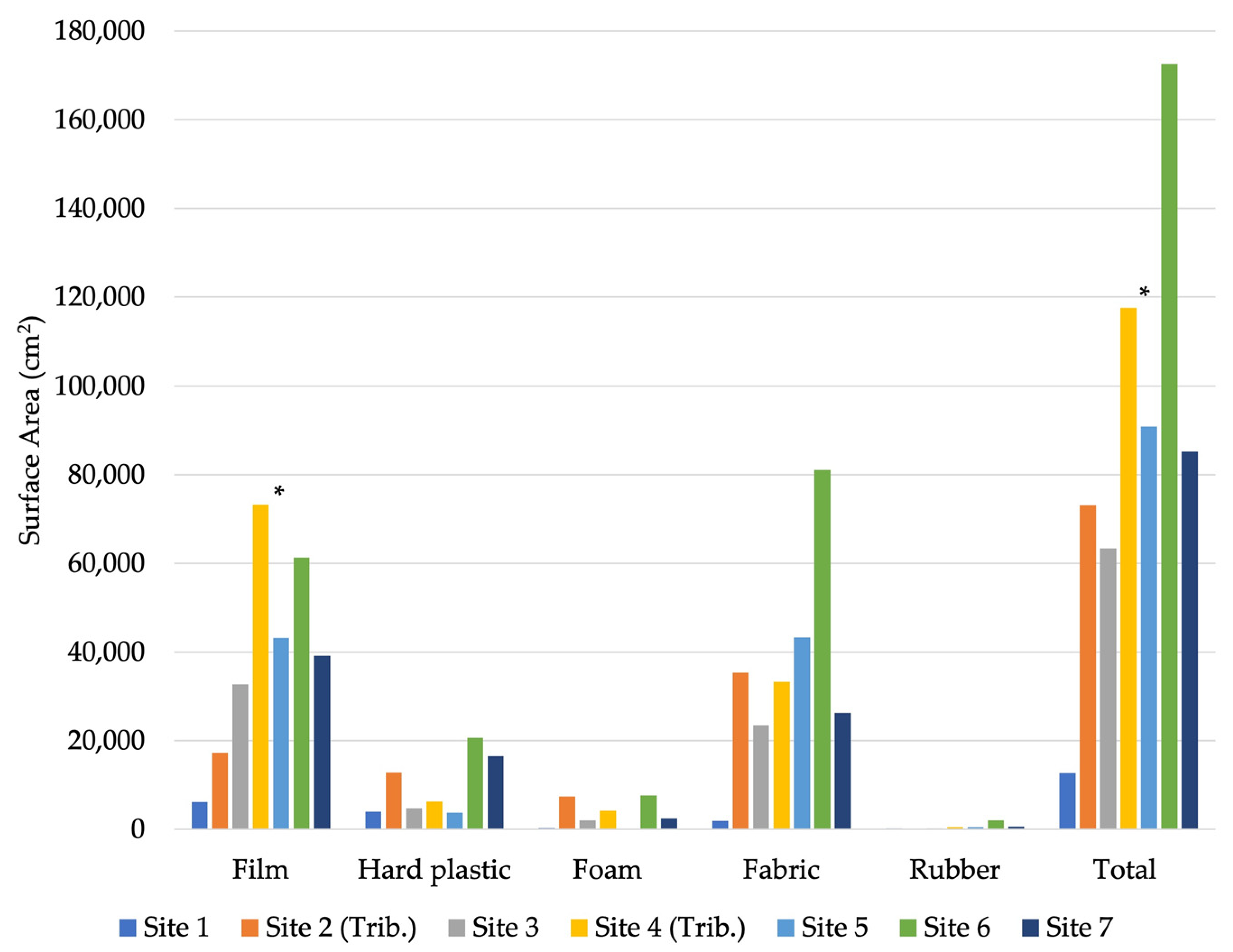
3.2.2. Characteristics of the Plastic Debris
3.3. Microplastic Production
4. Discussion
4.1. Temporal and Spatial Variations in Plastic Abundance
4.2. Functional Origin and Material Type of the Plastic Debris
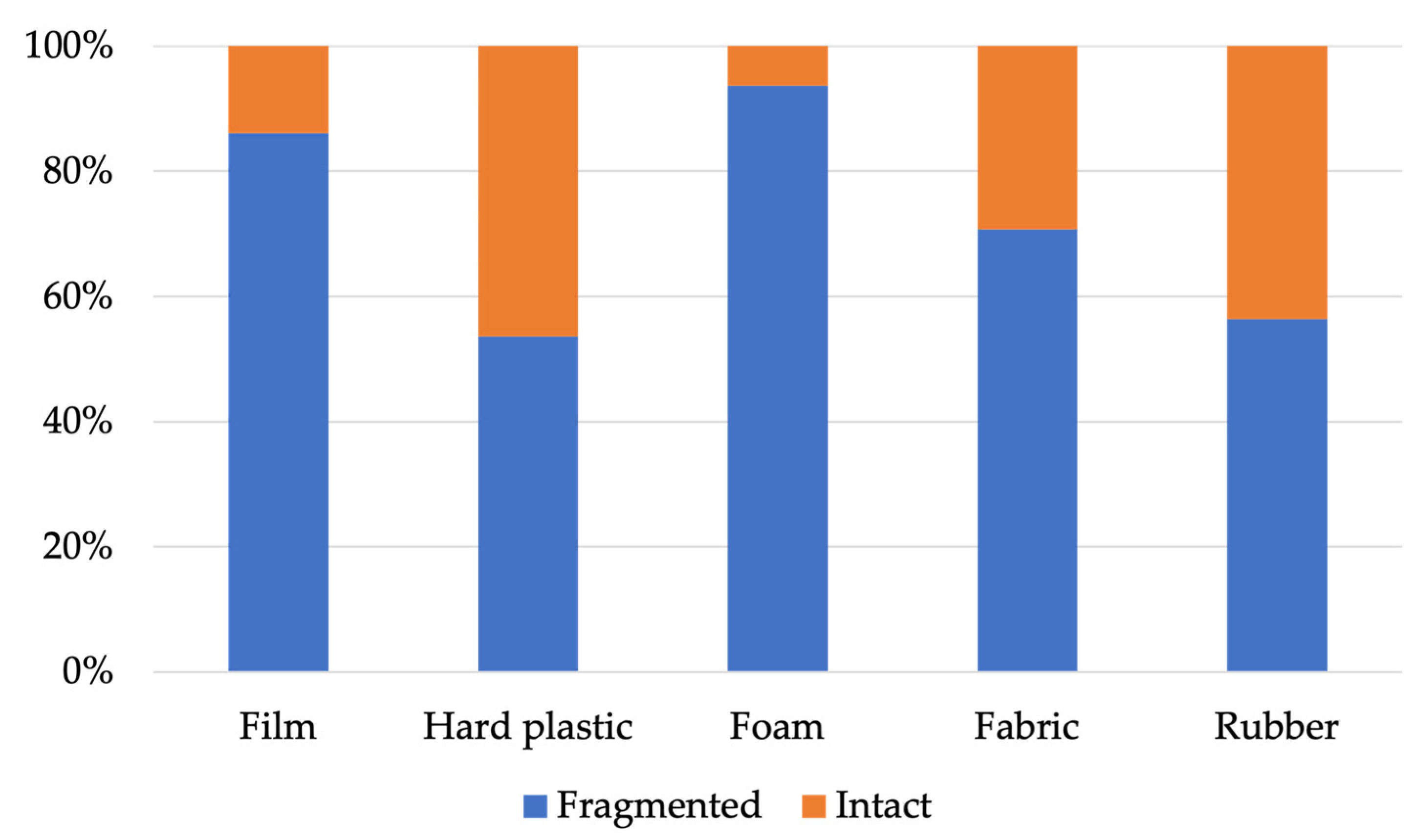
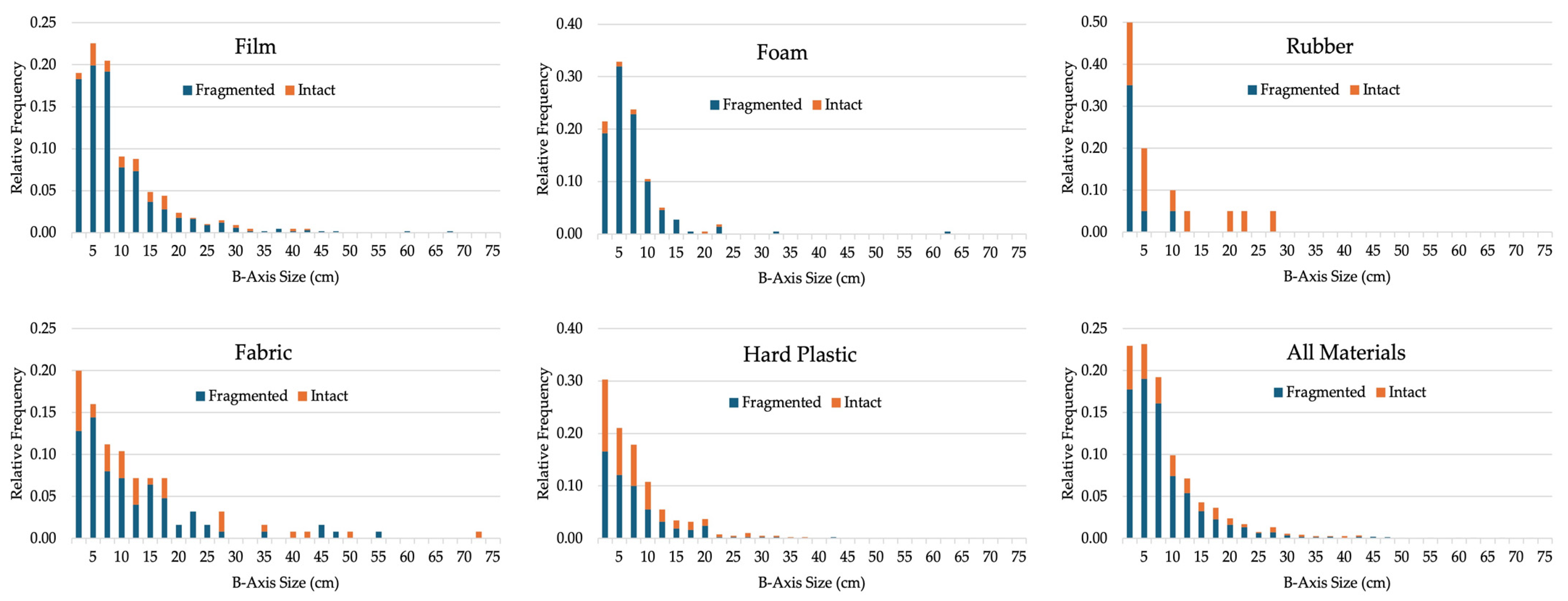
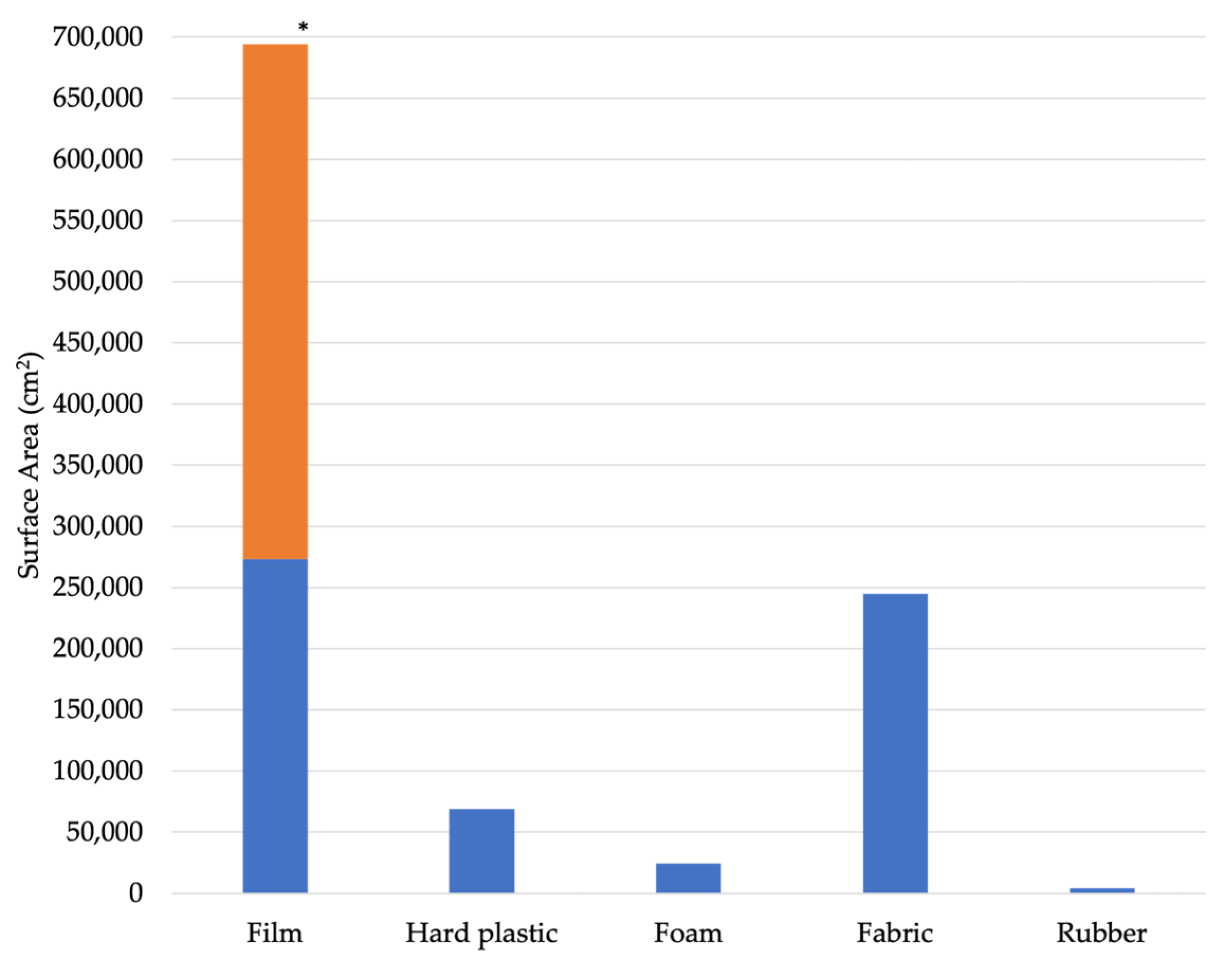
4.3. Item Size, Abundance, and Fragmentation
4.4. Meso- and Macroplastic Decomposition and Microplastic Production
4.5. Resin Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publications: Paris, Frances, 2022. [Google Scholar]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Diggle, A.; Walker, T.R. Environmental and Economic Impacts of Mismanaged Plastics and Measures for Mitigation. Environments 2022, 9, 15. [Google Scholar] [CrossRef]
- Galgani, L.; Loiselle, S.A. Plastic Accumulation in the Sea Surface Microlayer: An Experiment-Based Perspective for Future Studies. Geosciences 2019, 9, 66. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; Van Harmelen, T. Sources, Transport, and Accumulation of Different Types of Plastic Litter in Aquatic Environments: A Review Study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of Beach Sediments of a Subalpine Lake with Microplastic Particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-Levels of Microplastic Pollution in a Large, Remote, Mountain Lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith Jr, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The Rise in Ocean Plastics Evidenced from a 60-Year Time Series. Nat. Commun. 2019, 10, 1622. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global Ecological, Social and Economic Impacts of Marine Plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Honorato-Zimmer, D.; Kiessling, T.; Gatta-Rosemary, M.; Campodónico, C.K.; Núñez-Farías, P.; Rech, S.; Thiel, M. Mountain Streams Flushing Litter to the Sea–Andean Rivers as Conduits for Plastic Pollution. Environ. Pollut. 2021, 291, 118166. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.B.; Wolman, M.G.; Miller, J.P.; Wohl, E.E. Fluvial Processes in Geomorphology; Courier Dover Publications: Mineola, NY, USA, 2020; ISBN 0-486-84552-4. [Google Scholar]
- U.S. Census Bureau Decennial Census. 2020. Available online: https://data.census.gov/all?q=Waynesville%20town,%20North%20Carolina (accessed on 7 September 2024).
- U.S. Geological Survey USGS The National Map. Available online: https://www.usgs.gov/programs/national-geospatial-program/national-map (accessed on 18 August 2024).
- Gasperi, J.; Dris, R.; Bonin, T.; Rocher, V.; Tassin, B. Assessment of Floating Plastic Debris in Surface Water along the Seine River. Environ. Pollut. 2014, 195, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Lattin, G.L.; Zellers, A.F. Quantity and Type of Plastic Debris Flowing from Two Urban Rivers to Coastal Waters and Beaches of Southern California. Rev. Gest. Costeira Integr. J. Integr. Coast. Zone Manag. 2011, 11, 65–73. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so Colourful: A Potpourri of Plastic Litter Outnumbers Fish Larvae in Europe’s Second Largest River. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Sadri, S.S.; Thompson, R.C. On the Quantity and Composition of Floating Plastic Debris Entering and Leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014, 81, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A River Runs through It. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef]
- Lippiatt, S.; Opfer, S.; Arthur, C. Marine Debris Monitoring and Assessment: Recommendations for Monitoring Debris Trends in the Marine Environment. In NOAA Technical Memorandum NOS-OR&R; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2013. [Google Scholar]
- Blettler, M.C.; Ulla, M.A.; Rabuffetti, A.P.; Garello, N. Plastic Pollution in Freshwater Ecosystems: Macro-, Meso-, and Microplastic Debris in a Floodplain Lake. Environ. Monit. Assess. 2017, 189, 581. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H. The Ecology of Interfaces: Riparian Zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Ríos, J.M.; Tesitore, G.; de Mello, F.T. Does Color Play a Predominant Role in the Intake of Microplastics Fragments by Freshwater Fish: An Experimental Approach with Psalidodon Eigenmanniorum. Environ. Sci. Pollut. Res. 2022, 29, 49457–49464. [Google Scholar] [CrossRef] [PubMed]
- Yanoviak, S.P. Container Color and Location Affect Macroinvertebrate Community Structure in Artificial Treeholes in Panama. Fla. Entomol. 2001, 84, 265–271. [Google Scholar] [CrossRef]
- Negishi, J.N.; Nakagawa, T.; Nakamura, F. Exceptional Color Preferences for Flying Adult Aquatic Insects. Aquat. Ecol. 2022, 56, 1–6. [Google Scholar] [CrossRef]
- McArtor, J.D.; Detmer, T.M.; Porreca, A.P.; Parkos III, J.J.; Wahl, D.H. Do Freshwater Macroinvertebrates Select for Different Substrates Used in Fisheries Habitat Enhancement? Trans. Ill. State Acad. Sci. 2021, 114, 325–330. [Google Scholar]
- Driedger, A.G.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic Debris in the Laurentian Great Lakes: A Review. J. Gt. Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Teegarden, D.M. Polymer Chemistry: Introduction to an Indispensable Science; NSTA Press: Richmond, VA, USA, 2004; ISBN 0-87355-221-0. [Google Scholar]
- Miller, J.; Love, J.; O’Brien, C.; Gray, A.; Youker, R.; Cahoon, S. Source, Transport Rates, and Transport Dynamics of Plastic Particles in Small Headwater Basins of the Southern Appalachians, Community Collaborative Research Grant; NC Sea Grant and WRRI Program: Raleigh, NC, USA, 2023; Final Report, Contract No. 22-CCRG-04; p. 54. [Google Scholar]
- Dewitz, J. National Land Cover Database (NLCD) 2019 Products. US Geol. Surv. 2021, 10, P9KZCM54. [Google Scholar]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic Ingestion by Planktivorous Fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Codina-García, M.; Militão, T.; Moreno, J.; González-Solís, J. Plastic Debris in Mediterranean Seabirds. Mar. Pollut. Bull. 2013, 77, 220–226. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental Implications of Plastic Debris in Marine Settings—Entanglement, Ingestion, Smothering, Hangers-on, Hitch-Hiking and Alien Invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Sheavly, S.B.; Register, K.M. Marine Debris & Plastics: Environmental Concerns, Sources, Impacts and Solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic Ingestion Decreases Energy Reserves in Marine Worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P. The Ecotoxicology of Nanoparticles in Daphnia Magna. Ph.D. Thesis, Edinburgh Napier University, Edinburgh, UK, 2010. [Google Scholar]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017; ISBN 92-5-109882-4. [Google Scholar]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–Toxic Chemical Interaction: A Review Study on Quantified Levels, Mechanism and Implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Sánchez, C.; Hortal, M.; Aliaga, C.; Devis, A.; Cloquell-Ballester, V.A. Recyclability Assessment of Nano-Reinforced Plastic Packaging. Waste Manag. 2014, 34, 2647–2655. [Google Scholar] [CrossRef]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First Evidence of Microplastics in the African Great Lakes: Recovery from Lake Victoria Nile Perch and Nile Tilapia. J. Gt. Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and Occurrence of Micro (Nano) Plastics in Soils: Knowledge Gaps and Possible Risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ocean Conservancy the International Coastal Cleanup 2020 Report. International Coastal Cleanup Report 2020. 2020. Available online: https://oceanconservancy.org/wp-content/uploads/2020/09/2020-Report_-FINAL.pdf (accessed on 7 September 2024).
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic Debris in Seafood: Plastic Debris and Fibers from Textiles in Fish and Bivalves Sold for Human Consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Lavers, J.L.; Bond, A.L. Exceptional and Rapid Accumulation of Anthropogenic Debris on One of the World’s Most Remote and Pristine Islands. Proc. Natl. Acad. Sci. USA 2017, 114, 6052–6055. [Google Scholar] [CrossRef]
- Law, K.L.; Morét-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic Accumulation in the North Atlantic Subtropical Gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.P. Reducing Single-Use Plastic Shopping Bags in the USA. Waste Manag. 2017, 70, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zbyszewski, M.; Corcoran, P.L.; Hockin, A. Comparison of the Distribution and Degradation of Plastic Debris along Shorelines of the Great Lakes, North America. J. Gt. Lakes Res. 2014, 40, 288–299. [Google Scholar] [CrossRef]
- Miller, J.; Barrett, N.; Gray, A.; Love, J.; Youker, R.; Randall, G.; McGraw, E.; Milner, S. Spatial and Temporal Variations in Microplastic Concentrations in Small Headwater Basins, Southern Blue Ridge Mountains, North Carolina. In Proceedings of the Water Resources Research Institute Annual Conference, Raleigh, NC, USA, 21–22 March 2024. [Google Scholar]
- Providence City Hall Rhode Island’s Retail Plastic Bag Ban. Available online: https://www.providenceri.gov/sustainability/rhodeisland-retail-plastic-bag-ban/ (accessed on 20 August 2024).
- Colorado General Assembly HB21-1162 Management of Plastic Products. Available online: https://leg.colorado.gov/bills/hb21-1162 (accessed on 20 August 2024).
- NCSL State Plastic Bag Legislation. Available online: https://www.ncsl.org/environment-and-natural-resources/state-plastic-bag-legislation (accessed on 20 August 2024).
- Parkinson, L. US State Actions Concerning Food Packaging and Chemicals in 2023. Available online: https://www.foodpackagingforum.org/news/us-state-actions-concerning-food-packaging-and-chemicals-in-2023 (accessed on 20 August 2024).
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Miller, J.; Love, J.; Orbock Miller, S.M.; Cahoon, S.; Youker, R.; Gray, A. Source, Transport Rates, and Transport Dynamics of Plastic Particles in Small Headwater Basins of the Southern Appalachians. In Proceedings of the 4th World Congress of Geology and Earth Sciences, Rome, Italy, 4–6 September 2023. [Google Scholar]
- Corcoran, P.L. Benthic Plastic Debris in Marine and Fresh Water Environments. Environ. Sci. Process. Impacts 2015, 17, 1363–1369. [Google Scholar] [CrossRef]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Transport of Microplastics in Coastal Seas. Estuar. Coast. Shelf Sci. 2017, 199, 74–86. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and Temporal Distribution of Microplastics in Water and Sediments of a Freshwater System (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Peleg, E.; Teitelbaum, Y.; Arnon, S. Exploring the Influence of Sediment Motion on Microplastic Deposition in Streambeds. Water Res. 2024, 249, 120952. [Google Scholar] [CrossRef]
- Frias, J.P.; Gago, J.; Otero, V.; Sobral, P. Microplastics in Coastal Sediments from Southern Portuguese Shelf Waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and Health Hazards of Chemicals in Plastic Polymers and Products. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.R.; Rorrer, J.E.; Singh, A.; Konev, M.O.; Rorrer, N.A.; Carpenter, A.C.; Jacobsen, A.J.; Román-Leshkov, Y.; Beckham, G.T. The Critical Role of Process Analysis in Chemical Recycling and Upcycling of Waste Plastics. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 301–324. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Thapliyal, D.; Karale, M.; Diwan, V.; Kumra, S.; Arya, R.K.; Verros, G.D. Current Status of Sustainable Food Packaging Regulations: Global Perspective. Sustainability 2024, 16, 5554. [Google Scholar] [CrossRef]






| Resin | Abbreviation | Density (g/cm3) 2 | Common Type | Common Functional Uses |
|---|---|---|---|---|
| Low-density polyethylene | LDPE | 0.89–0.93 | Film | Squeeze bottles, container lids, six-pack beverage holders, diapers, bags |
| High-density polyethylene | HDPE | 0.94–0.98 | Film, Hard | Grocery bags, milk jugs, recycling bins, detergent and cleaner bottles, buoys |
| Polypropylene | PP | 0.85–0.92 | Hard | Food wrappers and containers, dishware, bottle caps, straws, auto parts |
| Expanded polystyrene | EPS | 0.01–0.04 | Foam | Foam cups, plates, trays, clamshell food containers |
| Polystyrene | PS | 1.04–1.08 | Hard | Plates, cutlery, toys |
| Polyvinyl chloride | PVC | 1.38–1.41 | Hard | Pipe, fencing, flooring, tampon applicators |
| Polyethylene terephthalate (including polyester) | PET | 1.38–1.41 | Hard, Fabric | Textiles, beverage bottles, strapping |
| Sample Location and Date | Film | Hard Plastic | Foam | Fabric | Rubber | Total Number | Concentration 1 (Items/m) | Accumulation Rate 2 (Items/Day) |
|---|---|---|---|---|---|---|---|---|
| Site 1 | ||||||||
| 28 Sep. 2022 | 25 | 9 | 9 | 11 | 1 | 55 | 1.10 | |
| 24 Mar. 2023 | 6 | 7 | 2 | 1 | 3 | 19 | 0.38 | 0.11 |
| Site 2 (Tributary) | ||||||||
| 13 Dec. 2022 | 40 | 42 | 35 | 13 | 0 | 130 | 2.60 | |
| 24 Mar. 2023 | 26 | 14 | 4 | 7 | 0 | 51 | 1.02 | 0.50 |
| Site 3 | ||||||||
| 28 Sep. 2022 | 20 | 6 | 0 | 1 | 1 | 28 | 0.56 | |
| 24 Mar. 2023 | 49 | 13 | 9 | 15 | 1 | 87 | 1.74 | 0.49 |
| 20 Sep. 2023 | 64 | 32 | 4 | 9 | 5 | 114 | 2.28 | 0.63 |
| Site 4 (Tributary) | ||||||||
| 28 Sep. 2022 | 51 | 13 | 1 | 1 | 3 | 69 | 1.38 | |
| 24 Mar. 2023 | 36 | 18 | 6 | 8 | 1 | 69 | 1.38 | 0.39 |
| 20 Sep. 2023 | 28 | 7 | 3 | 5 | 0 | 43 | 0.86 | 0.24 |
| Site 5 | ||||||||
| 28 Sep. 2022 | 50 | 11 | 1 | 16 | 1 | 79 | 1.58 | |
| 24 Mar. 2023 | 104 | 37 | 0 | 39 | 9 | 189 | 3.78 | 1.06 |
| Site 6 | ||||||||
| 13 Dec. 2022 | 122 | 62 | 76 | 24 | 2 | 286 | 5.72 | |
| Site 7 | ||||||||
| 28 Sep. 2022 | 45 | 22 | 7 | 8 | 0 | 82 | 1.64 | |
| 24 Mar. 2023 | 114 | 61 | 41 | 8 | 2 | 226 | 4.52 | 1.27 |
| Total Period | 863 | 432 | 220 | 187 | 35 | 1737 | 4.96 |
| Sample Location | Shopping Bag | Food Wrapper | Clothing | Bottle | Other 1 | Unknown 2 |
|---|---|---|---|---|---|---|
| Site 1 | 22 | 21 | 12 | 0 | 24 | 15 |
| Site 2 (Tributary) | 39 | 10 | 17 | 2 | 23 | 15 |
| Site 3 | 83 | 29 | 9 | 1 | 86 | 60 |
| Site 4 (Tributary) | 70 | 15 | 3 | 5 | 61 | 27 |
| Site 5 | 47 | 43 | 9 | 3 | 64 | 63 |
| Site 6 | 141 | 16 | 6 | 3 | 109 | 33 |
| Site 7 | 113 | 47 | 19 | 18 | 170 | 129 |
| Total | 515 | 181 | 75 | 32 | 537 | 342 |
| Sample Location | White | Transparent | Black | Brown | Blue | Green | Red | Yellow |
|---|---|---|---|---|---|---|---|---|
| Site 1 | 24 | 7 | 16 | 11 | 6 | 4 | 4 | 0 |
| Site 2 (Tributary) | 33 | 15 | 20 | 14 | 9 | 7 | 2 | 0 |
| Site 3 | 62 | 70 | 30 | 26 | 9 | 12 | 8 | 8 |
| Site 4 (Tributary) | 49 | 48 | 32 | 18 | 8 | 11 | 9 | 4 |
| Site 5 | 79 | 68 | 47 | 18 | 19 | 9 | 20 | 4 |
| Site 6 | 110 | 51 | 56 | 30 | 33 | 11 | 6 | 3 |
| Site 7 | 247 | 77 | 40 | 41 | 39 | 22 | 20 | 2 |
| Total | 604 | 336 | 241 | 158 | 123 | 76 | 69 | 21 |
| Material | Item Desc. | Area (cm2) 1 | Counts 2 | Type 3 | Color 4 |
|---|---|---|---|---|---|
| Fabric 1 | Carpet | 32 | >400 | Fiber | Transparent, black |
| Fabric 2 | Brown fabric | 32 | >400 | Fiber | Transparent, brown |
| Fabric 3 | Colorful fabric | 32 | >400 | Fiber | Transparent, red, black, blue |
| Film 1 | New grocery bag | 64 | >400 | Film | Blue |
| Film 2 | Old grocery bag | 64 | >400 | Film | White, red |
| Foam 1 | Old foam to-go container | 32 | >400 | Foam | White |
| Foam 2 | New foam cup | 32 | 6 (Below LOD 5) | N/A | N/A |
| Blank | N/A | N/A | 6 | Fibers | Blue, black |
| River (Reference) | Sampling Method | Dominant Types of Items | Dominant Resin Compositions 1,2 |
|---|---|---|---|
| Richland Creek (this study) | Surveys of deposited Items | Bags, food wrappers, bottles, fabrics | HDPE, LDPE, PET, PP; less abundant—PS, EPS, PVC |
| Parana Floodplain Lakes (Blettler et al.) [25] | Surveys of deposited Items | Food wrappers, bags, bottles, Styrofoam™ food containers | PP, PS, HDPE, LPE, PET |
| Seine River (Gasperi et al.) [19] | Floating detention boom | Food wrappers and containers, plastic cutlery | PP, PE, & less amounts of PET |
| Thames River (Morritt et al.) [23] | Eel fyke nets | Food wrappers, tobacco packaging, sanitary towel components, cups, cutlery | --- |
| Tamar Estuary (Sadri and Thompson) [22] | Towed net (surface) | Not specified | PE, PS, PP; minor amounts of PVC, PET (polyester), Nylon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrett, N.; Miller, J.; Orbock-Miller, S. Quantification and Categorization of Macroplastics (Plastic Debris) within a Headwaters Basin in Western North Carolina, USA: Implications to the Potential Impacts of Plastic Pollution on Biota. Environments 2024, 11, 195. https://doi.org/10.3390/environments11090195
Barrett N, Miller J, Orbock-Miller S. Quantification and Categorization of Macroplastics (Plastic Debris) within a Headwaters Basin in Western North Carolina, USA: Implications to the Potential Impacts of Plastic Pollution on Biota. Environments. 2024; 11(9):195. https://doi.org/10.3390/environments11090195
Chicago/Turabian StyleBarrett, Nathaniel, Jerry Miller, and Suzanne Orbock-Miller. 2024. "Quantification and Categorization of Macroplastics (Plastic Debris) within a Headwaters Basin in Western North Carolina, USA: Implications to the Potential Impacts of Plastic Pollution on Biota" Environments 11, no. 9: 195. https://doi.org/10.3390/environments11090195





