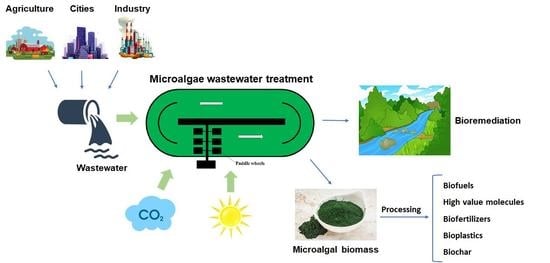
A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe
Abstract
:1. Introduction
1.1. State of the Art
1.2. Goal of the Present Study
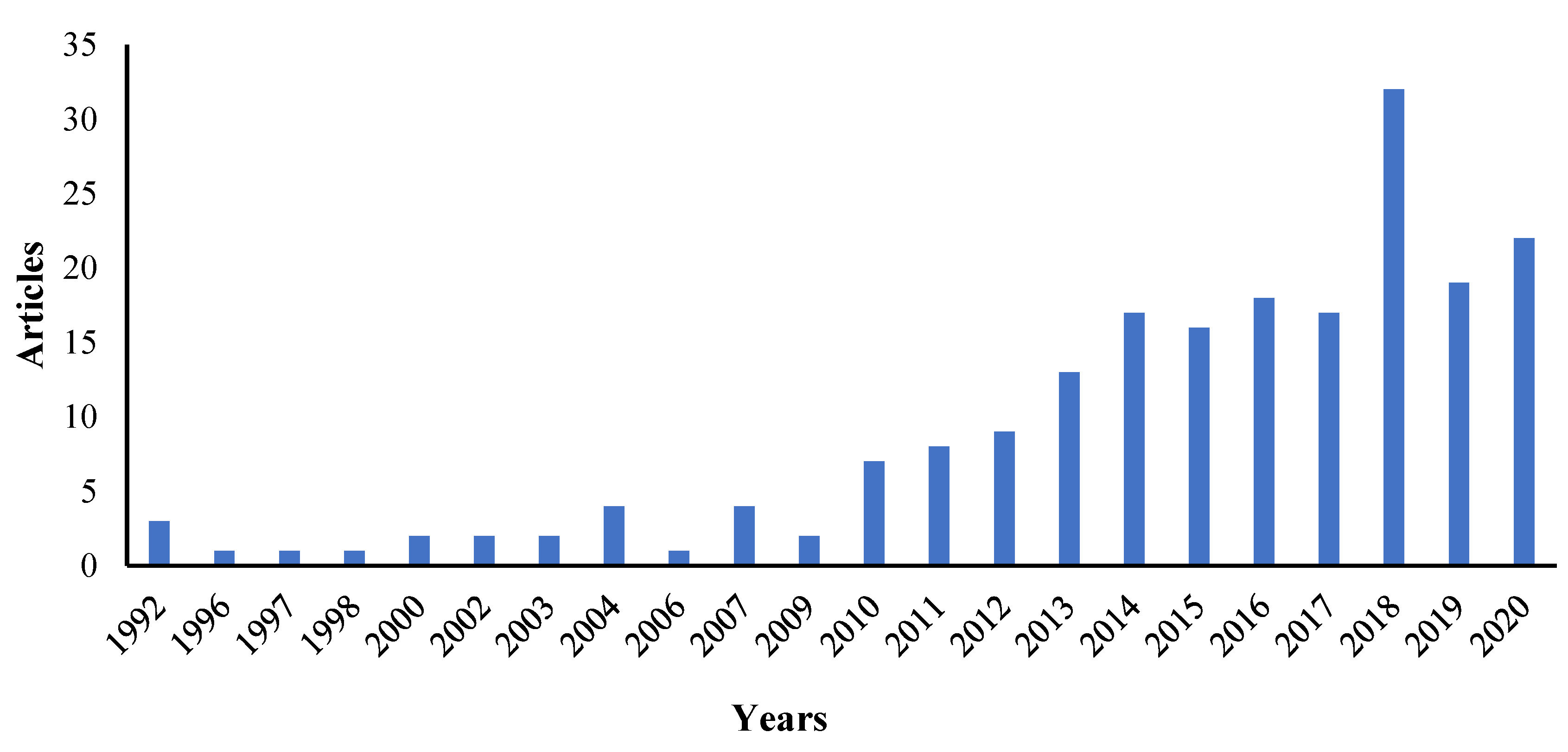
2. Methods
3. Results
3.1. Microalgae Cultivation Technologies
3.1.1. Extensive Systems
3.1.2. High-Rate Algal Pond Systems (HRAPs)
3.1.3. PBRs (Photobioreactors)
3.2. Cultivation on Different Effluents
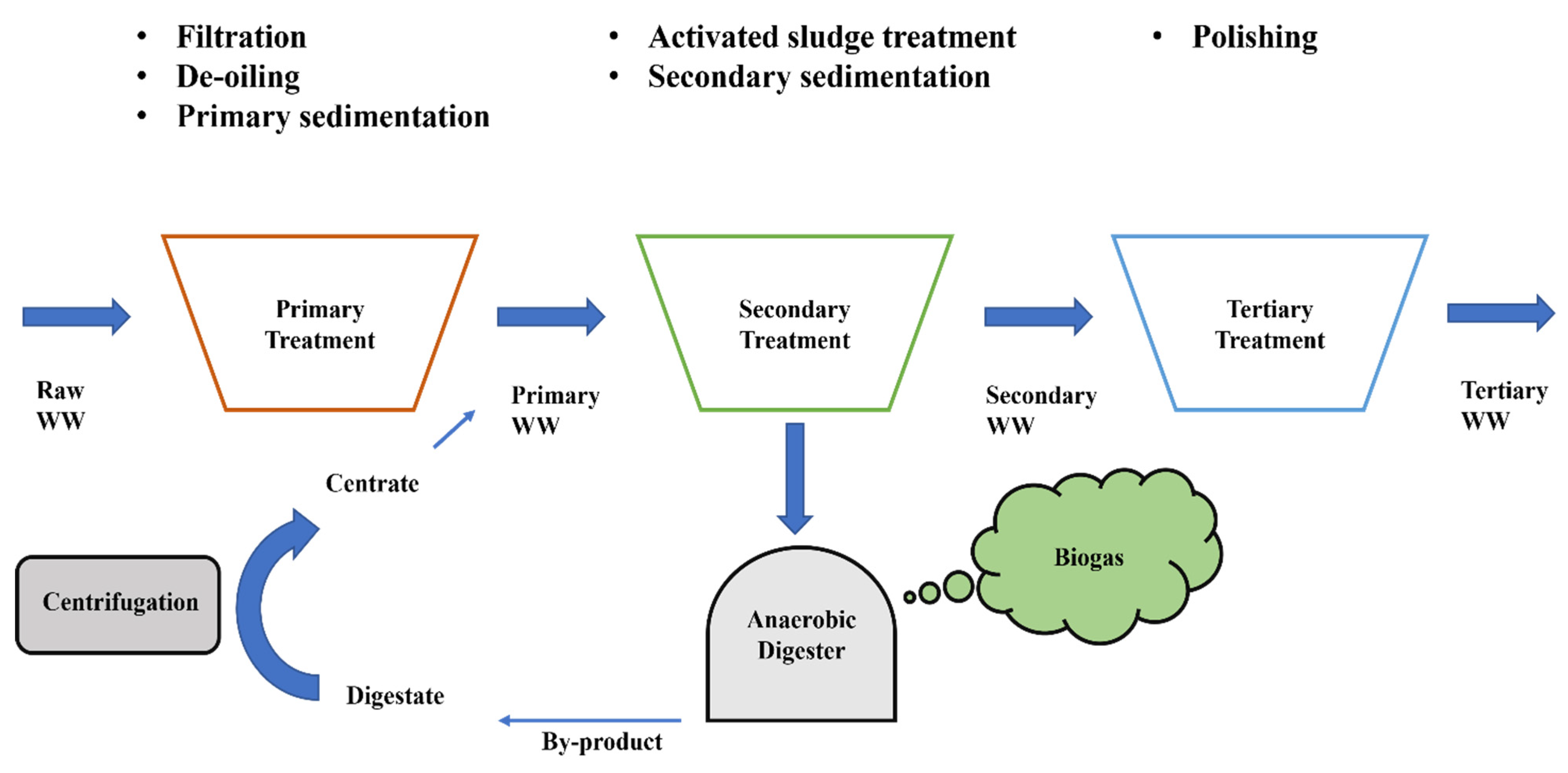
3.2.1. Raw and Primary Wastewaters
3.2.2. Secondary Wastewaters
3.2.3. Urban Wastewaters with Other Substrates: Glycerol/Glucose, Centrate and Secondary Effluents from Anaerobic Digestion
4. Discussion
4.1. Microalgae Genera
4.2. Driving Forces in Wastewater Bioremediation by Microalgae
4.3. Removal of Contaminants from Wastewater
4.3.1. Removal of N, P and COD
4.3.2. Removal of Heavy Metals, Xenobiotics and Pathogens
4.4. Secondary Treatment with Microalgae
4.4.1. Secondary Treatment with Cocultivation Microalgae–Bacteria
4.4.2. Secondary Treatment with Microalgae with Mixotrophic Metabolism
4.5. Potential Products Obtainable from Algal Biomass
4.6. Main Bottlenecks and Future Perspectives
4.6.1. Soil Requirement
4.6.2. Availability of Light
4.6.3. Biomass Harvesting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMW | Anaerobic digestate municipal wastewater |
| BioH2 | Biohydrogen |
| BOD | Biochemical oxygen demand |
| BPBR | Bubble column photobioreactors |
| BRT | Biomass retention time |
| CO2 | Carbon dioxide |
| COD | Chemical oxygen demand |
| ESP | Sticky polymeric exopolysaccharides |
| EPS | Extracellular polymeric substances |
| H2 | Hydrogen |
| H2O | Water |
| HRAP | High-rate algal pond systems |
| HRAP-noPT | Algal ponds without primary treatment |
| HRAP-PT | Algal ponds with primary treatment |
| HRTs | Hydraulic retention time |
| N | Nitrogen |
| NH4+ | Ammonia |
| NO3 | Nitrates |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| O2 | Oxygen |
| P | Phosporus |
| PBR | Photobioreactor |
| PHB | Polyhydroxybutarrate |
| PO43− | Phosphates |
| PSBR | Photosequencing batch reactors |
| PTFE | Polytetrafluoroethylene |
| RBCs | Rotating biological contactors |
| SBR | Sequence Batch Reactor |
| SS | Suspended solids |
| SWW | Synthetic wastewater |
| TSS | Total suspended solids |
| TL | Twin-Layer |
| VSS | Volatile suspended solids |
| WSP | Waste stabilization ponds |
References
- Lima, S.; Villanova, V.; Grisafi, F.; Caputo, G.; Brucato, A.; Scargiali, F. Autochthonous microalgae grown in municipal wastewaters as a tool for effectively removing nitrogen and phosphorous. J. Water Process Eng. 2020, 38, 101647. [Google Scholar] [CrossRef]
- Paddock, M.B. Microalgae Wastewater Treatment: A Brief History. Preprints 2019, 2019120377. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Arbib, Z.; Álvarez-Díaz, P.D.; Garrido-Pérez, C.; Barragán, J.; Perales, J.A. Photobiotreatment model (PhBT): A kinetic model for microalgae biomass growth and nutrient removal in wastewater. Environ. Technol. 2013, 34, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.G. Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Bioresour. Technol. 2014, 169, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zamalloa, C.; Boon, N.; Verstraete, W. Decentralized two-stage sewage treatment by chemical-biological flocculation combined with microalgae biofilm for nutrient immobilization in a roof installed parallel plate reactor. Bioresour. Technol. 2013, 130, 152–160. [Google Scholar] [CrossRef]
- Abinandan, S.; Shanthakumar, S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2015, 52, 123–132. [Google Scholar] [CrossRef]
- European Commission. European Commission Directive 91/271/EEC on Urban Wastewater Treatment. 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31991L0271 (accessed on 9 July 2021).
- Commission Directive 98/15/EC of 27 February 1998 Amending Council Directive 91/271/EEC with Respect to Certain Requirements Established in Annex I thereof (Text with EEA Relevance)—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/ff7ec087-8cc3-4619-bffc-b08ea4883d2c (accessed on 16 November 2021).
- Urban Waste Water Treatment in Europe—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/urban-waste-water-treatment/urban-waste-water-treatment-assessment-5 (accessed on 9 November 2020).
- Ho, L.; Goethals, P.L.M. Municipal wastewater treatment with pond technology: Historical review and future outlook. Ecol. Eng. 2020, 148, 105791. [Google Scholar] [CrossRef]
- Petrini, S.; Foladori, P.; Donati, L.; Andreottola, G. Comprehensive respirometric approach to assess photosynthetic, heterotrophic and nitrifying activity in microalgal-bacterial consortia treating real municipal wastewater. Biochem. Eng. J. 2020, 161, 107697. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020, 710, 135583. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater photobiotreatment with microalgae in a continuously operated photobioreactor: Growth, nutrient removal kinetics and biomass coagulation–flocculation. Environ. Technol. 2019, 40, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res. 2020, 52, 102090. [Google Scholar] [CrossRef]
- Ruiz, J.; Álvarez-Díaz, P.D.; Arbib, Z.; Garrido-Pérez, C.; Barragán, J.; Perales, J.A. Performance of a flat panel reactor in the continuous culture of microalgae in urban wastewater: Prediction from a batch experiment. Bioresour. Technol. 2013, 127, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Miksch, K.; Cema, G.; Corvini, P.F.-X.; Felis, E.; Sochacki, A.; Surmacz-Górska, J.; Wiszniowski, J.; Zabczynski, S. R&D priorities in the field of sustainable remediation and purification of agro-industrial and municipal wastewater. New Biotechnol. 2015, 32, 128–132. [Google Scholar] [CrossRef]
- Ferro, L.; Gorzsás, A.; Gentili, F.G.; Funk, C. Subarctic microalgal strains treat wastewater and produce biomass at low temperature and short photoperiod. Algal Res. 2018, 35, 160–167. [Google Scholar] [CrossRef]
- Peralta, E.; Jerez, C.G.; Figueroa, F.L. Centrate grown Chlorella fusca (Chlorophyta): Potential for biomass production and centrate bioremediation. Algal Res. 2019, 39, 101458. [Google Scholar] [CrossRef]
- Sforza, E.; Ramos-Tercero, E.A.; Gris, B.; Bettin, F.; Milani, A.; Bertucco, A. Integration of Chlorella protothecoides production in wastewater treatment plant: From lab measurements to process design. Algal Res. 2014, 6, 223–233. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Solimeno, A.; García, J. Microalgae and bacteria dynamics in high rate algal ponds based on modelling results: Long-term application of BIO_ALGAE model. Sci. Total Environ. 2019, 650, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Bussa, M.; Zollfrank, C.; Röder, H. Life-cycle assessment and geospatial analysis of integrating microalgae cultivation into a regional economy. J. Clean. Prod. 2020, 243, 118630. [Google Scholar] [CrossRef]
- Biomass Production, Supply, Uses and Flows in the European Union: First Results from an Integrated Assessment|EU Science Hub. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/biomass-production-supply-uses-and-flows-european-union-first-results-integrated-assessment (accessed on 16 January 2021).
- Robles, Á.; Capson-Tojo, G.; Galès, A.; Ruano, M.V.; Sialve, B.; Ferrer, J.; Steyer, J.-P. Microalgae-bacteria consortia in high-rate ponds for treating urban wastewater: Elucidating the key state indicators under dynamic conditions. J. Environ. Manag. 2020, 261, 110244. [Google Scholar] [CrossRef] [PubMed]
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The environmental biorefinery: Using microalgae to remediate wastewater, a win-win paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Romero Villegas, G.I.; Fiamengo, M.; Acién Fernández, F.G.; Molina Grima, E. Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res. 2017, 25, 538–548. [Google Scholar] [CrossRef]
- Development of an Innovative Algae Based Tertiary Wastewater Treatment and Value Recovery System|INDALG Project|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/733718 (accessed on 1 August 2021).
- Microalgae Protein Ingredients for the Food and Feed of the Future|Profuture Project|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/862980 (accessed on 1 August 2021).
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process Saf. Environ. Prot. 2015, 94, 285–306. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef]
- Faleschini, M.; Esteves, J.L.; Camargo Valero, M.A. The Effects of Hydraulic and Organic Loadings on the Performance of a Full-Scale Facultative Pond in a Temperate Climate Region (Argentine Patagonia). Water Air Soil Pollut. 2011, 223, 2483–2493. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.-T.; Suleiman Al Ahmad, M.; Yeh, R.Y.-L.; Liu, R.L.-H.; Fu, Y.-P. Oxidation pond for municipal wastewater treatment. Appl. Water Sci. 2015, 7, 31–51. [Google Scholar] [CrossRef] [Green Version]
- González-Camejo, J.; Ferrer, J.; Seco, A.; Barat, R. Outdoor microalgae-based urban wastewater treatment: Recent advances, applications, and future perspectives. Wiley Interdiscip. Rev. Water 2021, 8, e1518. [Google Scholar] [CrossRef]
- Ramos Tercero, E.A.; Sforza, E.; Morandini, M.; Bertucco, A. Cultivation of Chlorella protothecoides with urban wastewater in continuous photobioreactor: Biomass productivity and nutrient removal. Appl. Biochem. Biotechnol. 2014, 172, 1470–1485. [Google Scholar] [CrossRef] [PubMed]
- Thorin, E.; Olsson, J.; Schwede, S.; Nehrenheim, E. Co-digestion of sewage sludge and microalgae—Biogas production investigations. Appl. Energy 2018, 227, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Arbib, Z.; Ruiz, J.; álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Long term outdoor operation of a tubular airlift pilot photobioreactor and a high rate algal pond as tertiary treatment of urban wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Doria, E.; Longoni, P.; Scibilia, L.; Iazzi, N.; Cella, R.; Nielsen, E. Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J. Appl. Phycol. 2012, 24, 375–383. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale twin-layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef]
- Kotoula, D.; Iliopoulou, A.; Irakleous-Palaiologou, E.; Gatidou, G.; Aloupi, M.; Antonopoulou, P.; Fountoulakis, M.S.; Stasinakis, A.S. Municipal wastewater treatment by combining in series microalgae Chlorella sorokiniana and macrophyte Lemna minor: Preliminary results. J. Clean. Prod. 2020, 271, 122704. [Google Scholar] [CrossRef]
- Robles, Á.; Capson-Tojo, G.; Gales, A.; Viruela, A.; Sialve, B.; Seco, A.; Steyer, J.-P.; Ferrer, J. Performance of a membrane-coupled high-rate algal pond for urban wastewater treatment at demonstration scale. Bioresour. Technol. 2020, 301, 122672. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, L.T.; Ferrer, I.; Rousseau, D.P.L.; Van Hulle, S.W.H.; Garfí, M. The effect of primary treatment of wastewater in high rate algal pond systems: Biomass and bioenergy recovery. Bioresour. Technol. 2019, 280, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ferro, L.; Colombo, M.; Posadas, E.; Funk, C.; Muñoz, R. Elucidating the symbiotic interactions between a locally isolated microalga Chlorella vulgaris and its co-occurring bacterium Rhizobium sp. in synthetic municipal wastewater. J. Appl. Phycol. 2019, 31, 2299–2310. [Google Scholar] [CrossRef]
- Sforza, E.; Pastore, M.; Spagni, A.; Bertucco, A. Microalgae-bacteria gas exchange in wastewater: How mixotrophy may reduce the oxygen supply for bacteria. Environ. Sci. Pollut. Res. 2018, 25, 28004–28014. [Google Scholar] [CrossRef] [PubMed]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.D.; Ruiz, J.; Arbib, Z.; Barragán, J.; Garrido-Pérez, M.C.; Perales, J.A. Freshwater microalgae selection for simultaneous wastewater nutrient removal and lipid production. Algal Res. 2017, 24, 477–485. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Rossi, S.; Fornaroli, R.; Ficara, E.; Mezzanotte, V. Outdoor pilot trial integrating a sidestream microalgae process for the treatment of centrate under non optimal climate conditions. Algal Res. 2019, 39, 101430. [Google Scholar] [CrossRef] [Green Version]
- Şirin, S.; Sillanpää, M. Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour. Technol. 2015, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Caporgno, M.P.; Taleb, A.; Olkiewicz, M.; Font, J.; Pruvost, J.; Legrand, J.; Bengoa, C. Microalgae cultivation in urban wastewater: Nutrient removal and biomass production for biodiesel and methane. Algal Res. 2015, 10, 232–239. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Eriksson, K.; Sellstedt, A. Mixotrophic and heterotrophic production of lipids and carbohydrates by a locally isolated microalga using wastewater as a growth medium. Bioresour. Technol. 2018, 257, 260–265. [Google Scholar] [CrossRef]
- Tao, R.; Kinnunen, V.; Praveenkumar, R.; Lakaniemi, A.-M.; Rintala, J.A. Comparison of Scenedesmus acuminatus and Chlorella vulgaris cultivation in liquid digestates from anaerobic digestion of pulp and paper industry and municipal wastewater treatment sludge. J. Appl. Phycol. 2017, 29, 2845–2856. [Google Scholar] [CrossRef]
- Hultberg, M.; Olsson, L.E.; Birgersson, G.; Gustafsson, S.; Sievertsson, B. Microalgal growth in municipal wastewater treated in an anaerobic moving bed biofilm reactor. Bioresour. Technol. 2016, 207, 19–23. [Google Scholar] [CrossRef]
- Evans, L.; Hennige, S.J.; Willoughby, N.; Adeloye, A.J.; Skroblin, M.; Gutierrez, T. Effect of organic carbon enrichment on the treatment efficiency of primary settled wastewater by Chlorella vulgaris. Algal Res. 2017, 24, 368–377. [Google Scholar] [CrossRef]
- Hodaifa, G.; Sánchez, S.; Martínez, M.E.; Órpez, R. Biomass production of Scenedesmus obliquus from mixtures of urban and olive-oil mill wastewaters used as culture medium. Appl. Energy 2013, 104, 345–352. [Google Scholar] [CrossRef]
- Mendez, L.; Sialve, B.; Tomás-Pejó, E.; Ballesteros, M.; Steyer, J.P.; González-Fernández, C. Comparison of Chlorella vulgaris and cyanobacterial biomass: Cultivation in urban wastewater and methane production. Bioprocess Biosyst. Eng. 2016, 39, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae biodiesel production in China: A preliminary economic analysis. Renew. Sustain. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Ledda, C.; Romero Villegas, G.I.; Adani, F.; Acién Fernández, F.G.; Molina Grima, E. Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Arbib, Z.; Ruiz, J.; Alvarez, P.; Garrido, C.; Barragan, J.; Perales, J.A. Chlorella stigmatophora for Urban Wastewater Nutrient Removal and CO2 Abatement. Int. J. Phytoremediat. 2012, 14, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Szwaja, S.; Zieliński, M.; Kisielewska, M.; Stańczyk-Mazanek, E. The Influence of Anaerobic Digestion Effluents (ADEs) Used as the Nutrient Sources for Chlorella sp. Cultivation on Fermentative Biogas Production. Waste Biomass Valoriz. 2017, 8, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Uggetti, E.; Sialve, B.; Hamelin, J.; Bonnafous, A.; Steyer, J.-P. CO2 addition to increase biomass production and control microalgae species in high rate algal ponds treating wastewater. J. CO2 Util. 2018, 28, 292–298. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Effect of pH control by means of flue gas addition on three different photo-bioreactors treating urban wastewater in long-term operation. Ecol. Eng. 2013, 57, 226–235. [Google Scholar] [CrossRef]
- Iasimone, F.; De Felice, V.; Panico, A.; Pirozzi, F. Experimental study for the reduction of CO2 emissions in wastewater treatment plant using microalgal cultivation. J. CO2 Util. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Andersson, V.; Broberg Viklund, S.; Hackl, R.; Karlsson, M.; Berntsson, T. Algae-based biofuel production as part of an industrial cluster. Biomass Bioenergy 2014, 71, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Foladori, P.; Petrini, S.; Andreottola, G. How suspended solids concentration affects nitrification rate in microalgal-bacterial photobioreactors without external aeration. Heliyon 2020, 6, e03088. [Google Scholar] [CrossRef] [Green Version]
- Galès, A.; Bonnafous, A.; Carré, C.; Jauzein, V.; Lanouguère, E.; Le Floc’h, E.; Pinoit, J.; Poullain, C.; Roques, C.; Sialve, B.; et al. Importance of ecological interactions during wastewater treatment using High Rate Algal Ponds under different temperate climates. Algal Res. 2019, 40, 101508. [Google Scholar] [CrossRef]
- Koreiviene, J.; Valčiukas, R.; Karosiene, J.; Baltrenas, P. Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J. Environ. Eng. Landsc. Manag. 2014, 22, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the Heat Tolerance of Photosynthesis and the Thermal Stability of Rubisco Activase in Plants from Contrasting Thermal Environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [Green Version]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Khiewwijit, R.; Rijnaarts, H.; Temmink, H.; Keesman, K.J. Glocal assessment of integrated wastewater treatment and recovery concepts using partial nitritation/Anammox and microalgae for environmental impacts. Sci. Total Environ. 2018, 628–629, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ferro, L.; Gentili, F.G.; Funk, C. Isolation and characterization of microalgal strains for biomass production and wastewater reclamation in Northern Sweden. Algal Res. 2018, 32, 44–53. [Google Scholar] [CrossRef]
- Di Termini, I.; Prassone, A.; Cattaneo, C.; Rovatti, M. On the nitrogen and phosphorus removal in algal photobioreactors. Ecol. Eng. 2011, 37, 976–980. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Niemi, C.; Ferro, L.; Gorzsas, A.; Gentili, F.G.; Funk, C.; Sellstedt, A. Screening suitability of northern hemisphere algal strains for heterotrophic cultivation and fatty acid methyl ester production. Molecules 2020, 25, 2107. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Pistocchi, R. Nutrient removal efficiency and physiological responses of Desmodesmus communis at different HRTs and nutrient stress condition using different sources of urban wastewater effluents. Appl. Biochem. Biotechnol. 2014, 173, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.H.; Cheng, C.L.; Guo, W.Q.; Nagarajan, D.; Ren, N.Q.; Lee, D.J.; Chang, J.S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.M.Y.; Wong, Y.S.; Tam, N.F.Y. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 2000, 41, 251–257. [Google Scholar] [CrossRef]
- Bellucci, M.; Marazzi, F.; Naddeo, L.S.; Piergiacomo, F.; Beneduce, L.; Ficara, E.; Mezzanotte, V. Disinfection and nutrient removal in laboratory-scale photobioreactors for wastewater tertiary treatment. J. Chem. Technol. Biotechnol. 2020, 95, 959–966. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Caskan, N.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Algal-based, single-step treatment of urban wastewaters. Bioresour. Technol. 2015, 189, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Sforza, E.; Pastore, M.; Santeufemia Sanchez, S.; Bertucco, A. Bioaugmentation as a strategy to enhance nutrient removal: Symbiosis between Chlorella protothecoides and Brevundimonas Diminuta. Bioresour. Technol. Rep. 2018, 4, 153–158. [Google Scholar] [CrossRef]
- Kohlheb, N.; van Afferden, M.; Lara, E.; Arbib, Z.; Conthe, M.; Poitzsch, C.; Marquardt, T.; Becker, M.-Y. Assessing the life-cycle sustainability of algae and bacteria-based wastewater treatment systems: High-rate algae pond and sequencing batch reactor. J. Environ. Manag. 2020, 264, 110459. [Google Scholar] [CrossRef]
- Pastore, M.; Sforza, E. Exploiting symbiotic interactions between Chlorella protothecoides and Brevundimonas diminuta for an efficient single-step urban wastewater treatment. Water Sci. Technol. 2018, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Foladori, P.; Beghini, F.; Armanini, F.; Segata, N.; Andreottola, G. How inoculation affects the development and the performances of microalgal-bacterial consortia treating real municipal wastewater. J. Environ. Manag. 2020, 263, 110427. [Google Scholar] [CrossRef] [PubMed]
- Krustok, I.; Odlare, M.; Truu, J.; Nehrenheim, E. Inhibition of nitrification in municipal wastewater-treating photobioreactors: Effect on algal growth and nutrient uptake. Bioresour. Technol. 2016, 202, 238–243. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour. Technol. 2016, 200, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Gross, W.; Schnarrenberger, C. Heterotrophic Growth of Two Strains of the Acido-Thermophilic Red Alga Galdieria sulphuraria. Plant Cell Physiol. 1995, 36, 633–638. [Google Scholar] [CrossRef]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.I.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Ruiz, J.; Álvarez, P.; Arbib, Z.; Garrido, C.; Barragán, J.; Perales, J.A. Effect of nitrogen and phosphorus concentration on their removal kinetic in treated urban wastewater by Chlorella Vulgaris. Int. J. Phytoremediat. 2011, 13, 884–896. [Google Scholar] [CrossRef]
- Catone, C.M.; Ripa, M.; Geremia, E.; Ulgiati, S. Bio-products from algae-based biorefinery on wastewater: A review. J. Environ. Manag. 2021, 293, 112792. [Google Scholar] [CrossRef] [PubMed]
- Kudahettige, N.P.; Pickova, J.; Gentili, F.G. Stressing algae for biofuel production: Biomass and biochemical composition of Scenedesmus dimorphus and Selenastrum minutum grown in municipal untreated wastewater. Front. Energy Res. 2018, 6, 132. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Alvarado-Morales, M.; Kovalovszki, A.; Corbière, M.; Angelidaki, I. Energy recovery from wastewater microalgae through anaerobic digestion process: Methane potential, continuous reactor operation and modelling aspects. Biochem. Eng. J. 2018, 139, 1–7. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.K.; Kalia, V.C. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int. J. Hydrogen Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microbial Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheswari, N.U.; Ahilandeswari, K. Production of bioplastic using Spirulina platensis and comparison with commercial plastic. Res. Environ. Life Sci. 2011, 4, 133–136. [Google Scholar]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Herrero, N.; Siles, J.Á.; Chica, A.F.; Ángeles Martín, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Posadas, E.; Muñoz, R.; Guieysse, B. Integrating nutrient removal and solid management restricts the feasibility of algal biofuel generation via wastewater treatment. Algal Res. 2017, 22, 39–46. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Passos, F.; Ferrer, I.; Uggetti, E.; García, J. Harvesting microalgae from wastewater treatment systems with natural flocculants: Effect on biomass settling and biogas production. Algal Res. 2015, 9, 204–211. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Ferrer, I.; Uggetti, E.; Arnabat, C.; Salvadó, H.; García, J. Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds. Algal Res. 2016, 16, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater treatment by seven species of microalgae and analgal bloom: Biomass production, N and P removal kinetics andharvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, B.; Gálvez, J.Á.; Michel, M.; Bartual, A. Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2017, 228, 210–217. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Ferrer, I.; González-Molina, A.; Salvadó, H.; García, J.; Uggetti, E. Microalgae recycling improves biomass recovery from wastewater treatment high rate algal ponds. Water Res. 2016, 106, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Garbowski, T.; Charazińska, S.; Pulikowski, K.; Wiercik, P. Application of microalgae cultivated on pine bark for the treatment of municipal wastewater in cylindrical photobioreactors. Water Environ. J. 2020, 34, 949–959. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario analysis of nutrient removal from municipal wastewater by microalgal biofilms. Water 2012, 4, 460–473. [Google Scholar] [CrossRef]

| Item | Authors | Title | Main Topic | Source | Citations |
|---|---|---|---|---|---|
| 1 | Ho & Goethals. (2020) [10] | Municipal wastewater treatment with pond technology: Historical review and future outlook. | Historical and general overview of pond technology that treats wastewater. Assessment of future challenges. | Ecological Engineering | 5 |
| 2 | Arias et al. (2020) [20] | Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. | Ability of cyanobacteria to grow in wastewater and the capacity to produce polymers from biomass. | New Biotechnology | 16 |
| 3 | Välitalo et al. (2017) [29] | Toxicological impacts of antibiotics on aquatic micro-organisms: A mini review. | Overview of antibiotics of high ecotoxicological risk for aquatic microorganisms. | Hygiene and Environmental Health | 64 |
| 4 | Acién et al. (2016) [30] | Wastewater treatment using microalgae: how realistic a contribution might it be to significant urban wastewater treatment? | Main bottlenecks that are limiting the use of microalgae on urban wastewater. | Applied Microbiology and Biotechnology | 85 |
| 5 | Whitton et al. (2015) [31] | Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. | Technological challenges in tertiary treatment with the use of microalgae. | Environmental Technology | 61 |
| 6 | Hassard et al. (2015) [32] | Rotating biological contactors for wastewater treatment–A review. | Developments of RBCs (rotating biological contactors) for wastewater treatment. | Process Safety and Environmental Protection | 53 |
| 7 | Singh & Olsen. (2011) [33] | A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. | Advantages and disadvantages of algal biofuel production, sustainability and life cycle assessment. | Applied Energy | 275 |
| 8 | Rizzo. (2011) [34] | Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. | Bioassays as a tool to evaluate oxidation processes in treating wastewater. | Water Research | 269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. https://doi.org/10.3390/environments8120136
Geremia E, Ripa M, Catone CM, Ulgiati S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments. 2021; 8(12):136. https://doi.org/10.3390/environments8120136
Chicago/Turabian StyleGeremia, Eugenio, Maddalena Ripa, Claudio Marcello Catone, and Sergio Ulgiati. 2021. "A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe" Environments 8, no. 12: 136. https://doi.org/10.3390/environments8120136
APA StyleGeremia, E., Ripa, M., Catone, C. M., & Ulgiati, S. (2021). A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments, 8(12), 136. https://doi.org/10.3390/environments8120136