Prenatal Exposure to Persistent Organic Pollutants and Maternal Folic Acid Supplementation: Their Impact on Glucose Homeostasis in Male Rat Descendants
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. POP Mixture
2.3. Diet Formulation
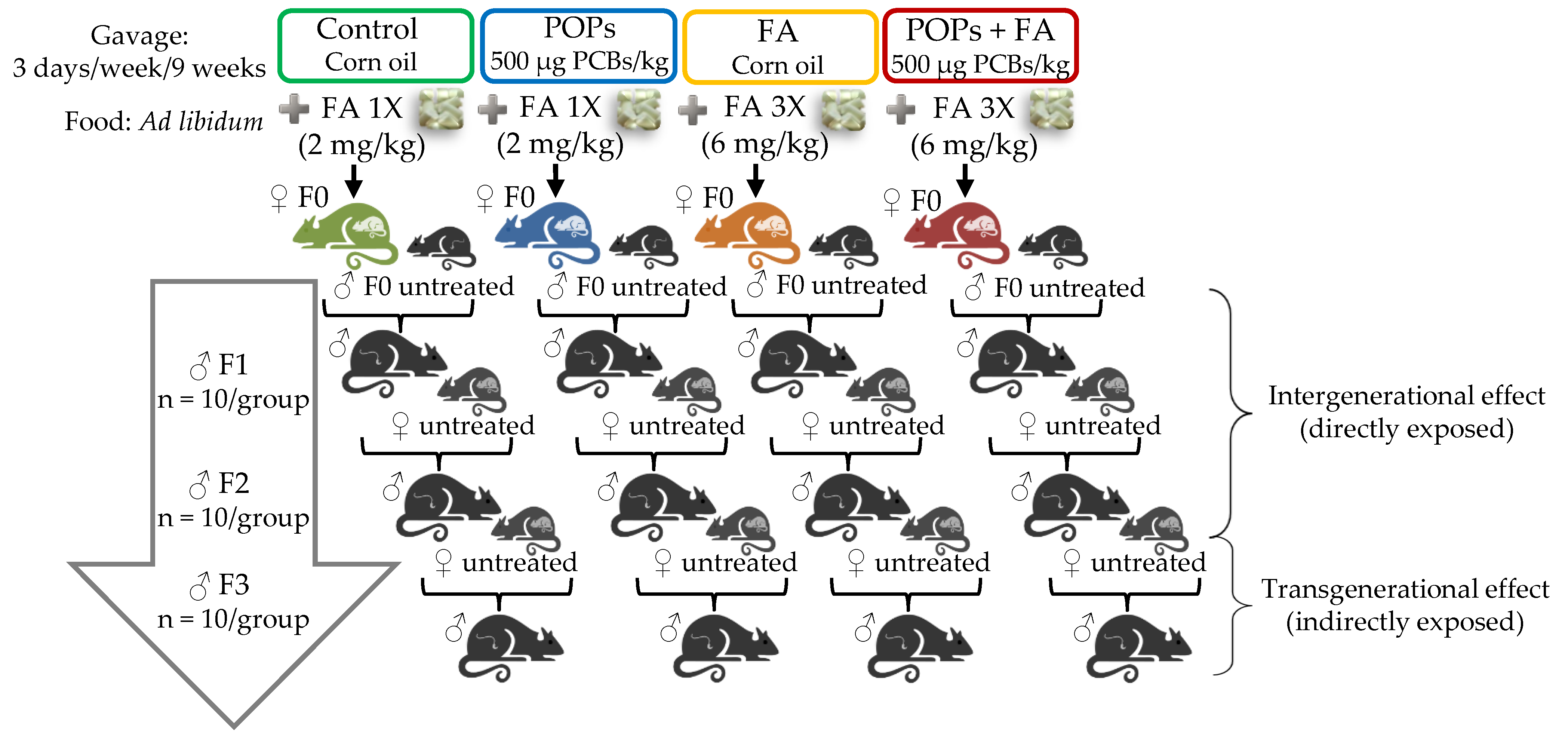
2.4. Experimental Design
2.4.1. F0 Generation
2.4.2. F1, F2 and F3 Generations
2.5. Blood Collection
2.6. Plasma Biochemistry, Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and Matsuda Indexes
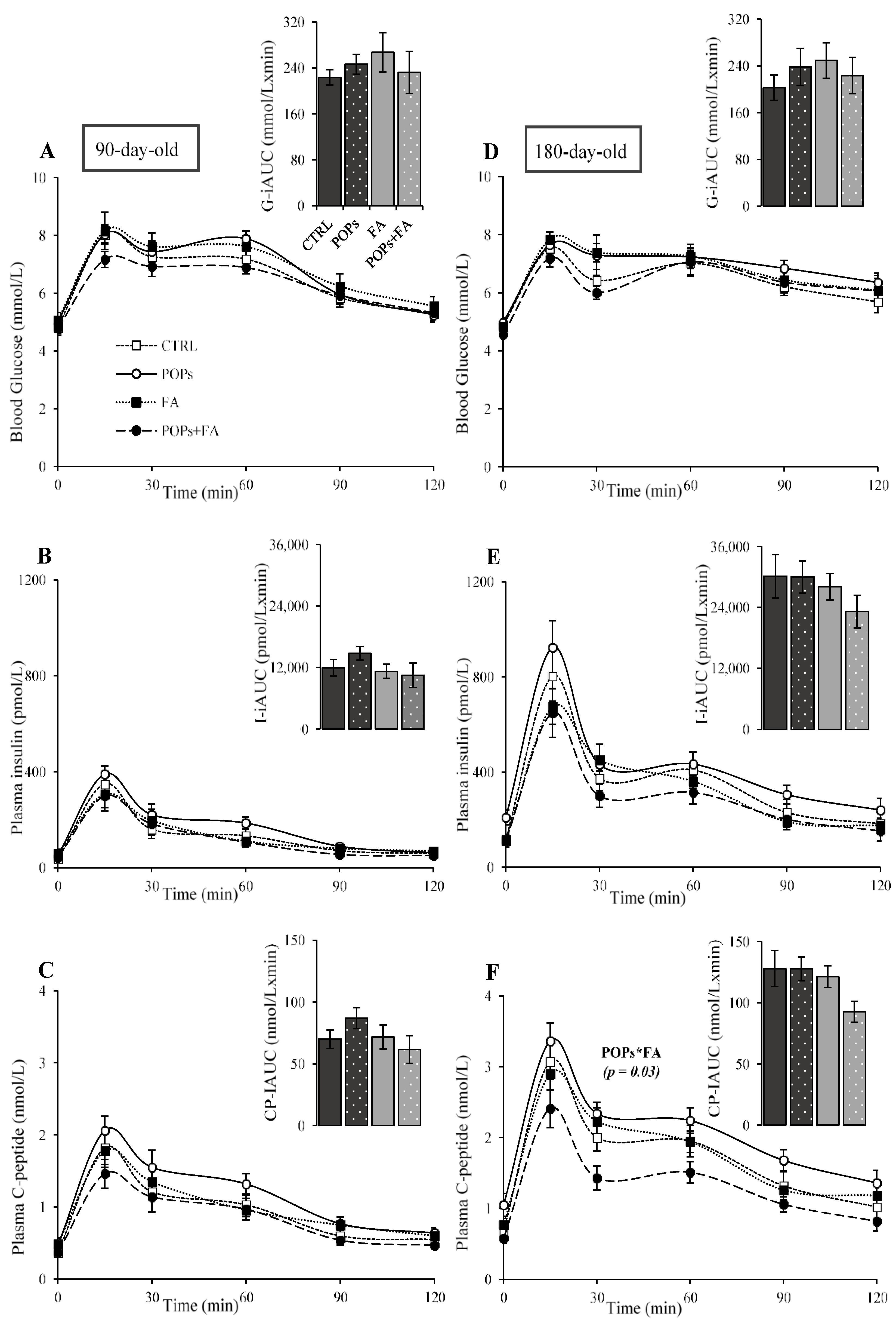
2.7. Glucose Tolerance Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Body Weight and Food Intake
3.2. Exposure to POPs
3.3. Folic Acid Supplementation
3.4. Exposure to POPs Combined with FA Supplementation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- Tait, H. Aboriginal Peoples Survey, 2006: Inuit Health and Social Conditions; Statistics Canada: Ottawa, ON, Canada, 2008; ISBN 978-1-100-11396-8. [Google Scholar]
- Wallace, S. Inuit Health: Selected Findings from the 2012 Aboriginal Peoples Survey; Statistics Canada: Ottawa, ON, Canada, 2014; ISBN 978-1-100-22738-2. [Google Scholar]
- Hopping, B.N.; Erber, E.; Mead, E.; Sheehy, T.; Roache, C.; Sharma, S. Socioeconomic Indicators and Frequency of Traditional Food, Junk Food, and Fruit and Vegetable Consumption among Inuit Adults in the Canadian Arctic. J. Hum. Nutr. Diet. 2010, 23, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Mead, E.; Erber, E.; Sheehy, C.; Roache, C.; Sharma, S. Dietary Adequacy of Inuit in the Canadian Arctic. J. Hum. Nutr. Diet. 2010, 23, 27–34. [Google Scholar] [CrossRef]
- Ritter, L.; Solomon, K.; Forget, J. A Review of Selected Persistent Organic Pollutants DDT-Aldrin-Dieldrin-Endrin-Chlordane Heptachlor-Hexachlorobenzene-Mïrex-Toxaphene Polychlorinated Biphenyls Dioxins and Furans. Available online: https://www.who.int/ipcs/assessment/en/pcs_95_39_2004_05_13.pdf (accessed on 21 January 2021).
- Weber, R.; Aliyeva, G.; Vijgen, J. The Need for an Integrated Approach to the Global Challenge of POPs Management. Environ. Sci. Pollut. Res. 2013, 20, 1901–1906. [Google Scholar] [CrossRef]
- Barrie, L.A.; Gregor, D.; Hargrave, B.; Lake, R.; Muir, D.; Shearer, R.; Tracey, B.; Bidleman, T. Arctic Contaminants: Sources, Occurrence and Pathways. Sci. Total Environ. 1992, 122, 1–74. [Google Scholar] [CrossRef]
- Jones, K.C.; De Voogt, P. Persistent Organic Pollutants (POPs): State of the Science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Muir, D.C.G.; Wagemann, R.; Hargrave, B.T.; Thomas, D.J.; Peakall, D.B.; Norstrom, R.J. Arctic Marine Ecosystem Contamination. Sci. Total Environ. 1992, 122, 75–134. [Google Scholar] [CrossRef]
- Johansen, P.; Muir, D.; Asmund, G.; Riget, F. Human Exposure to Contaminants in the Traditional Greenland Diet. Sci. Total Environ. 2004, 331, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, E.; Ayotte, P.; Bruneau, S.; Laliberte, C.; Muir, D.C.G.; Norstrom, R.J. Inuit Exposure to Organochlorines through the Aquatic Food Chain in Arctic Quebec. Environ. Health Perspect. 1993, 101, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Butler Walker, J.; Seddon, L.; McMullen, E.; Houseman, J.; Tofflemire, K.; Corriveau, A.; Weber, J.P.; Mills, C.; Smith, S.; Van Oostdam, J. Organochlorine Levels in Maternal and Umbilical Cord Blood Plasma in Arctic Canada. Sci. Total Environ. 2003, 302, 27–52. [Google Scholar] [CrossRef]
- Singh, K.; Chan, H.M. Persistent Organic Pollutants and Diabetes among Inuit in the Canadian Arctic. Environ. Int. 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Lee, D.H.; Steffes, M.W.; Sjödin, A.; Jones, R.S.; Needham, L.L.; Jacobs, D.R. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS ONE 2011, 6, e15977. [Google Scholar] [CrossRef]
- Dirinck, E.L.; Dirtu, A.C.; Govindan, M.; Covaci, A.; Van Gaal, L.F.; Jorens, P.G. Exposure to Persistent Organic Pollutants: Relationship with Abnormal Glucose Metabolism and Visceral Adiposity. Diabetes Care 2014, 37, 1951–1958. [Google Scholar] [CrossRef]
- Fujiyosh, P.T.; Michalek, J.E.; Matsumura, F. Molecular Epidemiologic Evidence for Diabetogenic Effects of Dioxin Exposure in U.S. Air Force Veterans of the Vietnam War. Environ. Health Perspect. 2006, 114, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.; Weir, D. The History of Folic Acid. Br. J. Haematol. 2001, 113, 579–589. [Google Scholar] [CrossRef]
- Lazalde-Ramos, B.P.; Zamora-Perez, A.L.; Sosa-Macías, M.; Guerrero-Velázquez, C.; Zúñiga-González, G.M. DNA and Oxidative Damages Decrease after Ingestion of Folic Acid in Patients with Type 2 Diabetes. Arch. Med. Res. 2012, 43, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Travisano, M.; Tahiliani, K.G.; Rached, M.T.; Mirza, S. Methyl Donor Supplementation Prevents Transgenerational Amplification of Obesity. Int. J. Obes. 2008, 32, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal Nutrient Supplementation Counteracts Bisphenol A-Induced DNA Hypomethylation in Early Development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Anas, M.-K.I.; Guillemette, C.; Ayotte, P.; Pereg, D.; Giguère, F.; Bailey, J.L. In Utero and Lactational Exposure to an Environmentally Relevant Organochlorine Mixture Disrupts Reproductive Development and Function in Male Rats. Biol. Reprod. 2005, 73, 414–426. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.E. Ancestral Dichlorodiphenyltrichloroethane (DDT) Exposure Promotes Epigenetic Transgenerational Inheritance of Obesity. BMC Med. 2013, 11, 228. [Google Scholar] [CrossRef]
- Khezri, A.; Lindeman, B.; Krogenaes, A.K.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E. Maternal Exposure to a Mixture of Persistent Organic Pollutants (POPs) Affects Testis Histology, Epididymal Sperm Count and Induces Sperm DNA Fragmentation in Mice. Toxicol. Appl. Pharmacol. 2017, 329, 301–308. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Muir, D.; Braune, B.; DeMarch, B.; Norstrom, R.; Wagemann, R.; Lockhart, L.; Hargrave, B.; Bright, D.; Addison, R.; Payne, J.; et al. Spatial and Temporal Trends and Effects of Contaminants in the Canadian Arctic Marine Ecosystem: A Review. Sci. Total Environ. 1999, 230, 83–144. [Google Scholar] [CrossRef]
- Swayne, B.G.; Kawata, A.; Behan, N.A.; Williams, A.; Wade, M.G.; MacFarlane, A.J.; Yauk, C.L. Investigating the Effects of Dietary Folic Acid on Sperm Count, DNA Damage and Mutation in Balb/c Mice. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2012, 737, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Swayne, B.G.; Behan, N.A.; Williams, A.; Stover, P.J.; Yauk, C.L.; MacFarlane, A.J. Supplemental Dietary Folic Acid Has No Effect on Chromosome Damage in Erythrocyte Progenitor Cells of Mice. J. Nutr. 2012, 142, 813–817. [Google Scholar] [CrossRef]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a Model of Insulin-Resistance Induced by a High-Fat Diet in Wistar Rats. Arch. Endocrinol. Metab. 2016, 60, 138–142. [Google Scholar] [CrossRef]
- Pacini, G.; Omar, B.; Ahrén, B. Methods and Models for Metabolic Assessment in Mice. J. Diabetes Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Airaksinen, R.; Rantakokko, P.; Eriksson, J.G.; Blomstedt, P.; Kajantie, E.; Kiviranta, H. Association between Type 2 Diabetes and Exposure to Persistent Organic Pollutants. Diabetes Care 2011, 34, 1972–1979. [Google Scholar] [CrossRef]
- Ngwa, E.N.; Kengne, A.P.; Tiedeu-Atogho, B.; Mofo-Mato, E.P.; Sobngwi, E. Persistent Organic Pollutants as Risk Factors for Type 2 Diabetes. Diabetol. Metab. Syndr. 2015, 7, 41. [Google Scholar] [CrossRef]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent Organic Pollutant Exposure Leads to Insulin Resistance Syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Chen, H.L.; Su, H.J.; Lee, C.C. Abdominal Obesity and Insulin Resistance in People Exposed to Moderate-to-High Levels of Dioxin. PLoS ONE 2016, 11, e0145818. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Fjære, E.; Lock, E.J.; Naville, D.; Amlund, H.; Meugnier, E.; Battistoni, B.L.M.; Frøyland, L.; Madsen, L.; Jessen, N.; et al. Chronic Consumption of Farmed Salmon Containing Persistent Organic Pollutants Causes Insulin Resistance and Obesity in Mice. PLoS ONE 2011, 6, e25170. [Google Scholar] [CrossRef]
- Howell, G.E.; Mulligan, C.; Meek, E.; Chambers, J.E. Effect of Chronic p,p’-Dichlorodiphenyldichloroethylene (DDE) Exposure on High Fat Diet-Induced Alterations in Glucose and Lipid Metabolism in Male C57BL/6H Mice. Toxicology 2015, 328, 112–122. [Google Scholar] [CrossRef]
- Gray, S.L.; Shaw, A.C.; Gagne, A.X.; Chan, H.M. Chronic Exposure to PCBs (Aroclor 1254) Exacerbates Obesity-Induced Insulin Resistance and Hyperinsulinemia in Mice. J. Toxicol. Environ. Health 2013, 76, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Ha, C.M.; Kim, S.A.; Thoudam, T.; Yoon, Y.R.; Kim, D.J.; Kim, H.C.; Moon, H.B.; Park, S.; Lee, I.K.; et al. Low-Dose Persistent Organic Pollutants Impair Insulin Secretory Function of Pancreatic b-Cells: Human and In Vitro Evidence. Diabetes 2017, 66, 2669–2680. [Google Scholar] [CrossRef]
- Gargari, B.P.; Aghamohammadi, V.; Aliasgharzadeh, A. Effect of Folic Acid Supplementation on Biochemical Indices in Overweight and Obese Men with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2011, 94, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The Role of the One-Carbon Cycle in the Developmental Origins of Type 2 Diabetes and Obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, L. Transgenerational Pancreatic Impairment with Igf2/H19 Epigenetic Alteration Induced by p,p’-DDE Exposure in Early Life. Toxicol. Lett. 2017, 280, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, C.E.; Foster, J.E.; Ramdath, D.D. A Maternal High-Fat, High-Sucrose Diet Alters Insulin Sensitivity and Expression of Insulin Signalling and Lipid Metabolism Genes and Proteins in Male Rat Offspring: Effect of Folic Acid Supplementation. Br. J. Nutr. 2017, 118, 580–588. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine Disruptors in the Etiology of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.; Karey, E.; Moshier, E.; Lindtner, C.; La Frano, M.R.; Newman, J.W.; Buettner, C. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring. PLoS ONE 2014, 9, e103337. [Google Scholar] [CrossRef] [PubMed]
- Dahri, S.; Snoeck, A.; Reusens-Billen, B.; Remacle, C.; Hoet, J.J. Islet Function in Offspring of Mothers on Low-Protein Diet during Gestation. Diabetes 1991, 40, 115–120. [Google Scholar] [CrossRef]
- Rando, O.J.; Simmons, R.A. I’m Eating for Two: Parental Dietary Effects on Offspring Metabolism. Cell 2015, 161, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Bettermann, I.; Hechtl, C.; Gäbele, E.; Hellerbrand, C.; Schölmerich, J.; Bollheimer, L.C. Dietary Folic Acid Activates AMPK and Improves Insulin Resistance and Hepatic Inflammation in Dietary Rodent Models of the Metabolic Syndrome. Horm. Metab. Res. 2010, 42, 769–774. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic Reprogramming in Plant and Animal Development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Belleau, P.; Deschênes, A.; Scott-Boyer, M.P.; Lambrot, R.; Dalvai, M.; Kimmins, S.; Bailey, J.; Droit, A. Inferring and Modeling Inheritance of Differentially Methylated Changes across Multiple Generations. Nucleic Acids Res. 2018, 46, e85. [Google Scholar] [CrossRef] [PubMed]
- Siklenka, K.; Erkek, S.; Godmann, M.; Lambrot, R.; McGraw, S.; Lafleur, C.; Cohen, T.; Xia, J.; Suderman, M.; Hallett, M.; et al. Disruption of Histone Methylation in Developing Sperm Impairs Offspring Health Transgenerationally. Science 2015, 350, aab2006. [Google Scholar] [CrossRef]
- Consales, C.; Toft, G.; Leter, G.; Bonde, J.P.E.; Uccelli, R.; Pacchierotti, F.; Eleuteri, P.; Jönsson, B.A.G.; Giwercman, A.; Pedersen, H.S.; et al. Exposure to Persistent Organic Pollutants and Sperm DNA Methylation Changes in Arctic and European Populations. Environ. Mol. Mutagen. 2016, 57, 200–209. [Google Scholar] [CrossRef]
- Rusiecki, J.A.; Baccarelli, A.; Bollati, V.; Tarantini, L.; Moore, L.E.; Bonefeld-Jorgensen, E.C. Global DNA Hypomethylation Is Associated with High Serum-Persistent Organic Pollutants in Greenlandic Inuit. Environ. Health Perspect. 2008, 116, 1547–1552. [Google Scholar] [CrossRef]
- Kandi, V.; Vadakedath, S. Effect of DNA Methylation in Various Diseases and the Probable Protective Role of Nutrition: A Mini-Review. Cureus 2015, 7, e309. [Google Scholar] [CrossRef]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low Paternal Dietary Folate Alters the Mouse Sperm Epigenome and Is Associated with Negative Pregnancy Outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef]
- Herst, P.M.; Dalvai, M.; Lessard, M.; Charest, P.L.; Navarro, P.; Joly-Beauparlant, C.; Droit, A.; Trasler, J.M.; Kimmins, S.; MacFarlane, A.J.; et al. Folic Acid Supplementation Reduces Multigenerational Sperm MiRNA Perturbation Induced by in Utero Environmental Contaminant Exposure. Environ. Epigenetics 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Chen, H.; Muzumdar, R.H.; Einstein, F.H.; Yan, X.; Yue, L.Q.; Barzilai, N.; Quon, M.J. Comparison between Surrogate Indexes of Insulin Sensitivity/Resistance and Hyperinsulinemic Euglycemic Clamp Estimates in Rats. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1023–E1029. [Google Scholar] [CrossRef] [PubMed]
- Petriello, M.C.; Newsome, B.J.; Dziubla, T.D.; Hilt, J.Z.; Bhattacharyya, D.; Hennig, B. Modulation of Persistent Organic Pollutant Toxicity through Nutritional Intervention: Emerging Opportunities in Biomedicine and Environmental Remediation. Sci. Total Environ. 2014, 491–492, 11–16. [Google Scholar] [CrossRef] [PubMed]



| Compound | CAS Number | Origin 1 | Quantity in the Mix (%) | Administered Dose (µg/kg of Body Weight) |
|---|---|---|---|---|
| Aroclor and congener neat mix 2 | 57-74-9 | AccuStandard | 32.4 | 500 |
| Technical grade chlordane | 72-55-9 | AccuStandard | 21.4 | 330.3 |
| p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) | 50-29-3 | Sigma-Aldrich | 19.3 | 297.8 |
| p,p’-dichlorodiphenyltrichloroethane (p,p’-DDT) | 8001-35-2 | SigmaAldrich | 6.8 | 104.9 |
| Technical grade toxaphene | 319-84-6 | AccuStandard | 6.5 | 100.0 |
| α-hexachlorocyclohexane (α-HCH) | 309-00-2 | Sigma-Aldrich | 6.2 | 95.7 |
| Aldrin | 60-57-1 | Sigma-Aldrich | 2.5 | 38.6 |
| Dieldrin | 95-94-3 | Sigma-Aldrich | 2.1 | 32.4 |
| 1,2,4,5-tetrachlorobenzene | 72-54-8 | Sigma-Aldrich | 0.9 | 13.9 |
| p, p’-dichlorodiphenyldichloroethane (p, p’-DDD) | 319-85-7 | Sigma-Aldrich | 0.5 | 7.7 |
| β-hexachlorocyclohexane (β-HCH) | 118-74-1 | Sigma-Aldrich | 0.4 | 6.2 |
| Hexachlorobenzene | 2385-85-5 | AccuStandard | 0.4 | 6.2 |
| Mirex | 58-89-9 | Sigma-Aldrich | 0.2 | 3.1 |
| Lindane | 608-93-5 | Sigma-Aldrich | 0.2 | 3.1 |
| Pentachlorobenzene | 57-74-9 | Sigma-Aldrich | 0.2 | 3.1 |
| Ingredients | 1× Diet 1 (g/kg of Diet) | 3× Diet 2 (g/kg of Diet) |
|---|---|---|
| Casein 3 | 200 | 200 |
| L-Cystine | 3 | 3 |
| Sucrose | 100 | 100 |
| Cornstarch | 397.486 | 396.286 |
| Dyetrose 4 | 132 | 132 |
| Soybean oil | 70 | 70 |
| t-butylhydroquinone | 0.014 | 0.014 |
| Cellulose | 50 | 50 |
| Mineral Mix #210025 | 35 | 35 |
| Vitamin Mix #310025 | 10 | 0 |
| Vitamin Mix #317761 (no folate) | 0 | 10 |
| Folic acid premix (5 mg/g) | 0 | 1.2 |
| Choline bitartrate | 2.5 | 2.5 |
| Total | 1000 | 1000 |
| CTRL | POPs | FA | POPs+FA | |
|---|---|---|---|---|
| F0 | ||||
| Weight gain 1 (g) | 73 ± 6 | 73 ± 6 | 65 ± 8 | 84 ± 8 |
| Food intake (g/day) | 14 ± 0.2 | 15 ± 1 | 15 ± 0.3 | 14 ± 0.9 |
| F1 | ||||
| Weight on day 21 (g) | 51 ± 2 | 52 ± 1 | 48 ± 2 | 51 ± 0.6 |
| Weight on day 90 (g) | 386 ± 9 | 400 ± 10 | 384 ± 9 | 387 ± 12 |
| Weight on day 180 2 (g) | 493 ± 12 | 521 ± 14 | 499 ± 12 | 493 ± 18 |
| Total weight gain 2,3 (g) | 441 ± 39 | 469 ± 43 | 451 ± 36 | 442 ± 53 |
| Food intake 2 (g/day) | 18 ± 0.3 | 19 ± 0.4 | 18 ± 0.4 | 19 ± 0.6 |
| F2 | ||||
| Weight on day 21 (g) | 51 ± 2 | 50 ± 2 | 50 ± 1 | 51 ± 0.9 |
| Weight on day 90 (g) | 410 ± 11 | 408 ± 11 | 415 ± 11 | 408 ± 13 |
| Weight on day 180 2 (g) | 538 ± 15 | 522 ± 15 | 544 ± 15 | 520 ± 18 |
| Total weight gain 2,3 (g) | 486 ± 44 | 472 ± 44 | 494 ± 49 | 470 ± 58 |
| Food intake 2 (g/day) | 20 ± 0.6 | 19 ± 0.5 | 19 ± 0.6 | 19 ± 0.6 |
| F3 | ||||
| Weight on day 21 (g) | 52 ± 2 | 53 ± 2 | 52 ± 1 | 53 ± 2 |
| Weight on day 90 (g) | 411 ± 7 | 419 ± 8 | 403 ± 8 | 401 ± 9 |
| Weight on day 180 2 (g) | 537 ± 12 | 547 ± 15 | 526 ± 11 | 512 ± 17 |
| Total weight gain 2,3 (g) | 485 ± 37 | 494 ± 46 | 474 ± 33 | 461 ± 50 |
| Food intake 2 (g/day) | 19 ± 0.3 | 20 ± 0.3 | 20 ± 0.6 | 19 ± 0.6 |
| CTRL | POPs | FA | POPs+FA | POPs | FA | POPs*FA | |
|---|---|---|---|---|---|---|---|
| 90-day-old | p values | ||||||
| Insulin/glucose IAUC 1 (pmol/mmol) | 51 ± 13 | 74 ± 26 | 62 ± 13 | 51 ± 12 | ns | ns | ns |
| C-peptide/insulin IAUC 2 (nmol/pmol) × 10−3 | 5.3 ± 0.3 | 5.4 ± 0.9 | 4.2 ± 0.4 | 4.3 ± 0.5 | ns | ‡ | ns |
| HOMA-IR index | 1.4 ± 0.3 | 2.0 ± 0.4 | 0.9 ± 0.1 | 1.8 ± 0.5 | * | ns | ns |
| Matsuda index 3 | 9.9 ± 2.2 | 8.1 ± 1.5 | 11.7 ± 2.2 | 10.8 ± 1.9 | ns | ns | ns |
| 180-day-old | p values | ||||||
| Insulin/glucose IAUC 4 (pmol/mmol) | 135 ± 13 | 183 ± 67 | 176 ± 24 | 126 ± 21 | ns | ns | ns |
| C-peptide/insulin IAUC 5 (nmol/pmol) × 10−3 | 4.4 ab ± 0.3 | 3.6 c ± 0.3 | 3.9 bc ± 0.2 | 4.8 a ± 0.3 | ns | ns | * |
| HOMA-IR index 4 | 4.1 ± 0.8 | 4.8 ± 1.3 | 2.3 ± 0.2 | 2.4 ± 0.3 | ns | ns | ns |
| Matsuda index 4 | 3.6 ± 0.6 | 4.6 ± 1.3 | 4.5 ± 0.3 | 4.9 ± 0.5 | ns | ns | ns |
| CTRL | POPs | FA | POPs+FA | POPs | FA | POPs*FA | |
|---|---|---|---|---|---|---|---|
| 90-day-old | p values | ||||||
| Insulin/glucose IAUC (pmol/mmol) | 64 ± 9 | 123 ± 17 | 65 ± 12 | 83 ± 25 | ns | ns | ns |
| C-peptide/insulin IAUC (nmol/pmol) × 10−3 | 5.0 ± 0.3 | 4.6 ± 0.3 | 5.4 ± 0.6 | 4.9 ± 0.5 | ns | ns | ns |
| HOMA-IR index | 1.0 ± 0.1 | 1.6 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.1 | ns | ns | ns |
| Matsuda index | 9.3 ± 0.9 | 6.1 ± 0.5 | 9.0 ± 1.3 | 7.9 ± 0.5 | † | ns | ns |
| 180-day-old | p values | ||||||
| Insulin/glucose IAUC 1 (pmol/mmol) | 141 ± 31 | 150 ± 23 | 152 ± 41 | 138 ± 30 | ns | ns | ns |
| C-peptide/insulin IAUC 1 (nmol/pmol) × 10−3 | 4.9 ± 0.4 | 4.4 ± 0.2 | 4.6 ± 0.3 | 4.6 ± 0.5 | ns | ns | ns |
| HOMA-IR index 1 | 3.3 ± 0.8 | 3.9 ± 0.6 | 3.7 ± 0.9 | 3.5 ± 1 | ns | ns | ns |
| Matsuda index 1 | 4.7 ± 0.8 | 3.9 ± 0.6 | 5.6 ± 1.3 | 5.2 ± 1.0 | ns | ns | ns |
| CTRL | POPs | FA | POPs+FA | POPs | FA | POPs*FA | |
|---|---|---|---|---|---|---|---|
| 90-day-old | p values | ||||||
| Insulin/glucose IAUC (pmol/mmol) | 56 ± 9 | 62 ± 6 | 50 ± 10 | 65 ± 17 | ns | ns | ns |
| C-peptide/insulin IAUC (nmol/pmol) × 10−3 | 6.3 ± 0.5 | 6.0 ± 0.6 | 6.7 ± 0.7 | 7.2 ± 1.1 | ns | ns | ns |
| HOMA-IR index | 1.1 ± 0.2 | 1.6 ± 0.3 | 2.1 ± 0.9 | 1.4 ± 0.4 | ns | ns | ns |
| Matsuda index | 10.5 ± 1.4 | 7.3 ± 0.5 | 9.3 ± 1.5 | 12.3 ± 2.1 | ns | ns | ns |
| 180-day-old | p values | ||||||
| Insulin/glucose IAUC (pmol/mmol) | 154 ± 23 | 139 ± 20 | 143 ± 31 | 122 ± 20 | ns | ns | ns |
| C-peptide/insulin IAUC (nmol/pmol) × 10−3 | 4.4 ± 0.3 | 4.5 ± 0.3 | 4.5 ± 0.4 | 4.3 ± 0.3 | ns | ns | ns |
| HOMA-IR index | 3.8 ± 0.8 | 6.7 ± 1.0 | 3.6 ± 0.6 | 3.3 ± 0.8 | ns | ns | ns |
| Matsuda index | 3.9 a ± 0.6 | 2.4 b ± 0.3 | 4.1 a ± 0.8 | 4.9 a ± 0.8 | ns | ns | † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, P.; Dalvai, M.; L. Charest, P.; Herst, P.M.; Lessard, M.; Marcotte, B.; Leblanc, N.; Kimmins, S.; Trasler, J.; MacFarlane, A.J.; et al. Prenatal Exposure to Persistent Organic Pollutants and Maternal Folic Acid Supplementation: Their Impact on Glucose Homeostasis in Male Rat Descendants. Environments 2021, 8, 24. https://doi.org/10.3390/environments8030024
Navarro P, Dalvai M, L. Charest P, Herst PM, Lessard M, Marcotte B, Leblanc N, Kimmins S, Trasler J, MacFarlane AJ, et al. Prenatal Exposure to Persistent Organic Pollutants and Maternal Folic Acid Supplementation: Their Impact on Glucose Homeostasis in Male Rat Descendants. Environments. 2021; 8(3):24. https://doi.org/10.3390/environments8030024
Chicago/Turabian StyleNavarro, Pauline, Mathieu Dalvai, Phanie L. Charest, Pauline M. Herst, Maryse Lessard, Bruno Marcotte, Nadine Leblanc, Sarah Kimmins, Jacquetta Trasler, Amanda J. MacFarlane, and et al. 2021. "Prenatal Exposure to Persistent Organic Pollutants and Maternal Folic Acid Supplementation: Their Impact on Glucose Homeostasis in Male Rat Descendants" Environments 8, no. 3: 24. https://doi.org/10.3390/environments8030024
APA StyleNavarro, P., Dalvai, M., L. Charest, P., Herst, P. M., Lessard, M., Marcotte, B., Leblanc, N., Kimmins, S., Trasler, J., MacFarlane, A. J., Marette, A., L. Bailey, J., & Jacques, H. (2021). Prenatal Exposure to Persistent Organic Pollutants and Maternal Folic Acid Supplementation: Their Impact on Glucose Homeostasis in Male Rat Descendants. Environments, 8(3), 24. https://doi.org/10.3390/environments8030024






