Factors Affecting Alkali Activation of Laterite Acid Leaching Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis AAMs
2.3. Characterization of Raw Materials and the Produced AAMs
3. Results
3.1. Reactivity of Raw Materials
3.2. Properties of AAMs
3.2.1. Alkali Activation of Laterite Leaching Residues
3.2.2. Effect of the Addition of Metakaolin—Calcination of Leaching Residues
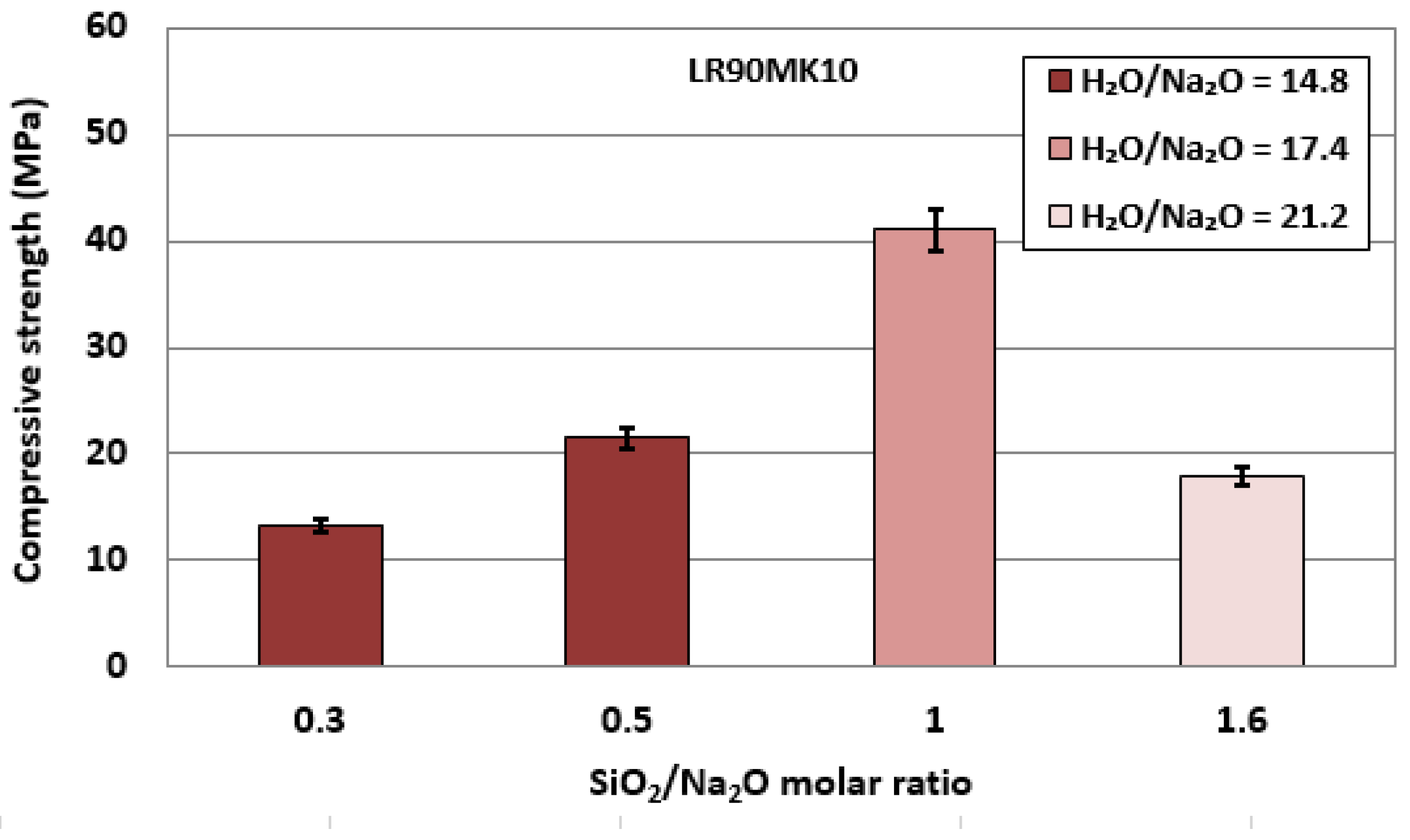
3.2.3. Effect of SiO2/Na2O and H2O/Na2O Molar Ratios in the Activating Solution
3.2.4. Effect of Pre-Curing and Curing Time
3.2.5. Porosity, Water Absorption, and Apparent Density of Selected AMMs
3.2.6. Structural Integrity of LR90MK10 AAM
3.2.7. Comparison with Other Studies
3.3. Mineralogy-Morphology of AAMs
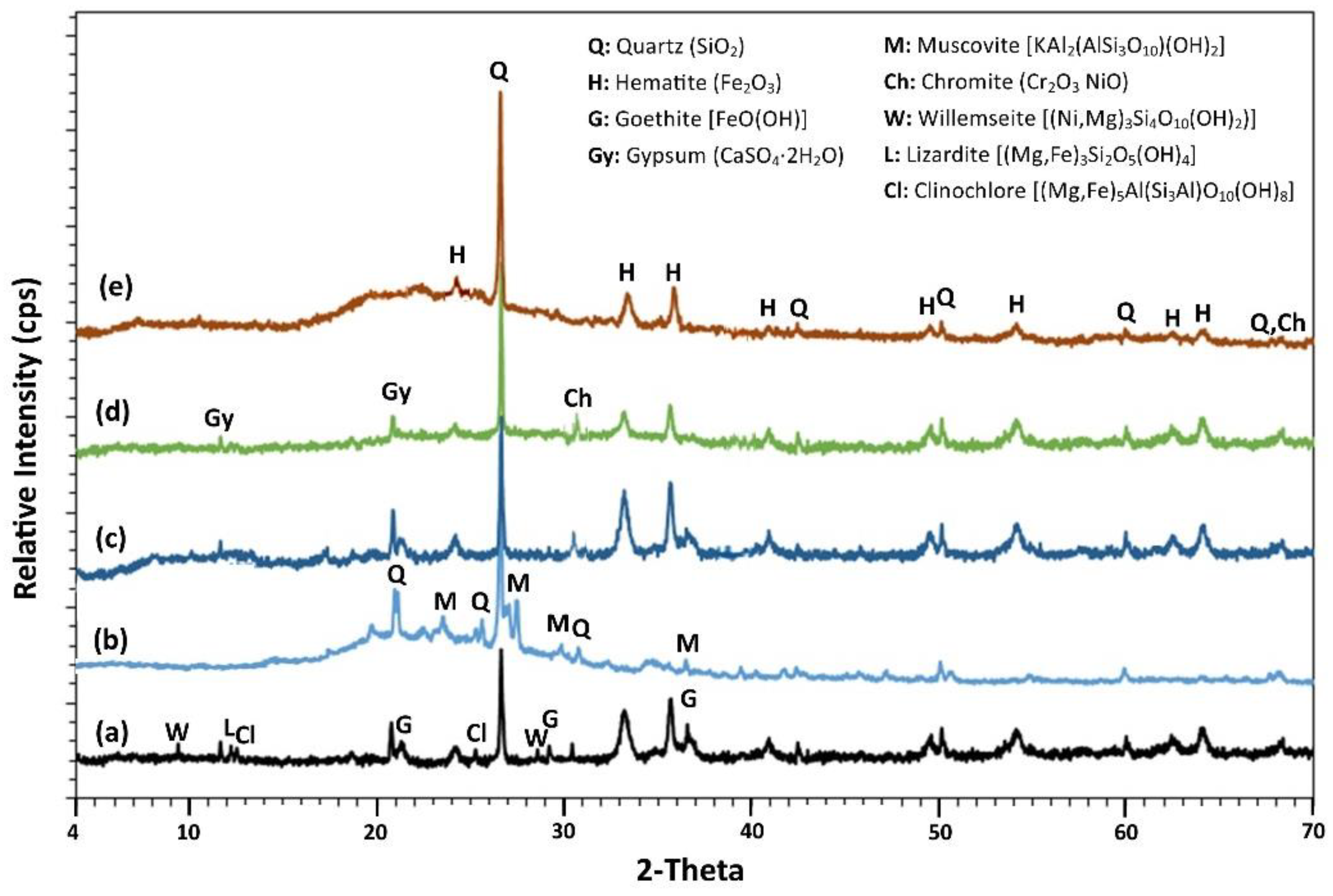
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
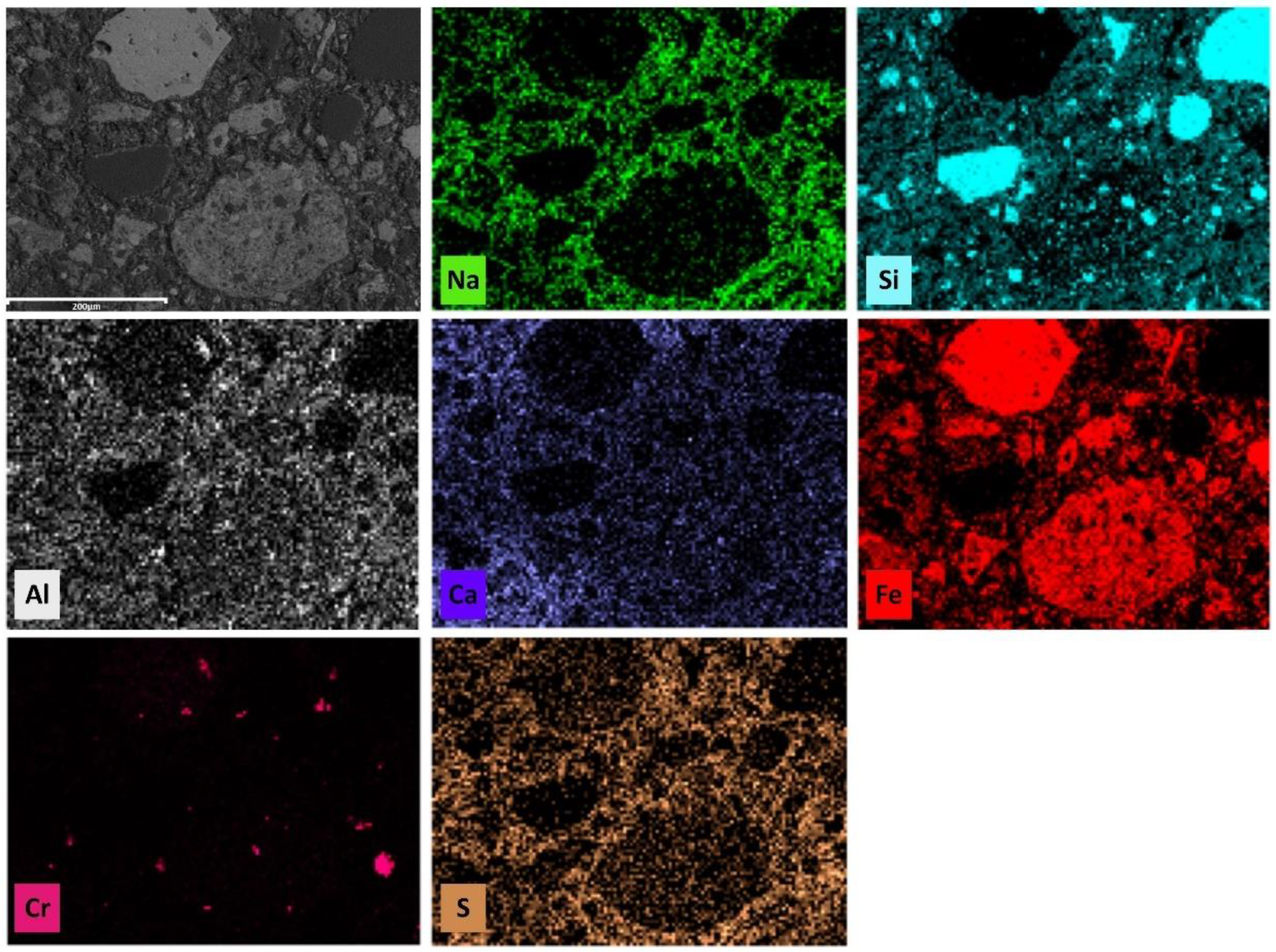
3.3.3. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oraby, E.A.; Eksteen, J.J.; Karrech, A.; Attar, M. Gold extraction from paleochannel ores using an aerated alkaline glycine lixiviant for consideration in heap and in-situ leaching applications. Miner. Eng. 2019, 138, 112–118. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Thenepalli, T.; Chilakala, R.; Habte, L.; Tuan, L.Q.; Kim, C.S. A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals. Sustainability 2019, 11, 3347. [Google Scholar] [CrossRef]
- Spooren, J.; Breemersch, K.; Dams, Y.; Mäkinen, J.; Lopez, M.; González-Moya, M.; Tripiana, M.; Pontikes, Y.; Kurylak, W.; Pietek, G.; et al. Near-zero-waste processing of low-grade, complex primary and secondary ores: Challenges and opportunities. Resour. Conserv. Recy. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Arpalahti, A.; Lundström, M. The leaching behavior of minerals from a pyrrhotite-rich pentlandite ore during heap leaching. Miner. Eng. 2018, 119, 116–125. [Google Scholar] [CrossRef]
- Komnitsas, C.; Pooley, F.D. Mineralogical characteristics and treatment of refractory gold ores. Miner. Eng. 1989, 2, 449–457. [Google Scholar] [CrossRef]
- Oxley, A.; Smith, M.E.; Caceres, O. Why heap leach nickel laterites? Miner. Eng. 2016, 88, 53–60. [Google Scholar] [CrossRef]
- Ram, R.; Beiza, L.; Becker, M.; Pownceby, M.I.; Chen, M.; Yang, Y.; Yang, S.; Petersen, J. Study of the leaching and pore evolution in large particles of a sulfide ore. Hydrometallurgy 2020, 192, 105261. [Google Scholar] [CrossRef]
- Ulrich, B.; Andrade, H.; Gardner, T. Lessons learnt from heap leaching operations in South America—An update. J. S. Afr. Inst. Min. Metall. 2003, 103, 23–28. [Google Scholar]
- Catalan, L.J.J.; Li, M.G. Decommissioning of copper heap-leach residues by rinsing with water and alkaline solutions—A pilot scale study. Environ. Eng. Sci. 2000, 17, 191–202. [Google Scholar] [CrossRef]
- Gaulier, C.; Billon, G.; Lesven, L.; Falantin, C.; Superville, P.J.; Baeyens, W.; Gao, Y. Leaching of two northern France slag heaps: Influence on the surrounding aquatic environment. Environ. Pollut. 2020, 257, 113601. [Google Scholar] [CrossRef] [PubMed]
- Lupo, J.F. Sustainable issues related to heap leaching operations. J. S. Afr. Inst. Min. Metall. 2012, 112, 1021–1030. [Google Scholar]
- McDonald, G.R.; Li, J.; Austin, P.J. High temperature pressure oxidation of a low-grade nickel sulfide concentrate with control of the residue composition. Minerals 2020, 10, 249. [Google Scholar] [CrossRef]
- Zevgolis, E.; Zografidis, C.; Halikia, I. The reducibility of the Greek nickeliferous laterites: A review. Miner. Process. Ext. Metall. 2010, 119, 9–17. [Google Scholar] [CrossRef]
- Petrakis, E.; Karmali, V.; Komnitsas, K. Factors affecting nickel upgrade during selective grinding of low–grade limonitic laterites. Miner. Process. Ext. Metall. 2018, 1–10. [Google Scholar] [CrossRef]
- Putzolu, F.; Balassone, G.; Boni, M.; Maczurad, M.; Mondillo, N.; Najorka, J.; Pirajno, F. Mineralogical association and Ni-Co deportment in the Wingellina oxide-type laterite deposit (Western Australia). Ore Geol. Rev. 2018, 97, 21–34. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Pantelaki, O.; Kritikaki, A. Column leaching of saprolitic laterites with sulphuric acid. In Proceedings of the 10th European Metallurgical Conference, EMC 2019, Dusseldorf, Germany, 23–26 June 2019; Volume 1, pp. 273–284. [Google Scholar]
- Bartzas, G.; Komnitsas, K. Life cycle assessment of ferronickel production in Greece. Resour. Conserv. Recy. 2015, 105, 113–122. [Google Scholar] [CrossRef]
- Panagiotopoulos, N.; Agatzini, S.; Kontopoulos, A. Extraction of nickel and cobalt from serpentinic type laterites by atmospheric pressure sulfuric acid leaching. In Proceedings of the 115th TMS-AIME Annual Meeting, New Orleans, LA, USA, 2–6 March 1986; p. A86-30. [Google Scholar]
- Mystrioti, C.; Papassiopi, N.; Xenidis, A.; Komnitsas, K. Counter-Current Leaching of Low-Grade Laterites with Hydrochloric Acid and Proposed Purification Options of Pregnant Solution. Minerals 2018, 8, 599. [Google Scholar] [CrossRef]
- Kontopoulos, A.; Komnitsas, K. Sulphuric acid pressure leaching of low-grade Greek laterites. In Proceedings of the 1st International Symposium on Hydrometallurgy, Beijing, China, 12–15 October 1988; Zheng, Y., Xu, J., Eds.; Pergamon Press: Oxford, UK, 1988; pp. 140–144. [Google Scholar]
- Agatzini-Leonardou, S.; Zafiratos, J.G.; Spathis, D. Beneficiation of a Greek serpentinic nickeliferous ore: Part I. Mineral processing. Hydrometallurgy 2004, 74, 259–265. [Google Scholar] [CrossRef]
- Agatzini-leonardou, S.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Pantelaki, O.; Kritikaki, A. Column leaching of Greek low-grade limonitic laterites. Minerals 2018, 8, 377. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part 1–Physical evolution. J. Non Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali—Activated fly ashes, a cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation. A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Melo, U.C.; Cui, X.-M. Recent developments on inorganic polymers synthesis and applications. Ceram. Int. 2016, 42, 15142–15159. [Google Scholar] [CrossRef]
- Peys, A.; Douvalis, A.P.; Hallet, V.; Rahier, H.; Blanpain, B.; Pontikes, Y. Inorganic polymers from CaO-FeOx-SiO2 Slag: The start of oxidation of Fe and the formation of a mixed valence binder. Front. Mater. Sci. 2019, 6, 212. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewics, J. Assessment of alkali activation potential of a Polish ferronickel slag. Sustainability 2019, 11, 1863. [Google Scholar] [CrossRef]
- Azevedo, A.G.D.S.; Strecker, K. Brazilian fly ash based inorganic polymers production using different alkali activator solutions. Ceram. Int. 2017, 43, 9012–9018. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Gisèle, L.-N.; Djétéli, G.; Blanchart, P. Kaolin–based geopolymers: Effect of mechanical activation and curing process. Constr. Build. Mater. 2013, 42, 105–113. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin–based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Soultana, A.; Valouma, A.; Bartzas, G.; Komnitsas, K. Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag. Minerals 2019, 9, 551. [Google Scholar] [CrossRef]
- Alshaaer, M. Synthesis and characterization of self-healing geopolymer composite. Constr. Build. Mater. 2020, 245, 118432. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud)–Putting things in perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Studies on eco–friendly concrete incorporating industrial waste as aggregates. Int. J. Sustain. Built Environ. 2015, 4, 378–390. [Google Scholar] [CrossRef]
- Peys, A.; Douvalis, A.P.; Siakati, C.; Rahier, H.; Blanpain, B.; Pontikes, Y. The influence of air and temperature on the reaction mechanism and molecular structure of Fe-silicate inorganic polymers. J. Non Cryst. Solids 2019, 526, 119675. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 229–327. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.F.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Oyelami, C.A.; Van Rooy, J.L. A review of the use of lateritic soils in the construction/development of sustainable housing in Africa: A geological perspective. J. Afr. Earth Sci. 2016, 119, 226–237. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Bartzas, G.; Karmali, V. Column leaching of low–grade saprolitic laterites and valorization of leaching residues. Sci. Total Environ. 2019, 665, 347–357. [Google Scholar] [CrossRef]
- Konan, K.L.; Peyratout, C.; Smith, A.; Bonnet, J.-P.; Rossignol, S.; Oyetola, S. Comparison of surface properties between kaolin and metakaolin in concentrated lime solutions. J. Colloid Interface Sci. 2009, 339, 103–109. [Google Scholar] [CrossRef]
- Kakali, G.; Perrraki, T.; Tsivilis, S.; Badogiannis, E. Thermal Treatment of Kaolin: The Effect of Mineralogy on the Pozzolanic Activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Shvarzman, A.; Kovler, K.; Grader, G.S.; Shter, G.E. The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite. Cem. Concr. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- British Standards Institute. BS EN 1936: Natural Stone Test Methods. Determination of Real Density and Apparent Density and of Total and Open Porosity; NP EN 1936:2006; BSI: London, UK, 2007. [Google Scholar]
- Ascensão, G.; Marchi, M.; Segata, M.; Faleschini, F.; Pontikes, Y. Reaction kinetics and structural analysis of alkali activated Fe–Si–Ca rich materials. J. Clean. Prod. 2020, 246, 119065. [Google Scholar] [CrossRef]
- Aughenbaugh, K.L.; Williamson, T.; Juenger, M.C.G. Critical evaluation of strength prediction methods for alkali-activated fly ash. Mater. Struct. 2015, 48, 607–620. [Google Scholar] [CrossRef]
- Bumanis, G.; Vitola, L.; Bajare, D.; Dembovska, L.; Pundiene, I. Impact of reactive SiO2/Al2O3 ratio in precursor on durability of porous alkali activated materials. Ceram. Int. 2017, 43, 5471–5477. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, A.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.-H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Tennakoon, C.; De Silva, P.; Sagoe-Crentsil, K.; Sanjayan, J.G. Influence and role of feedstock Si and Al content in Geopolymer synthesis. J. Sust. Cem Based Mater. 2014, 4, 129–139. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 11852. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Dong, T.; Ouyang, G. Structural and mechanical properties of geopolymers made of aluminosilicate powder with different SiO2/Al2O3 ratio: Molecular dynamics simulation and microstructural experimental study. Constr. Build. Mater. 2020, 240, 117935. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, T.; Yang, Z.; Wang, C.; Wu, H.-C. Influence of different activators on microstructure and strength of alkali-activated nickel slag cementitious materials. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Kpinsoton, G.M.R.; Karoui, H.; Richardson, Y.; Koffi, B.N.D.S.; Yacouba, H.; Motuzas, J.; Drobek, M.; Lawane Gana, A. New insight into the microstructure of natural calcined laterites and their performance as heterogeneous Fenton catalyst for methylene blue degradation. Reac. Kinet. Mech. Cat. 2018, 124, 931–956. [Google Scholar] [CrossRef]
- Mascarin, L. Characterization and Thermodynamic Modelling of Alkali-Activated Calcined Clays: Potentiality of Cameroon’s Laterites as Eco-Sustainable Binders. Master’s Thesis, Department of Geosciences, University of Padua, Padova, Italy, 19 July 2018. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Kamseu, E.; Billong, N.; Melo, U.C.; Cui, X.-M. Effect of slag and calcium carbonate addition on the development of geopolymer from indurated laterite. Appl. Clay Sci. 2017, 148, 109–117. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and microstructure of metakaolin based geopolymers: Effect of fly ash and liquid/solid contents. Materials (Basel) 2019, 12, 3485. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; He, P.; Jia, D.; Yang, C.; Zhang, Y.; Yan, S.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effect of curing temperature and SiO2/K2O molar ratio on the performance of metakaolin-based geopolymers. Ceram. Int. 2016, 42, 16184–16190. [Google Scholar] [CrossRef]
- Jackson, P.R.; Radford, D.W. Effect of initial cure time on toughness of geopolymer matrix composites. Ceram. Int. 2017, 43, 9884–9890. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Chinje Melo, U.F.; Delplancke, M.-P.; Rahier, H. Influence of the activating solution composition on the stability and thermo-mechanical properties of inorganic polymers (geopolymers) from volcanic ash. Constr. Build. Mater. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Sietins, J.M.; Granados-Focil, S.; Pepi, M.S.; Yan Xu, Y.; Tao, M. Reaction kinetics of red mud-fly ash based geopolymers: Effects of curing temperature on chemical bonding, porosity, and mechanical strength. Cem. Concr. Comp. 2018, 93, 175–185. [Google Scholar] [CrossRef]
- Kaze, C.R.; Venyite, P.; Nana, A.; Juvenal, D.N.; Tchakoute, H.K.; Rahier, H.; Kamseu, E.; Melo, U.C.; Leonelli, C. Meta–halloysite to improve compactness in iron–rich laterite–based alkali activated materials. Mater. Chem. Phys. 2020, 239, 122268. [Google Scholar] [CrossRef]
- Heap, M.J.; Lavallée, Y.; Laumann, A.; Hess, K.-U.; Meredith, P.G.; Dingwell, D.B.; Huismann, S.; Weise, F. The influence of thermal-stressing (up to 1000 °C) on the physical, mechanical, and chemical properties of siliceous-aggregate, high-strength concrete. Constr. Build. Mater. 2013, 42, 248–265. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Romagnoli, M.; Pollastri, S.; Gualtieri, A.F. Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: Mechanical and microstructural properties. Cem. Concr. Res. 2015, 67, 259–270. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Sukmak, P.; Chinkulkijniwat, A.; Arulrajah, A.; Shen, S.-L. Stabilisation of marginal lateritic soil using high calcium fly ash–based geopolymer. Road Mater. Pavement Des. 2016, 17, 877–891. [Google Scholar] [CrossRef]
- Nkwaju, R.Y.; Djobo, J.N.Y.; Nouping, J.N.F.; Huisken, P.W.M.; Deutou, J.G.N.; Courard, L. Iron–rich laterite-bagasse fibers based geopolymer composite: Mechanical, durability and insulating properties. Appl. Clay Sci. 2019, 183, 105333. [Google Scholar] [CrossRef]
- Obonyo, E.A.; Kamseu, E.; Lemougna, P.N.; Tchamba, A.B.; Melo, U.C.; Leonelli, C.A. Sustainable approach for the geopolymerization of natural iron–rich aluminosilicate. Sustainability 2014, 6, 5535–5553. [Google Scholar] [CrossRef]
- Yi, C.; Boluk, Y.; Bindiganavile, V. Enhancing alkali-activation of metakaolin-based geopolymers using dry water. J. Clean. Prod. 2020, 258, 120676. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Comparison of metakaolin-based geopolymer cements from commercial sodium waterglass and sodium waterglass from rice husk ash. J. Sol Gel Sci. Technol. 2016, 78, 492–506. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and Mechanical Properties of Alkali Activated Colombian Raw Materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Kaze, C.R.; Tchakoute, H.K.; Mbakop, T.T.; Mache, J.R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Synthesis and properties of inorganic polymers (geopolymers) derived from Cameroon–meta–halloysite. Ceram. Int. 2018, 44, 18499–18508. [Google Scholar] [CrossRef]
- Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Yliniemi, J.; Illikainen, M. Mechanical trans-formation of phyllite mineralogy toward its use as alkali-activated binderprecursor. Miner. Eng. 2020, 145, 106093. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Vimmrová, A.; Keppert, M.; Michalko, O.; Černý, R. Calcined gypsum–lime–metakaolin binders: Design of optimal composition. Cem. Concr. Comp. 2014, 52, 91–96. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Gluth, G.J.G.; Pistol, K. In-situ thermo-mechanical testing of fly ash geopolymer concretes made with quartz and expanded clay aggregates. Cem. Concr. Res. 2016, 80, 33–43. [Google Scholar] [CrossRef]






| Component (wt%) | Leaching Residues (LR) | Metakaolin (MK) |
|---|---|---|
| SiO2 | 30.8 | 54.2 |
| Al2O3 | 2.3 | 40.3 |
| Cr2O3 | 2.5 | 0.02 |
| Na2O | - | 1.3 |
| Fe2O3 | 50.9 | 0.6 |
| MgO | 2.1 | 0.3 |
| NiO | 0.1 | - |
| K2O | - | 2.4 |
| TiO2 | 0.6 | 0.4 |
| CoO | 0.02 | <0.00097 |
| MnO | 0.3 | 0.01 |
| CaO | 3.7 | 0.1 |
| P2O5 | - | 0.5 |
| SO3 | - | 0.3 |
| ZnO | - | <0.00003 |
| LOI * | 6.2 | - |
| Total | 99.5 | 100.1 |
| Particle Size | μm | μm |
| d90 | 48.2 | 25.5 |
| d50 | 9.9 | 8.8 |
| AAM Code | Solids (wt%) | NaOH (M) | NaOH (wt%) | H2O (wt%) | Na2SiO3 (wt%) | L/S Ratio * | H2O/Na2O ** | SiO2/Na2O ** |
|---|---|---|---|---|---|---|---|---|
| LR1 | 78.1 | 6 | 2.2 | 8.8 | 10.9 | 0.25 | 21.2 | 1 |
| LR2 | 75.6 | 8 | 3.1 | 9.1 | 12.2 | 0.27 | 17.4 | 1 |
| LR3 | 73.4 | 10 | 4.0 | 9.3 | 13.3 | 0.29 | 14.8 | 1 |
| MK | 52.0 | 8 | 6.0 | 18.0 | 24.0 | 0.92 | 17.4 | 1 |
| 800LR | 76.0 | 8 | 3.0 | 8.9 | 12.0 | 0.26 | 17.4 | 1 |
| 1000LR | ||||||||
| LR95MK5 | 74.6 | 8 | 3.2 | 9.5 | 12.7 | 0.29 | 17.4 | 1 |
| 800LR95MK5 | ||||||||
| 1000LR95M5 | ||||||||
| LR90MK10 | 75.8 | 8 | 3.1 | 9.0 | 12.1 | 0.27 | 17.4 | 1 |
| 800LR90MK10 | ||||||||
| 1000LR90MK10 | ||||||||
| LR90MK10-1 | 82.7 | 8 | 3.5 | 10.4 | 3.4 | 0.16 | 14.8 | 0.3 |
| LR90MK10-2 | 80.8 | 3.4 | 10.2 | 5.6 | 0.19 | 14.8 | 0.5 | |
| LR90MK10-3 | 75.0 | 2.0 | 7.0 | 16.0 | 0.30 | 21.2 | 1.6 |
| Raw Material | Si (mg L−1) | Al (mg L−1) | Si/Al (Molar Ratio) |
|---|---|---|---|
| Leaching residue | 20.1 ± 1.7 | 2.4 ± 0.4 | 8.4 ± 2.2 |
| Leaching residue calcined at 800 °C | 12.9 ± 0.9 | 0.6 ± 0.1 | 21.5 ± 5.1 |
| Leaching residue calcined at 1000 °C | 2.0 ± 0.3 | 0.4 ± 0.1 | 5.4 ± 2.0 |
| Metakaolin | 58.7 ± 3.2 | 41.9 ± 2.3 | 1.4 ± 0.2 |
| AAM Code | Compressive Strength (MPa) * | Porosity (%) | Water Absorption (%) | Apparent Density (g cm−3) |
|---|---|---|---|---|
| LR2 | 1.43 | 36.8 | 30.7 | 1.8 |
| LR95MK5 | 13.3 | 27.9 | 13.1 | 2.2 |
| LR90MK10 | 41.0 | 21.3 | 5.1 | 2.3 |
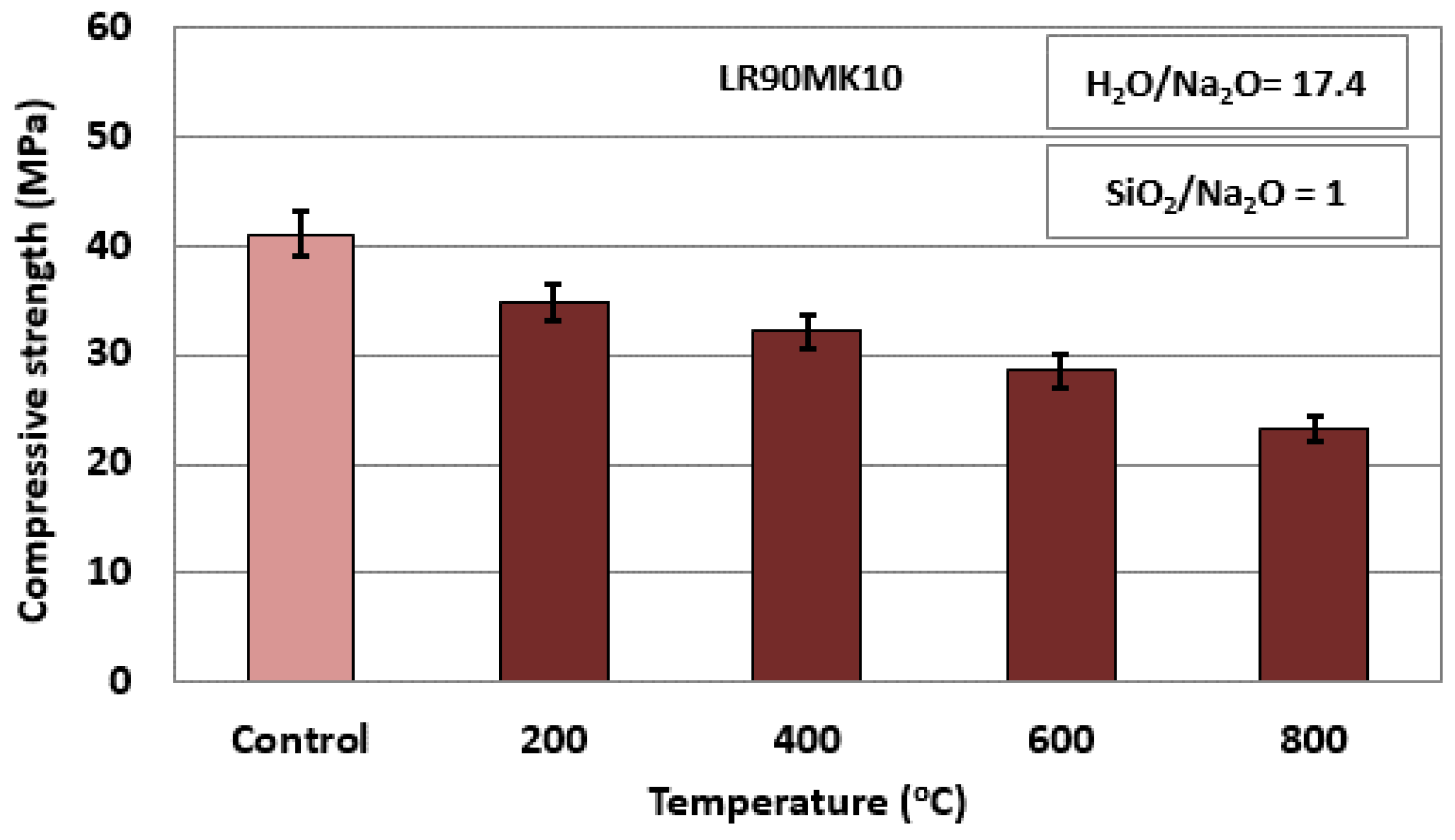
| Temperature (°C) | Compressive Strength (MPa) | Weight Loss (%) | Shrinkage (%) | Apparent Density (g cm−3) | Porosity (%) | Water Absorption (%) |
|---|---|---|---|---|---|---|
| Control AAM | 41.0 | - | - | 2.3 | 21.3 | 5.1 |
| 200 | 34.8 | 6.3 | 3.2 | 2.0 | 24.8 | 8.2 |
| 400 | 32.1 | 7.6 | 4.3 | 2.1 | 25.1 | 10.8 |
| 600 | 28.5 | 11.2 | 7.3 | 1.8 | 25.0 | 11.3 |
| 800 | 23.1 | 12.1 | 7.5 | 1.8 | 27.2 | 14.8 |
| Raw Materials | Sio2/Na2O Molar Ratio | Conditions | Compressive Strength (MPa) | Reference | |
|---|---|---|---|---|---|
| Temperature (°C) | Ageing Period (Days) | ||||
| Laterite (calcined at 700 °C for 2 h) and metakaolin | 3.0 | 60 | 28 | 4 | [76] |
| Marginal lateritic soil and fly ash | 5.6 | 27–30 | 7 | 7.1 | [77] |
| Iron-rich laterite (calcined at 700 °C for 4 h) and bagasse fibers | 1.4 | 25 | 28 | 14–50 | [78] |
| Laterite (calcined at 700 °C for 2 h) and slag | 1.6 | 25 | 28 | 65 | [67] |
| Laterite (calcined at 600 °C) and metahalloysite (calcined halloysite clay at 600 °C) | 0.75 | 25 ± 3 | 28 | 45 | [73] |
| Laterite leaching residues and metakaolin | 1.0 | 80 | 7 | 45 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E. Factors Affecting Alkali Activation of Laterite Acid Leaching Residues. Environments 2021, 8, 4. https://doi.org/10.3390/environments8010004
Komnitsas K, Bartzas G, Karmali V, Petrakis E. Factors Affecting Alkali Activation of Laterite Acid Leaching Residues. Environments. 2021; 8(1):4. https://doi.org/10.3390/environments8010004
Chicago/Turabian StyleKomnitsas, Konstantinos, Georgios Bartzas, Vasiliki Karmali, and Evangelos Petrakis. 2021. "Factors Affecting Alkali Activation of Laterite Acid Leaching Residues" Environments 8, no. 1: 4. https://doi.org/10.3390/environments8010004
APA StyleKomnitsas, K., Bartzas, G., Karmali, V., & Petrakis, E. (2021). Factors Affecting Alkali Activation of Laterite Acid Leaching Residues. Environments, 8(1), 4. https://doi.org/10.3390/environments8010004








