Cytosolic Distribution of Metals (Cd, Cu) and Metalloids (As, Se) in Livers and Gonads of Field-Collected Fish Exposed to an Environmental Contamination Gradient: An SEC-ICP-MS Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Collection and Selection of Individuals for SEC-ICP-MS Analysis
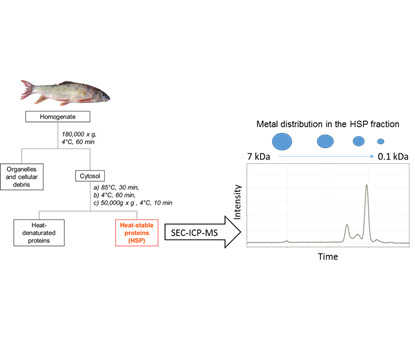
2.2. HSP Fraction Isolation for the Liver and Gonads of Selected Fish
2.3. SEC-ICP-MS Analysis of HSP Fractions
2.4. Metal Quantification in Hepatic and Gonadal HSP Fractions
2.5. Quality Control
2.6. Data Processing
3. Results and Discussion
3.1. Biomolecule UV Profiles of White Sucker Liver and Gonads
3.2. Distribution of Trace Elements in Hepatic and Gonadal HSP Fractions of White Suckers ([M]HSP)
3.2.1. Copper (Cu)
3.2.2. Cadmium (Cd)
3.2.3. Arsenic (As)
3.2.4. Selenium (Se)
4. Conclusions
Implications Regarding How the HSP Fraction Should Be Interpreted in Future Studies of Subcellular Metal Partitioning
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Total Metal Concentrations Determination in the Whole Liver and Gonads of Collected White Suckers
References
- Wang, W.-X. Prediction of metal toxicity in aquatic organisms. Chin. Sci. Bull. 2013, 58, 194–202. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Hare, L. Metal detoxification in freshwater animals. Roles of metallothioneins. In Metallothioneins and Related Chelators; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Royal Society of Chemistry: Cambridge, UK, 2009; Volume 5, pp. 239–277. [Google Scholar]
- Mason, A.Z.; Jenkins, K.D. Metal detoxification in aquatic organisms. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D., Eds.; IUPAC Series on Analytical and Physical Chemistry of Environmental Systems; J. Wiley & Sons: Chichester, UK, 1995; pp. 479–608. [Google Scholar]
- Wallace, W.G.; Lee, B.-G.; Luoma, S.N. Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rainbow, P.S. Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ. Chem. 2006, 3, 395–399. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Vasak, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 1992, 22, 81–113. [Google Scholar] [CrossRef]
- Giguère, A.; Campbell, P.G.C.; Hare, L.; Couture, P. Sub-cellular partitioning of cadmium, copper, nickel and zinc in indigenous yellow perch (Perca flavescens) sampled along a polymetallic gradient. Aquat. Toxicol. 2006, 77, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Urien, N.; Cooper, S.; Caron, A.; Sonnenberg, H.; Rozon-Ramilo, L.; Campbell, P.G.C.; Couture, P. Subcellular partitioning of metals and metalloids (As, Cd, Cu, Se and Zn) in liver and gonads of wild white suckers (Catostomus commersonii) collected downstream from a mining operation. Aquat. Toxicol. 2018, 202, 105–116. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 30 August 2018).
- Klaverkamp, J.F.; Dutton, M.D.; Majewski, H.S.; Hunt, R.V.; Wesson, L.J. Evaluating the effectiveness of metal pollution controls in a smelter by using metallothionein and other biochemical responses in fish. In Metal Ecotoxicology—Concepts and Applications; Newman, M.C., McIntosh, A.W., Eds.; Lewis Publishers Ltd.: Chelsea, MI, USA, 1991; pp. 33–64. [Google Scholar]
- Van Campenhout, K.; Infante, H.G.; Goemans, G.; Belpaire, C.; Adams, F.; Blust, R.; Bervoets, L. A field survey of metal binding to metallothionein and other cytosolic ligands in liver of eels using an on-line isotope dilution method in combination with size exclusion (SE) high pressure liquid chromatography (HPLC) coupled to Inductively Coupled Plasma time-of-flight Mass Spectrometry (ICP-TOFMS). Sci. Total Environ. 2008, 394, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Infante, H.G.; Campenhout, K.V.; Schaumlöffel, D.; Blust, R.; Adams, F.C. Multi-element speciation of metalloproteins in fish tissue using size-exclusion chromatography coupled “on-line” with ICP-isotope dilution-time-of-flight-mass spectrometry. Analyst 2003, 128, 651–657. [Google Scholar] [CrossRef]
- Krasnići, N.; Dragun, Z.; Erk, M.; Ramani, S.; Jordanova, M.; Rebok, K.; Kostov, V. Size-exclusion HPLC analysis of trace element distributions in hepatic and gill cytosol of Vardar chub (Squalius vardarensis Karaman) from mining impacted rivers in north-eastern Macedonia. Sci. Total Environ. 2018, 613–614, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Krasnići, N.; Dragun, Z.; Erk, M.; Raspor, B. Distribution of selected essential (Co, Cu, Fe, Mn, Mo, Se, and Zn) and nonessential (Cd, Pb) trace elements among protein fractions from hepatic cytosol of European chub (Squalius cephalus L.). Environ. Sci. Pollut. Res. 2013, 20, 2340–2351. [Google Scholar] [CrossRef] [PubMed]
- Infante, H.G.; Van Campenhout, K.; Blust, R.; Adams, F.C. Anion-exchange high performance liquid chromatography hyphenated to inductively coupled plasma-isotope dilution-time-of-flight mass spectrometry for speciation analysis of metal complexes with metallothionein isoforms in gibel carp (Carassius auratus gibelio) exposed to environmental metal pollution. J. Chromatogr. A 2006, 1121, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Rosabal, M.; Drevet, O.; Couture, P.; Campbell, P.G.C. Binding of trace elements (Ag, Cd, Co, Cu, Ni, and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal contamination gradients. Environ. Toxicol. Chem. 2018, 37, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Krasnići, N.; Dragun, Z.; Erk, M.; Raspor, B. Distribution of Co, Cu, Fe, Mn, Se, Zn, and Cd among cytosolic proteins of different molecular masses in gills of European chub (Squalius cephalus L.). Environ. Sci. Pollut. Res. 2014, 13512–13521. [Google Scholar] [CrossRef]
- Albores, A.; Koropatnick, J.; Cherian, M.G.; Zelazowski, A.J. Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem. Biol. Interact. 1992, 85, 127–140. [Google Scholar] [CrossRef]
- Kreppel, H.; Bauman, J.W.; Liu, J.; McKim, J.M., Jr.; Klaassen, C.D. Induction of metallothionein by arsenicals in mice. Fundam. Appl. Toxicol. 1993, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; Santos, H.M.; Costa, P.M.; Peres, I.; Costa, M.H.; Capelo, J.L. Metallothionein responses in the Asiatic clam (Corbicula fluminea) after exposure to trivalent arsenic. Biomarkers 2007, 12, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.M.; Wang, W.X. Biotransformation and detoxification of inorganic arsenic in a marine juvenile fish Terapon jarbua after waterborne and dietborne exposure. J. Hazard. Mater. 2012, 221, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.-F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; MacDonald, T.C.; Saibu, Y.; George, G.N.; Niyogi, S.; (University of Saskatchewan, Saskatoon, SK, Canada). Personal communication, 2017.
- Ballihaut, G.; Pécheyran, C.; Mounicou, S.; Preud’homme, H.; Grimaud, R.; Lobinski, R. Multimode detection (LA-ICP-MS, MALDI-MS and nanoHPLC-ESI-MS2) in 1D and 2D gel electrophoresis for selenium-containing proteins. Trends Anal. Chem. 2007, 26, 183–190. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. FEBS J. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [Green Version]
- Pappas, A.C.; Zoidis, E.; Surai, P.F.; Zervas, G. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Lemly, A.D. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Monit. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.M.; DeForest, D.K.; Brooks, M.L.; Chapman, P.M.; Gilron, G.; Hoff, D.; Hopkins, W.A.; McIntyre, D.O.; Mebane, C.A.; Palace, V.P. Selenium toxicity to aquatic organisms. In Ecological Assessment of Selenium in the Aquatic Environment; Chapman, P.M., Adams, W.J., Brooks, M.L., Delos, C.G., Luoma, S.N., Maher, W.A., Ohlendorf, H.M., Presser, T.S., Shaw, D.P., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 141–231. ISBN 978-1-4398-2678-2. [Google Scholar]
- Muscatello, J.R.; Bennett, P.M.; Himbeault, K.T.; Belknap, A.M.; Janz, D.M. Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ. Sci. Technol. 2006, 40, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.; Behne, D. Selenium-containing proteins in mammals and other forms of life. In Reviews of Physiology, Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Blaustein, M.P., Grunicke, H., Jahn, R., Lederer, W.J., Miyajima, A., Murer, H., Pfanner, N., Schultz, G., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–46. ISBN 3-540-43520-4. [Google Scholar]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Yamashita, M. Identification of a Novel Selenium-containing Compound, Selenoneine, as the Predominant Chemical Form of Organic Selenium in the Blood of Bluefin Tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anan, Y.; Ishiwata, K.; Suzuki, N.; Tanabe, S.; Ogra, Y. Speciation and identification of low molecular weight selenium compounds in the liver of sea turtles. J. Anal. At. Spectrom. 2011, 26, 80–85. [Google Scholar] [CrossRef]
- Adams, W.J.; Blust, R.; Borgmann, U.; Brix, K.V.; DeForest, D.K.; Green, A.S.; Meyer, J.S.; McGeer, J.C.; Paquin, P.R.; Rainbow, P.S.; et al. Utility of tissue residues for predicting effects of metals on aquatic organisms. Integr. Environ. Assess. Manag. 2011, 7, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Rosabal, M.; Mounicou, S.; Hare, L.; Campbell, P.G.C. Metal (Ag, Cd, Cu, Ni, Tl, and Zn) Binding to Cytosolic Biomolecules in Field-Collected Larvae of the Insect Chaoborus. Environ. Sci. Technol. 2016, 50, 3247–3255. [Google Scholar] [CrossRef] [PubMed]





| Molecular Weight Standard | Retention Time (Rt, in min) | Molecular Weight (MW, in kDa) |
|---|---|---|
| Carbonic anhydrase (CA) | 13.8 | 10 |
| Rabbit metallothionein-2 (MT) | 15.8 | 6.8 |
| Cobalamin (B12) | 26 | 1.3 |
| Cysteine (Cys) | 29.3 | 0.12 |
| Organ | Sex | n | Lake | Cu | HSP Contribution | As | HSP Contribution | Se | HSP Contribution | Cd | HSP Contribution | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Female | 4 | Reference | 426 (140) | A | 64% | 0.42 (0.02) | A | 36% | 3.63 (0.59) | A | 12% | 0.435 (0.1) * | A | 61% |
| 3 | Exposed | 678 (142) | A | 63% | 0.59 (0.04) | B | 31% | 40.8 (4.7) | B | 11% | 4.86 (1.71) | B | 68% | ||
| [M]max/[M]min | 1.6 | 1.4 | 11.2 | 11.2 | |||||||||||
| Male | 2 | Reference | 629 (41) | A | 69% | 0.53 (0.21) | A | 29% | 4.31 (0.35) | A | 14% | 0.813 (0.032) | A | 70% | |
| 3 | Exposed | 630 (104) | A | 61% | 0.85 (0.06) | B | 45% | 32.5 (1.5) | B | 13% | 3.803 (0.767) | B | 63% | ||
| [M]max/[M]min | 1.0 | 1.6 | 7.5 | 4.7 | |||||||||||
| Gonads | Female | 4 | Reference | 133 (15) | A | 68% | 0.92 (0.14) | A | 55% | 3.65 (0.15) | A | 25% | 0.053 (0.016) | A | 26% |
| 3 | Exposed | 221 (74) | A | 68% | 0.90 (0.09) | A | 52% | 26.5 (5.9) | B | 12% | 0.116 (0.006) | B | 37% | ||
| [M]max/[M]min | 1.7 | 1.0 | 7.3 | 2.2 | |||||||||||
| Male | 2 | Reference | 7.1 (5.6) | A | 17% | 0.42 (0.06) | A | 35% | 1.38 (0.73) | A | 21% | 0.017 (0.002) | A | 14% | |
| 3 | Exposed | 11 (2) | A | 23% | 1.05 (0.10) | B | 32% | 20.9 (1.5) | B | 15% | 0.091 (0.016) * | B | 19% | ||
| [M]max/[M]min | 1.5 | 2.5 | 15.1 | 5.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urien, N.; Jacob, S.; Couture, P.; Campbell, P.G.C. Cytosolic Distribution of Metals (Cd, Cu) and Metalloids (As, Se) in Livers and Gonads of Field-Collected Fish Exposed to an Environmental Contamination Gradient: An SEC-ICP-MS Analysis. Environments 2018, 5, 102. https://doi.org/10.3390/environments5090102
Urien N, Jacob S, Couture P, Campbell PGC. Cytosolic Distribution of Metals (Cd, Cu) and Metalloids (As, Se) in Livers and Gonads of Field-Collected Fish Exposed to an Environmental Contamination Gradient: An SEC-ICP-MS Analysis. Environments. 2018; 5(9):102. https://doi.org/10.3390/environments5090102
Chicago/Turabian StyleUrien, Nastassia, Sabrina Jacob, Patrice Couture, and Peter G. C. Campbell. 2018. "Cytosolic Distribution of Metals (Cd, Cu) and Metalloids (As, Se) in Livers and Gonads of Field-Collected Fish Exposed to an Environmental Contamination Gradient: An SEC-ICP-MS Analysis" Environments 5, no. 9: 102. https://doi.org/10.3390/environments5090102
APA StyleUrien, N., Jacob, S., Couture, P., & Campbell, P. G. C. (2018). Cytosolic Distribution of Metals (Cd, Cu) and Metalloids (As, Se) in Livers and Gonads of Field-Collected Fish Exposed to an Environmental Contamination Gradient: An SEC-ICP-MS Analysis. Environments, 5(9), 102. https://doi.org/10.3390/environments5090102