Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Stool Sample Collection
2.2. Collection of Drinking Water, Seafood, Staple Foods, Table Salts, and Toothpaste Samples
2.3. Sample Preparation
2.4. Microplastic Analysis
2.5. Ethical Statement
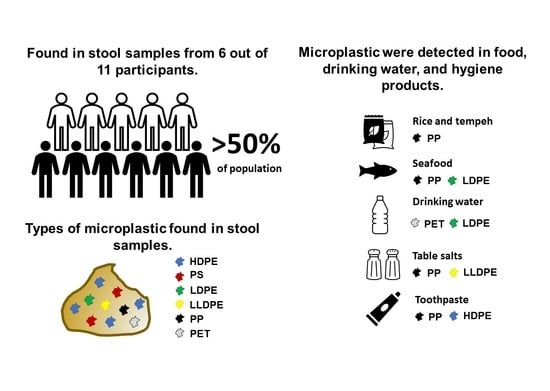
3. Results
3.1. Detection of Microplastic in Human Stools
3.2. Microplastic Contamination in Drinking Water
3.3. Widespread Microplastic Contamination in Seafood, Staple Foods, and Hygiene Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vriend, P.; Hidayat, H.; van Leeuwen, J.; Cordova, M.R.; Purba, N.P.; Löhr, A.J.; Faizal, I.; Ningsih, N.S.; Agustina, K.; Husrin, S.; et al. Plastic Pollution Research in Indonesia: State of Science and Future Research Directions to Reduce Impacts. Front. Environ. Sci. 2021, 9, 692907. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Lestari, P.; Trihadiningrum, Y. The impact of improper solid waste management to plastic pollution in Indonesian coast and marine environment. Mar. Pollut. Bull. 2019, 149, 110505. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Su, L.; Craig, N.J.; Du, F.; Wu, C.; Shi, H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- Marrone, A.; La Russa, M.F.; Randazzo, L.; La Russa, D.; Cellini, E.; Pellegrino, D. Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea. Int. J. Environ. Res. Public Health 2021, 18, 10712. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Kalavrouziotis, I.K. Microplastics in Water and Wastewater; IWA Publishing: London, UK, 2019; ISBN 9781789060027. [Google Scholar]
- Tudor, V.C.; Mocuta, D.N.; Teodorescu, R.F.; Smedescu, D.I. The Issue of Plastic and Microplastic Pollution in Soil. Mater. Plast. 2019, 56, 484–487. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Miino, M.C.; Caccamo, F.M.; Milanese, C. Microplastics in Sewage Sludge: A Known but Underrated Pathway in Wastewater Treatment Plants. Sustainability 2021, 13, 12591. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- Baalkhuyur, F.M.; Bin Dohaish, E.J.; Elhalwagy, M.E.; Alikunhi, N.M.; AlSuwailem, A.M.; Røstad, A.; Coker, D.J.; Berumen, M.; Duarte, C.M. Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 2018, 131, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.R.; Riani, E.; Shiomoto, A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 2020, 161, 111763. [Google Scholar] [CrossRef]
- Ismail, M.R.; Lewaru, M.W.; Prihadi, D.J. Microplastics Ingestion by Fish in the Pangandaran Bay, Indonesia. World News Nat. Sci. Int. Sci. J. 2019, 23, 173–181. [Google Scholar]
- Lusher, A.; McHugh, M.; Thompson, R. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Seafood Intended for Human Consumption: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 126002. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Zhang, N.; Bin Li, Y.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Wibowo, A.T.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Sugiyo, P.W.W.; Putro, Y.K.; Fauzia, F.N.; Santoso, H.; et al. Microplastic Contamination in the Human Gastrointestinal Tract and Daily Consumables Associated with an Indonesian Farming Community. Sustain. Sci. Pract. Policy 2021, 13, 12840. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Anuar, S.T.; Azmi, A.A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Turner, A. Human exposure to microplastics: A study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Galvão, L.D.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Hastuti, A.R.; Lumbanbatu, D.T.; Wardiatno, Y. The presence of microplastics in the digestive tract of commercial fishes off Pantai Indah Kapuk coast, Jakarta, Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Khoironi, A.; Anggoro, S. The Existence of Microplastic in Asian Green Mussels. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012050. [Google Scholar] [CrossRef]
- Dwiyitno, D.; Sturm, M.T.; Januar, H.I.; Schuhen, K. Influence of various production methods on the microplastic contamination of sea salt produced in Java, Indonesia. Environ. Sci. Pollut. Res. 2021, 28, 30409–30413. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, H.; Zhao, Y.; Zhu, Q.; Qiao, R.; Ren, H.; Zhang, Y. An efficient method for extracting microplastics from feces of different species. J. Hazard. Mater. 2020, 384, 121489. [Google Scholar] [CrossRef] [PubMed]
- Wahyuono, R.A.; Hesse, J.; Hipler, U.-C.; Elsner, P.; Böhm, V. Carotenoids of Indigenous Citrus Species from Aceh and Its in Vitro Antioxidant, Antidiabetic and Antibacterial Activities. Eur. Food Res. Technol. 2016, 242, 1869–1881. [Google Scholar]
- Wahyuono, R.A.; Hesse, J.; Hipler, U.-C.; Elsner, P.; Böhm, V. In Vitro Lipophilic Antioxidant Capacity, Antidiabetic and Antibacterial Activity of Citrus Fruits Extracts from Aceh, Indonesia. Antioxid. Redox Signal 2017, 6, 11. [Google Scholar]
- Radityaningrum, A.D.; Trihadiningrum, Y.; Soedjono, E.S.; Herumurti, W. Microplastic Contamination in Water Supply and the Removal Efficiencies of the Treatment Plants: A Case of Surabaya City, Indonesia. J. Water Process. Eng. 2021, 43, 102195. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, H.-J.; Kim, S.-K.; Kim, H.-J. Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef] [PubMed]
- Ustabaşı, G.S.; Baysal, A. Occurrence and risk assessment of microplastics from various toothpastes. Environ. Monit. Assess. 2019, 191, 438. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Abrantes, N.; Gonçalves, F.; Nogueira, H.S.; Marques, J.; Gonçalves, A.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-C.; Kim, J.H.; Orski, S.; Fairbrother, A.; Jacobs, D.; Perry, L.; Hunston, D.; White, C.; Sung, L. Micro and macroscopic mechanical behaviors of high-density polyethylene under UV irradiation and temperature. Polym. Degrad. Stab. 2020, 174, 109098. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, M.; Molina-Molina, J.-M.; Jiménez-Díaz, I.; Arrebola, J.P.; Sáenz, J.-M.; Fernández, M.F.; Olea, N. Screening of hormone-like activities in bottled waters available in Southern Spain using receptor-specific bioassays. Environ. Int. 2015, 74, 125–135. [Google Scholar] [CrossRef]

| Samples | Type of Microplastic | Content (µg/g) |
|---|---|---|
| Drinking water in gallon brand 1 | PET | 9.18 ± 1.30 |
| Drinking water in gallon brand 2 | nd | nd |
| Drinking water in gallon from refilling station 1 | nd | nd |
| Drinking water in gallon from refilling station 2 | nd | nd |
| Drinking water in gallon from refilling station 3 | LDPE | 30.21 ± 4.26 |
| Samples | Type of Microplastic | Content (µg/g) |
|---|---|---|
| Salted fish 1 | PP | 11.61 ± 4.68 |
| Salted fish 2 | nd | nd |
| Seawater catfish 1 | LDPE | 5.15 ± 2.48 |
| Seawater catfish 2 | nd | nd |
| Seawater catfish 3 | nd | nd |
| Mussel | nd | nd |
| Shrimp | nd | nd |
| Freshwater catfish | nd | nd |
| Tofu Brand 1 | nd | nd |
| Tofu Brand 2 | nd | nd |
| Tempeh Brand 1 | PP | 4.07 ± 1.35 |
| Tempeh Brand 2 | nd | nd |
| Rice Brand 1 | PP | 15.36 ± 4.79 |
| Rice Brand 2 | nd | nd |
| Samples | Type of Microplastic | Content (µg/g) |
|---|---|---|
| Table salt | ||
| Brand 1 | PP | 8.69 ± 1.26 |
| Brand 2 | LLDPE | 26.27 ± 3 |
| Toothpaste | ||
| Brand 1 | PP | 23.47 ± 5.77 |
| Brand 2 | HDPE | 14.79 ± 5.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138. https://doi.org/10.3390/environments8120138
Luqman A, Nugrahapraja H, Wahyuono RA, Islami I, Haekal MH, Fardiansyah Y, Putri BQ, Amalludin FI, Rofiqa EA, Götz F, et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments. 2021; 8(12):138. https://doi.org/10.3390/environments8120138
Chicago/Turabian StyleLuqman, Arif, Husna Nugrahapraja, Ruri Agung Wahyuono, Izzatul Islami, Muhammad Husain Haekal, Yasri Fardiansyah, Balqis Qonita Putri, Fahmi Ikhlasul Amalludin, Elsalisa Ainur Rofiqa, Friedrich Götz, and et al. 2021. "Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population" Environments 8, no. 12: 138. https://doi.org/10.3390/environments8120138
APA StyleLuqman, A., Nugrahapraja, H., Wahyuono, R. A., Islami, I., Haekal, M. H., Fardiansyah, Y., Putri, B. Q., Amalludin, F. I., Rofiqa, E. A., Götz, F., & Wibowo, A. T. (2021). Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments, 8(12), 138. https://doi.org/10.3390/environments8120138