Heavy Metal Toxicity on Daphnia magna
Abstract
1. Introduction
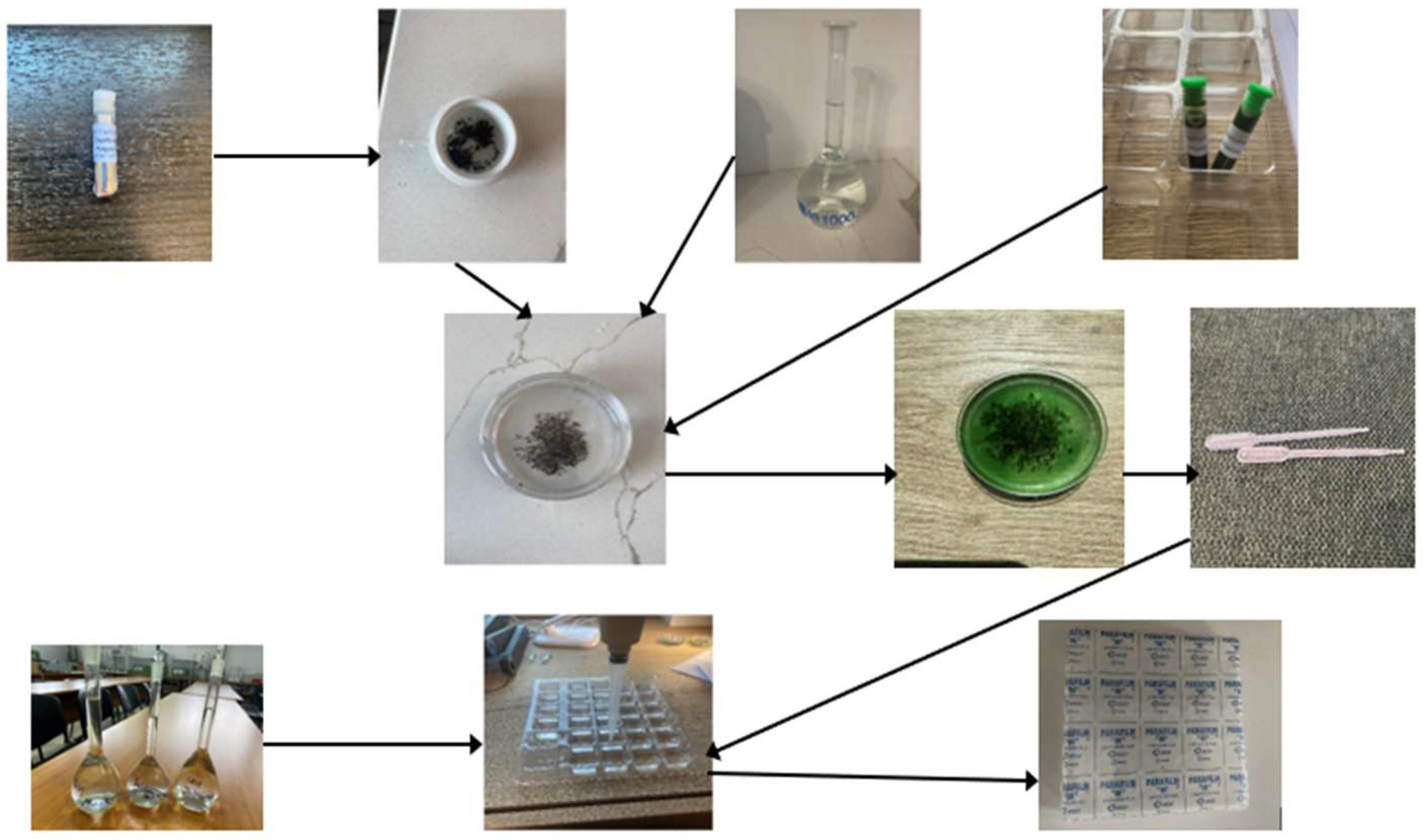
2. Materials and Methods
2.1. Preparation of Freshwater Solutions
2.2. Daphnia Magna Ephippia
2.3. Pollutant Solutions Used
2.4. Experimental Procedure
2.4.1. Hatching of Ephippia
2.4.2. Acute Toxicity of Heavy Metals
2.4.3. Determination of Lethal Concentration 50% (LC50)
2.5. Analysis of Experimental Data
3. Results and Discussion
3.1. Influence of Pollutants on Daphnia Magna Ephippia
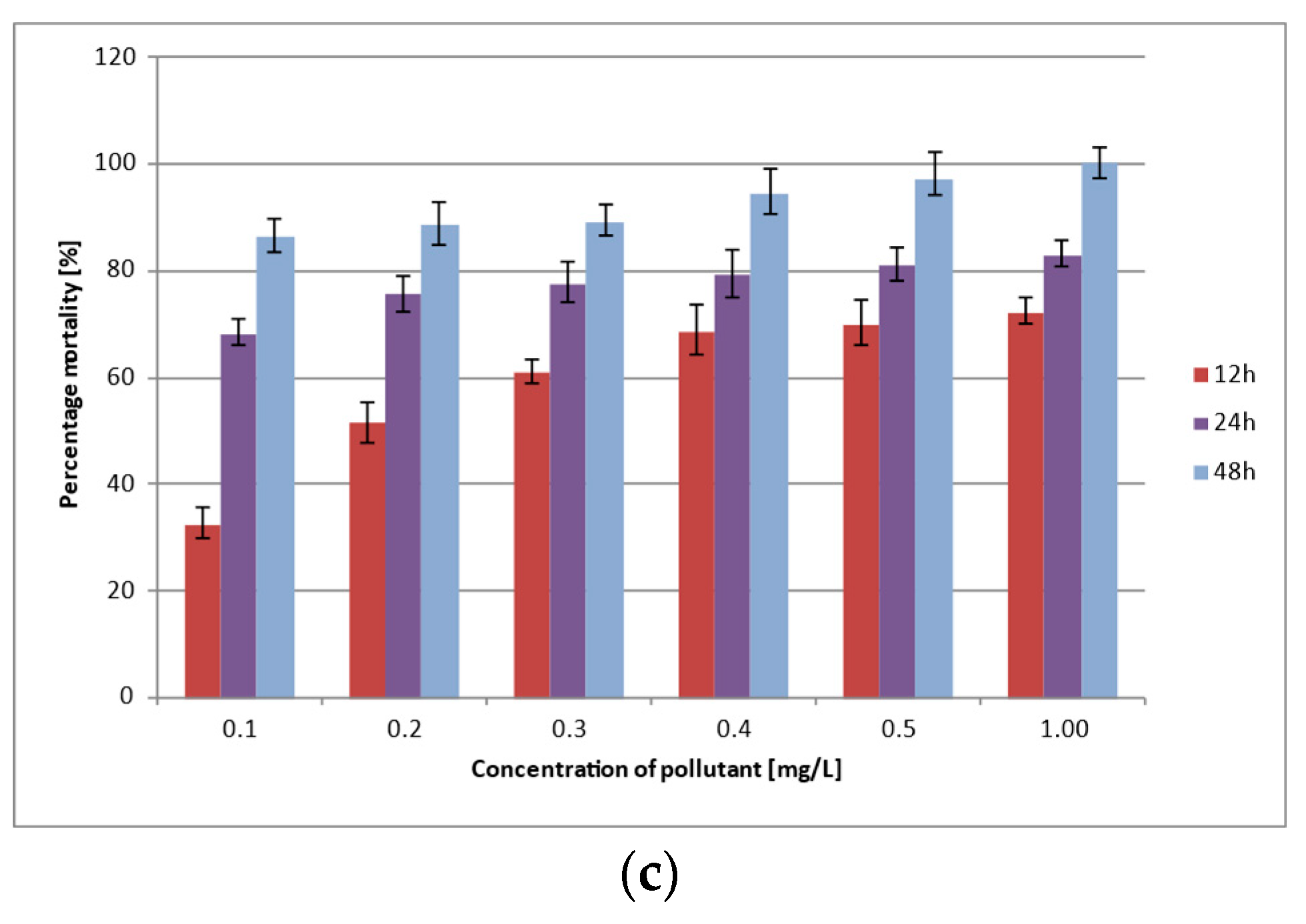
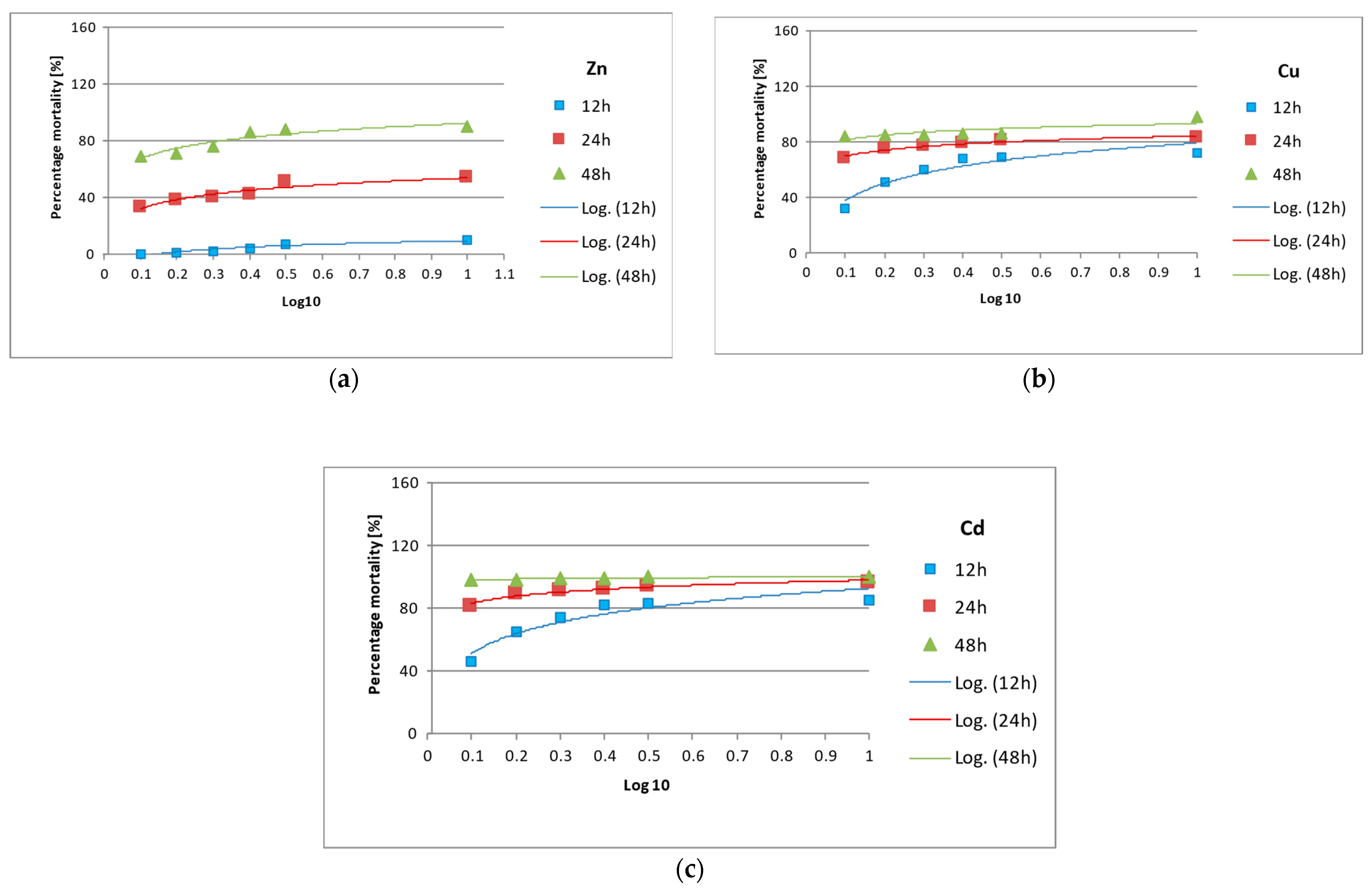
3.2. Lethality and Acute Toxicity of Pollutants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2005; ISBN 1-932811-06-0. [Google Scholar]
- Lampert, W.; Sommer, U. Limnoecology: The Ecology of Lakes and Streams; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Arao, T.; Kato, Y.; Nong, Q.D.; Yamamoto, H.; Watanabe, H.; Matsuura, T.; Tatarazako, N.; Tani, K.; Okamoto, A.; Matsumoto, T.; et al. Production of genome-edited Daphnia for heavy metal detection by fluorescence. Sci. Rep. 2020, 10, 21490. [Google Scholar] [CrossRef]
- Hooper, H.; Connon, R.; Callaghan, A.; Fryer, G.; Yarwood-Buchanan, S.; Biggs, J.; Maund, S.; Hutchinson, T.; Sibly, R. The ecological niche of Daphnia magna characterized using population growth rate. Ecology 2008, 89, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Clare, J. Daphnia: An aquarist’s Guide. Caudata Online. 2002. Available online: www.caudata.org/daphnia (accessed on 22 April 2020).
- Coors, A.; Vanoverbeke, J.; De Bie, T.; De Meester, L. Land use, genetic diversity and toxicant tolerance in natural populations of Daphnia magna. Aquat. Toxicol. 2009, 95, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Dhanker, R.; Saxena, A.; Goyal, S.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Toxicity evaluation of personal care and household products as silent killers on survival of Daphnia magna. Case Stud. Chem. Environ. Eng. 2021, 4, 100124. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A. Effect of phthalates on development, reproduction, fat metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2019, 654, 969–977. [Google Scholar] [CrossRef]
- Czech, B.; Jośko, I.; Oleszczuk, P. Ecotoxicological evaluation of selected pharmaceuticals to Vibrio fischeri and Daphnia magna before and after photooxidation process. Ecotoxicol. Environ. Saf. 2014, 104, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Valimaña-Traverso, J.; Amariei, G.; Boltes, K.; García, M.A.; Marina, M.L. Enantiomer stability and combined toxicity of duloxetine and econazole on Daphnia magna using real concentrations determined by capillary electrophoresis. Sci. Total Environ. 2019, 670, 770–778. [Google Scholar] [CrossRef]
- Canton, J.H.; Adema, D.M.M. Reproducibility of short-term and reproduction toxicity experiments with Daphnia magna and comparison of the sensitivity of Daphnia magna with Daphnia pulex and Daphnia cucullata in short-term experiments. Hydrobiologia 1978, 59, 135–140. [Google Scholar] [CrossRef]
- Steinkey, D.; Lari, E.; Woodman, S.G.; Luong, K.H.; Wong, C.S.; Pyle, G.G. Effects of gemfibrozil on the growth, reproduction, and energy stores of Daphnia magna in the presence of varying food concentrations. Chemosphere 2018, 192, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Watts, R.G.; Cheng, W. Acute Toxicity of Inorganic Chloramines to Daphnia magna in Two Types of Dilution Water. Bull. Environ. Contam. Toxicol. 2000, 65, 147–152. [Google Scholar] [CrossRef]
- Arienzo, M.; Albanese, S.; Lima, A.; Cannatelli, C.; Aliberti, F.; Cicotti, F.; Qi, S.; De Vivo, B. Assessment of the concentrations of polycyclic aromatic hydrocarbons and organochlorine pesticides in soils from the Sarno River basin, Italy, and ecotoxicological survey by Daphnia magna. Environ. Monit. Assess. 2015, 187, 52. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, K.B.; Cedergreen, N. Pesticide cocktails can interact synergistically on aquatic crustaceans. Environ. Sci. Pollut. Res. 2010, 17, 957–967. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wan, J.; Li, X.; Chu, S.; Sun, N.; Liu, R. Toxic effects of benzovindiflupyr, a new SDHI-type fungicide on earthworms (Eisenia fetida). Environ. Sci. Pollut. Res. 2021, 28, 62782–62795. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, K.; Sługocki, L. Short-term heat shock perturbation affects populations of Daphnia magna and Eurytemora carolleeae: A warning to the water thermal pollution. Sci. Rep. 2021, 11, 16909. [Google Scholar] [CrossRef]
- Margina, D.; Olaru, O.; Gutu, C.; Florea, L.; Gradinaru, D.; Purdel, C.; Ilie, M. Effects induced by natural extracts on Daphnia magna exposed to UV toxicity. Toxicol. Lett. 2015, 238, S286. [Google Scholar] [CrossRef]
- Zaldívar, J.M.; Baraibar, J. A biology-based dynamic approach for the reconciliation of acute and chronic toxicity tests: Application to Daphnia magna. Chemosphere 2011, 82, 1547–1555. [Google Scholar] [CrossRef]
- Rehman, A.; Chandio, A.A.; Hussain, I.; Jingdong, L. Fertilizer consumption, water availability and credit distribution: Major factors affecting agricultural productivity in Pakistan. J. Saudi Soc. Agric. Sci. 2020, 389, 122155. [Google Scholar] [CrossRef]
- Hare, L. Aquatic Insects and Trace Metals: Bioavailability, Bioaccumulation, and Toxicity. Crit. Rev. Toxicol. 1992, 22, 327–369. [Google Scholar] [CrossRef]
- Mebane, C.A.; Schmidt, T.S.; Miller, J.L.; Balistrieri, L.S. Bioaccumulation and Toxicity of Cadmium, Copper, Nickel, and Zinc and Their Mixtures to Aquatic Insect Communities. Environ. Toxicol. Chem. 2021, 40, 2374. [Google Scholar] [CrossRef]
- Cain, D.J.; Luoma, S.N.; Wallace, W.G. Linking metal bioaccumulation of aquatic insects to their distribution patterns in a mining-impacted river. Environ. Toxicol. Chem. 2004, 23, 1463–1473. [Google Scholar] [CrossRef]
- Closson, K.; Paul, E. Comparison of the toxicity of two chelated copper algaecides and copper sulfate to non-target fish. Bull. Environ. Contam. Toxicol. 2014, 93, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Mucci, M.; Fang, J.; Lürling, M. New is not always better: Toxicity of novel copper-based algaecides to Daphnia magna. Ecotoxicol. Environ. Saf. 2022, 241, 113817. [Google Scholar] [CrossRef]
- Fan, W.; Cui, M.; Liu, H.; Wang, C.; Shi, Z.; Tan, C.; Yang, X. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut. 2011, 159, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 63–147. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.V.; De Boeck, G.; Baken, S.; Fort, D.J. Adverse Outcome Pathways for Chronic Copper Toxicity to Fish and Amphibians. Environ. Toxicol. Chem. 2022, 41, 2911–2927. [Google Scholar] [CrossRef]
- Ishaque, A.B.; Johnson, L.; Gerald, T.; Boucaud, D.; Okoh, J. Assessment of Individual and Combined Toxicities of Four Non-Essential Metals (As, Cd, Hg and Pb) in the Microtox Assay. Int. J. Environ. Res. Public Health 2006, 3, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Cañizares-Villanueva, R.O.; Martínez-Jerónimo, F.; Espinosa-Chávez, F. Acute toxicity to Daphnia magna of effluents containing Cd, Zn, and a mixture Cd-Zn, after metal removal by Chlorella vulgaris. Environ. Toxicol. 2000, 15, 160–164. [Google Scholar] [CrossRef]
- Li, M.; Tang, T.; Yuan, F.; Zhang, Y.; Li, F.; Liu, F. Protective effects of small heat shock proteins in Daphnia magna against heavy metal exposure. Sci. Total Environ. 2022, 848, 157565. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated Reactive Oxygen Species Production and Oxidative Damage in Aquatic Organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Sanpradit, P.; Peerakietkhajorn, S. Disturbances in growth, oxidative stress, energy reserves and the expressions of related genes in Daphnia magna after exposure to ZnO under thermal stress. Sci. Total Environ. 2023, 869, 161682. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Canli, M.; Van Lierde, V.; Forrez, I.; Vanhaecke, F.; Janssen, C.R. Reproductive toxicity of dietary zinc to Daphnia magna. Aquat. Toxicol. 2004, 70, 233–244. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Muyssen, B.T.A.; Messiaen, M.; Janssen, C.R. Combined cadmium and temperature acclimation in Daphnia magna: Physiological and sub-cellular effects. Ecotoxicol. Environ. Saf. 2010, 73, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Heugens, E.H.W.; Jager, T.; Creyghton, R.; Kraak, M.H.S.; Hendriks, A.J.; Van Straalen, N.M.; Admiraal, W. Temperature-Dependent Effects of Cadmium on Daphnia magna: Accumulation versus Sensitivity. Environ. Sci. Technol. 2003, 37, 2145–2151. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Section 2. 2004, Test no. 202: Daphnia sp. Acute Imobilisation Test. Available online: https://doi.org/10.1787/9789264069947-en (accessed on 23 November 2024).
- Barata, C.; Baird, D.J.; Soares, A.M.V.M. Demographic responses of a tropical cladoceran to cadmium: Effects of food supply and density. Ecol. Appl. 2002, 12, 552–564. [Google Scholar] [CrossRef]
- Muyssen, B.T.A.; De Schamphelaere, K.A.C.; Janssen, C.R. Mechanisms of chronic waterborne Zn toxicity in Daphnia magna. Aquat. Toxicol. 2006, 77, 393–401. [Google Scholar] [CrossRef]
- Soininen, J.; Bartels, P.; Heino, J.; Luoto, M.; Hillebrand, H. Towards more integrated ecosystem research in aquatic and terrestrial environments. Environ. Pollut. 2015, 202, 6–10. [Google Scholar] [CrossRef]
- Baudouin, M.F.; Scoppa, P. The toxic effects of cadmium on Daphnia magna. Environ. Res. 1974, 7, 95–101. [Google Scholar]
- Bossuyt, B.T.A.; Janssen, C.R. Copper toxicity to different field-collected cladoceran species: Intra- and inter-species sensitivity. Environ. Pollut. 2005, 136, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bodar, C.W.; Hendriks, A.J.; Sijm, D.T. Chronic toxicity of copper and cadmium to Daphnia magna: Effect of exposure time on sensitivity. Ecotoxicol. Environ. Saf. 1988, 15, 72–78. [Google Scholar]
- Traudt, E.M.; Ranville, J.F.; Meyer, J.S. Effect of age on acute toxicity of cadmium, copper, nickel, and zinc in individual-metal exposures to Daphnia magna neonates, Environ. Toxicol. Chem. 2017, 36, 113–119. [Google Scholar] [CrossRef]
- Lari, E.; Gauthier, P.; Mohaddes, E.; Pyle, G. Interactive toxicity of Ni, Zn, Cu, and Cd on Daphnia magna at lethal and sub-lethal concentrations. J Hazard Mater. 2017, 334, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Paylar, B.; Bezabhe, Y.H.; Jass, J.; Olsson, P.-E. Exploring the Sublethal Impacts of Cu and Zn on Daphnia magna: A transcriptomic perspective. BMC Genom. 2024, 25, 790. [Google Scholar] [CrossRef]
- Rand, G.M. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Sprague, J.B. The ABCs of pollutant bioassay using fish. In Biological Methods for the Assessment of Water Quality; ASTM International: West Conshohocken, PA, USA, 1973. [Google Scholar]
- Newman, M.C.; Clements, W.H. Ecotoxicology: A Comprehensive Treatment; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- AAT Bioquest, Inc. Quest GraphTM LC50 Calculator. 2024. Available online: https://www.aatbio.com/tools/lc50-calculator (accessed on 20 November 2024).
- Rajaretnam, A.S.; Stanley, S.A. Studies on the toxicological effects of bimetals on the cladoceran, Daphnia magna and examination of histopathological effects through Transmission Electron Microscopy (TEM). J. Chem. Pharm. Res. 2015, 7, 506–511. [Google Scholar]
- Paylar, B.; Asnake, S.; Sjöberg, V.; Ragnvaldsson, D.; Jass, J.; Olsson, P.-E. Influence of water hardness on zinc toxicity in Daphnia magna. J. Appl. Toxicol. 2022, 42, 1510–1523. [Google Scholar] [CrossRef] [PubMed]


| Type of Pollutant | Heavy Metal Concentration in Water (mg/L) | Hatching Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| Time Range (Hours) | |||||||
| 42 h | 47 h | 52 h | 57 h | 62 h | 67 h | ||
| Cd2+ | 0.1 | 56 ± 1.30 | 65 ± 2.24 | 76 ± 6.26 | 88 ± 3.73 | 96 ± 2.73 | 99 ± 0.80 |
| 0.2 | 56 ± 2.27 | 60 ± 2.57 | 68 ± 4.38 | 79 ± 6.38 | 84 ± 3.34 | 92 ± 6.25 | |
| 0.3 | 56 ± 2.80 | 59 ± 0.32 | 63 ± 0.82 | 70 ± 0.77 | 75 ± 1.98 | 89 ± 7.64 | |
| 0.4 | 56 ± 2.83 | 57 ± 0.22 | 60 ± 0.35 | 72 ± 0.40 | 77 ± 0.86 | 82 ± 4.07 | |
| 0.5 | 48 ± 4.64 | 52 ± 0.56 | 58 ± 0.41 | 64 ± 0.46 | 68 ± 1.25 | 73 ± 2.11 | |
| 1.0 | 42 ± 3.66 | 46 ± 0.35 | 49 ± 0.39 | 52 ± 0.61 | 56 ± 1.23 | 58 ± 1.82 | |
| Zn2+ | 0.1 | 90 ± 4.23 | 94 ± 0.71 | 96 ± 2.20 | 97 ± 2.30 | 98 ± 0.71 | 99 ± 0.50 |
| 0.2 | 90 ± 2.07 | 96 ± 3.17 | 97 ± 3.04 | 98 ± 1.22 | 99 ± 0.14 | 99 ± 0.90 | |
| 0.3 | 90 ± 2.30 | 91 ± 1.62 | 93 ± 1.24 | 96 ± 3.02 | 98 ± 0.88 | 99 ± 0.72 | |
| 0.4 | 89 ± 1.80 | 90 ± 2.62 | 92 ± 1.35 | 94 ± 2.71 | 98 ± 1.26 | 99 ± 0.83 | |
| 0.5 | 89 ± 3.60 | 90 ± 1.87 | 91 ± 1.04 | 95 ± 1.96 | 97 ± 1.72 | 99 ± 0.96 | |
| 1.0 | 86 ± 2.83 | 88 ± 2.45 | 90 ± 0.96 | 93 ± 1.05 | 97 ± 0.96 | 99 ± 0.82 | |
| Cu2+ | 0.1 | 82 ± 1.13 | 86 ± 2.22 | 91 ± 4.06 | 95 ± 3.86 | 97 ± 2.02 | 99 ± 0.21 |
| 0.2 | 80 ± 1.72 | 83 ± 1.09 | 86 ± 3.43 | 89 ± 3.47 | 93 ± 1.48 | 96 ± 2.10 | |
| 0.3 | 77 ± 2.05 | 81 ± 1.12 | 83 ± 3.18 | 88 ± 2.35 | 90 ± 1.67 | 93 ± 1.82 | |
| 0.4 | 73 ± 3.52 | 76 ± 0.75 | 80 ± 2.09 | 82 ± 1.65 | 85 ± 1.08 | 87 ± 1.47 | |
| 0.5 | 70 ± 1.04 | 72 ± 0.63 | 77 ± 2.25 | 78 ± 1.12 | 79 ± 1.14 | 81 ± 0.75 | |
| 1.0 | 64 ± 1.08 | 68 ± 0.24 | 70 ± 0.72 | 72 ± 0.21 | 73 ± 0.23 | 74 ± 0.44 | |
| Type of Pollutant | Equation | LC50 | ||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |
| Zn2+ | 0.42 | 0.39 | 0.33 | |||
| Cd2+ | 0.19 | 0.03 | 0.31 | |||
| Cu2+ | 0.18 | 0.02 | 1.70 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, P.; Pastia, M.-C.; Biali, G.; Cojocaru, C. Heavy Metal Toxicity on Daphnia magna. Environments 2025, 12, 70. https://doi.org/10.3390/environments12030070
Cojocaru P, Pastia M-C, Biali G, Cojocaru C. Heavy Metal Toxicity on Daphnia magna. Environments. 2025; 12(3):70. https://doi.org/10.3390/environments12030070
Chicago/Turabian StyleCojocaru, Paula, Maria-Cătălina Pastia, Gabriela Biali, and Cristian Cojocaru. 2025. "Heavy Metal Toxicity on Daphnia magna" Environments 12, no. 3: 70. https://doi.org/10.3390/environments12030070
APA StyleCojocaru, P., Pastia, M.-C., Biali, G., & Cojocaru, C. (2025). Heavy Metal Toxicity on Daphnia magna. Environments, 12(3), 70. https://doi.org/10.3390/environments12030070







