Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research
Abstract
1. Introduction
2. Organic Residues as Soil Amendments
2.1. Origin, Characteristics and Impact on Soil Properties
2.2. European Legislation on the Use of Organic Residues as Soil Amendments
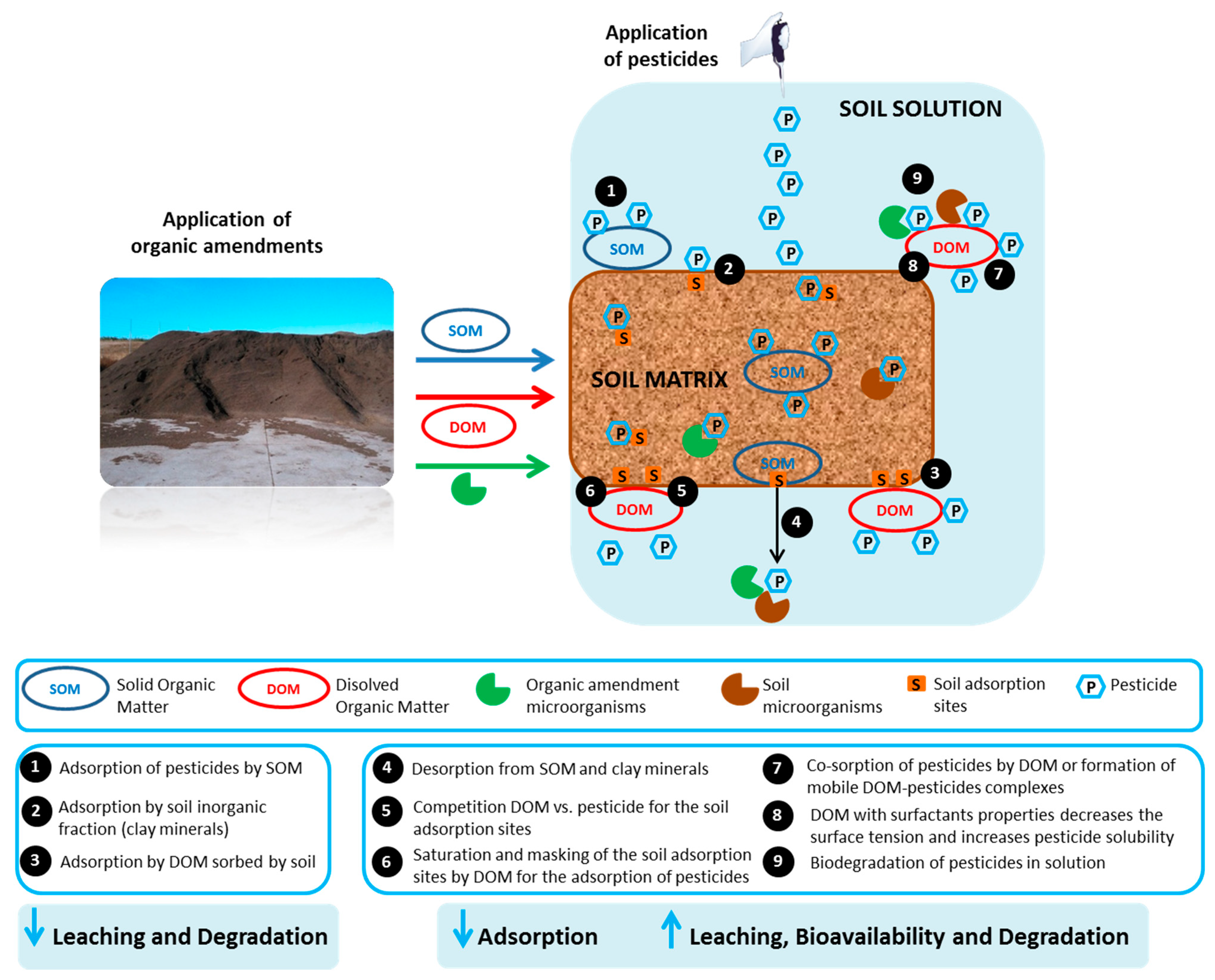
3. Effect of Organic Residues on the Fate of Pesticides in Soil
3.1. Effect of Organic Residues on the Adsorption-Desorption of Pesticides
3.2. Effect of Organic Residues on Pesticide Leaching
3.3. Effect of Organic Residues on Pesticide Dissipation
4. Conclusions
5. Future Perspectives on the Application of Pesticides and Organic Amendments in Soils
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Nyamwasa, I.; Li, K.; Rutikanga, A.; Rukazambuga, D.N.T.; Zhang, S.; Yin, J.; Ya-Zhong, C.; Zhang, X.X.; Sun, X. Soil insect crop pests and their integrated management in East Africa: A review. Crop Prot. 2018, 106, 163–176. [Google Scholar] [CrossRef]
- United Nations. Global Issues. Our Global Population. 2020. Available online: https://www.un.org/en/sections/issues-depth/population (accessed on 10 September 2020).
- Ahmad, A.; Shahid, M.; Khalid, S.; Zaffar, H.; Naqvi, T.; Pervez, A.; Bilal, M.; Ali, M.A.; Abbas, G.; Nasim, W. Residues of endosulfan in cotton growing area of Vehari, Pakistan: An assessment of knowledge and awareness of pesticide use and health risks. Environ. Sci. Pollut. 2018, 26, 20079–20091. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Preet, G.; Sidhu, S.; Handa, N. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Pesticide Reports. Pesticides Global Market Opportunities and Strategies to 2023. 2020. Available online: https://www.thebusinessresearchcompany.com/report/pesticides-market (accessed on 10 September 2020).
- Worldatlas. 2018. Available online: https://www.worldatlas.com/articles/top–pesticideconsuming–countries–of–the–world.html (accessed on 10 September 2020).
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, pesticides, personal care products and microplastics contamination assessment of Al-Hassa irrigation network (Saudi Arabia) and its shallow lakes. Sci. Total Environ. 2020, 701, 135021. [Google Scholar] [CrossRef] [PubMed]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. 2009, 16, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, S.; Pose-Juan, E.; Herrero-Hernández, E.; Álvarez-Martín, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, S. Pesticide residues in groundwaters and soils of agricultural areas in the Águeda River Basin from Spain and Portugal. Int. J. Environ. Anal. Chem. 2013, 93, 1585–1601. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S. Intra–annual trends of fungicide residues in waters from vineyard areas in La Rioja region of northern Spain. Environ. Sci. Pollut. Res. 2016, 23, 22924–22936. [Google Scholar] [CrossRef] [PubMed]
- AL-Ahmadi, M.S. Pesticides, anthropogenic activities, and the health of our environment safety. IntechOpen 2019. [Google Scholar] [CrossRef][Green Version]
- Baxter, J.; Cummings, S.P. The degradation of the herbicide bromoxynil and its impact on bacterial diversity in a top soil. J. Appl. Microbiol. 2008, 104, 1605–1616. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.; Singh, A. Pesticides use and its effect on soil bacteria and fungal populations; microbial biomass carbon and enzymatic activity. Curr. Sci. 2019, 116, 643–649. [Google Scholar] [CrossRef]
- Satapute, P.; Kamble, M.V.; Adhikari, S.S.; Jogaiah, S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. Sci. Total Environ. 2019, 651, 2334–2344. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Shen, Z.; Zhang, C.; Wang, Z.; He, T. Spatial and seasonal distribution of organochlorine pesticides in the sediments of the Yangtze Estuary. Chemosphere 2014, 114, 233–240. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Pose-Juan, E.; Sánchez-González, S.; Andrades, M.S.; Sánchez-Martín, M.J. Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Sci. Total Environ. 2017, 609, 161–171. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Barizon, R.R.M.; Figueiredo, R.O.; de Souza Dutra, D.R.C.; Regitano, J.B.; Ferracini, V.L. Pesticides in the surface waters of the Camanducaia River watershed, Brazil. J. Environ. Sci. Health B 2020, 55, 283–292. [Google Scholar] [CrossRef]
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci. Total Environ. 2019, 650, 2188–2198. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Simón-Egea, A.B.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Andrades, M.S. Monitoring and environmental risk assessment of pesticide residues and some of their degradation products in natural waters of the Spanish vineyard region included in the Denomination of Origin Jumilla. Environ. Pollut. 2020, 264, 114666. [Google Scholar] [CrossRef]
- Marsala, R.Z.; Capri, E.; Russo, E.; Bisagni, M.; Colla, R.; Lucini, L.; Gallo, A.; Suciu, N.A. First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Total Environ. 2020, 736, 139730. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union L 2008, 348, 84–97.
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Impact of spent mushroom substrates on the fate of pesticides in soil, and their use for preventing and/or controlling soil and water contamination: A review. Toxics 2016, 4, 17. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Application of a biosorbent to soil: A potential method for controlling water pollution by pesticides. Environ. Sci. Pollut. Res. 2016, 23, 9192–9203. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Dickerson, G. A Sustainable Approach to Recycling Urban and Agricultural Organic Wastes; Guide H–159, Cooperative Extension Service; College of Agriculture and Home Economics: Belen, NM, USA, 2000. [Google Scholar]
- Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.; Perez-Espinosa, A.; Rufete, B.; Paredes, C. Characterization of the organic matter pool in manures. Bioresour. Technol. 2005, 96, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in the sorption-desorption of fungicides over time in an amended sandy clay loam soil under laboratory conditions. J. Soils Sediments 2012, 12, 1111–1123. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.S.; Herrero-Hernández, E.; Ordax, J.M.; Marín-Benito, J.M.; Draoui, K.; Sánchez-Martín, M.J. Adsorption of pesticides by sewage sludge, grape marc, spent mushroom substrate and by amended soils. Int. J. Environ. Anal. Chem. 2012, 92, 933–948. [Google Scholar] [CrossRef]
- Castillo, J.M.; Beguet, J.; Martin-Laurent, F.; Romero, E. Multidisciplinary assessment of pesticide mitigation in soil amended with vermicomposted agroindustrial wastes. J. Hazard. Mater. 2016, 304, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Worrall, F.; Fernandez-Perez, M.; Johnson, A.C.; Flores-Cesperedes, F.; Gonzalez-Pradas, E. Limitations on the role of incorporated organic matter in reducing pesticide leaching. J. Contam. Hydrol. 2001, 49, 241–262. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Plant. Nutr. Soil Sci. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Lugato, E.; Bampa, F.; Panagos, P.; Montanarella, L.; Jones, A. Potential carbon sequestration of European arable soils estimated by modelling a comprehensive set of management practices. Glob. Change Biol. 2014, 20, 3557–3567. [Google Scholar] [CrossRef]
- Hijbeek, R.; Van Ittersum, M.K.; ten Berge, H.F.; Gort, G.; Spiegel, H.; Whitmore, A.P. Do organic inputs matter—A meta–analysis of additional yield effects for arable crops in Europe. Plant Soil 2017, 411, 293–303. [Google Scholar] [CrossRef]
- UNFCCC, United Nations Framework Convention on Climate Change. Join the 4/1000 Initiative. In Soils for Food Security and Climate; UNFCCC: Rio de Janeiro, Brazil; New York, NY, USA, 2015; Available online: https://unfccc.int/news/join–the–41000–initiative–soils–for–food–security–and–climate (accessed on 10 September 2020).
- Donn, S.; Wheatley, R.E.; McKenzie, B.M.; Loades, K.W.; Hallett, P.D. Improved soil fertility from compost amendment increases root growth and reinforcement of surface soil on slope. Ecol. Eng. 2014, 71, 458–465. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.; Ibrahim, M.H.; Hashim, R. Land Application of sewage sludge: Physicochemical and microbial response. Rev. Environ. Contam. Toxicol. 2011, 214, 41–61. [Google Scholar]
- Gómez-Sagasti, M.T.; Hernández, A.; Artetxe, U.; Garbisu, C.; Becerril, J.M. How Valuable Are Organic Amendments as Tools for the Phytomanagement of Degraded Soils? The Knowns, Known Unknowns, and Unknowns. Front. Sustain. Food Syst. 2018, 2, 68. [Google Scholar] [CrossRef]
- Fließbach, A.; Oberholzer, H.R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Domínguez, M.T.; Madejón, E.; Madejón, P.; Pastorelli, R.; Renella, G. Long-term effects of organic amendments on bacterial and fungal communities in a degraded Mediterranean soil. Geoderma 2018, 332, 20–28. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.D. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 2012, 7, 51897. [Google Scholar] [CrossRef] [PubMed]
- Memoli, V.; De Marco, A.; Baldantoni, D.; De Nicola, F.; Maisto, G. Short- and long-term effects of a single application of two organic amendments. Ecosphere 2017, 8, e02009. [Google Scholar] [CrossRef]
- Ventorino, V.; de Marco, A.; Pepe, O.; Virzo de Santo, A.; Moschetti, G. Impact of Innovative Agricultural Practices of Carbon Sequestration on Soil Microbial Community. Carbon Sequestration in Agricultural Soils. A Multidisciplinary Approach to Innovative Methods; Piccolo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–177. [Google Scholar] [CrossRef]
- Abbott, L.K.; Macdonald, L.M.; Wong, M.T.F.; Webb, M.J.; Jenkins, S.N.; Farrell, M. Potential roles of biological amendments for profitable grain production—A review. Agric. Ecosyst. Environ. 2018, 256, 34–50. [Google Scholar] [CrossRef]
- Edmeades, D.C. The long-term effects of manures and fertilizers on soil productivity and quality: A review. Nut. Cycl. Agroecosyst. 2003, 66, 165–180. [Google Scholar] [CrossRef]
- Leroy, B.L.M.; Herath, H.M.S.K.; Sleutel, S.; De Neve, S.; Gabriels, D.; Reheul, D.; Moens, M. The quality of exogenous organic matter: Short-term effects on soil physical properties and soil organic matter fractions. Soil Use Manag. 2008, 24, 139–147. [Google Scholar] [CrossRef]
- Scotti, R.; Conte, P.; Berns, A.E.; Alonzo, G.; Rao, M.A. Effect of organic amendments on the evolution of soil organic matter in soils stressed by intensive agricultural practices. Curr. Org. Chem. 2013, 17, 2998–3005. [Google Scholar] [CrossRef][Green Version]
- Six, J.; Paustian, K. Aggregate–associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Ingelmo-Sánchez, F.; Rubio-Delgado, J. Efecto de la aplicación del compost sobre las propiedades físicas y químicas del suelo. In Compostaje; Moreno-Casco, J., Moral-Herrero, R., Eds.; Mundi-Prensa: Madrid, Spain, 2008. [Google Scholar]
- Hernández, T.; García, E.; García, C. A strategy for marginal semiarid degraded soil restoration: A sole addition of compost at a high rate. A five-year field experiment. Soil Biol. Biochem. 2015, 89, 61–71. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Wu, L.; Huang, Y.; Christie, P. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming. Environ. Sci. Pollut. Res. Int. 2015, 22, 5908–5918. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, J.; Hu, B.; Chu, G. Ammonia-oxidizing bacteria are sensitive and not resilient to organic amendment and nitrapyrin disturbances, but ammonia-oxidizing archaea are resistant. Geoderma 2021, 384, 114814. [Google Scholar] [CrossRef]
- EUROSTAT, Statistical Office of the European Communities. Waste Statistics. 2020. Available online: https://ec.europa.eu/eurostat/statistics–explained/index.php/Waste_statistics (accessed on 10 September 2020).
- EC, European Commission Decision 2015/2099. Review of Waste Policy and Legislation. 2020. Available online: https://ec.europa.eu/environment/waste/target_review.htm (accessed on 10 September 2020).
- ECN, European Compost Network. Bio-Waste in Europe. 2020. Available online: https://www.compostnetwork.info/policy/biowaste–in–europe/ (accessed on 10 September 2020).
- EEA, European Environment Agency. Waste Generation in Europe. 2020. Available online: https://www.eea.europa.eu/data–and–maps/indicators/waste–generation–4/assessment#:~:text=More%20and%20more%20waste%20is,by%2070%20kg%20per%20capita (accessed on 10 September 2020).
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- EC (European Commission). Impact Assessment of the Thematic Strategy on Soil SEC 620. 2006. Available online: https://ec.europa.eu/environment/archives/soil/pdf/SEC_2006_620.pdf (accessed on 10 September 2020).
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef]
- Schreuder, R.; De Visser, C. EIP AGRI Focus Group Protein Crops: Final Report. European Innovation Partnership for Agricultural Productivity and Sustainability (EIP AGRI). Brussels. 2014. Available online: https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-protein-crops-final-report (accessed on 10 September 2020).
- Saveyn, H.; Eder, P. End of Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost and Digestate): Technical Proposal; © European Commission in 2014, EUR 26425; Joint Research Centre—Institute for Prospective Technological Studies: Luxembourg, 2014; p. 310. [Google Scholar]
- Büyüksönmez, F.; Rink, R.; Hess, T.; Bechinski, E. Occurrence, degradation and fate of pesticides during composting. Part I. Composting, pesticides, and pesticides degradation. Compost Sci. Util. 1999, 7, 66–82. [Google Scholar] [CrossRef]
- Büyüksönmez, F.; Rynk, R.; Hess, T.; Bechinski, E. Occurrence, degradation and fate of pesticides during composting. Part II. Occurrence and fate de pesticides in compost and composting systems. Compost Sci. Util. 2000, 8, 61–81. [Google Scholar] [CrossRef]
- Briceño, G.; Palma, G.; Durán, N. Influence of organic amendment on the biodegradation and movement of pesticides. Crit. Rev. Environ. Sci. Technol. 2007, 37, 233–271. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Assessment of spent mushroom substrate as sorbent of fungicides: Influence of sorbent and sorbate properties. J. Environ. Qual. 2012, 41, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Brown, C.D.; Herrero-Hernández, E.; Arienzo, M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Use of raw or incubated organic wastes as amendments in reducing pesticide leaching through soil columns. Sci. Total Environ. 2013, 463–464, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M. Effect of different organic amendments on the dissipation of linuron, diazinon and myclobutanil in an agricultural soil incubated for different time periods. Sci. Total Environ. 2014, 476–477, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J. Pesticides removal using conventional and low–cost adsorbents: A review. Clean-Soil Air Water 2011, 39, 1105–1119. [Google Scholar] [CrossRef]
- Garrido, I.; Vela, N.; Fenoll, J.; Navarro, G.; Pérez–Lucas, G.; Navarro, S. Testing of leachability and persistence of sixteen pesticides in three agricultural soils of a semiarid Mediterranean region. Span. J. Agric. Res. 2015, 13, 1104. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Sun, K.; Zhao, Y.; Lin, C. Sorption of Tetracycline to Sediments and Soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. FESE 2010, 4, 421–429. [Google Scholar] [CrossRef]
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–525. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.L.; Jia, L.X.; Liu, Y.; Pan, B.; Yang, Y.; Lin, Y. Effects of two different organic amendments addition to soil on sorption-desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum. aestivum L.). J. Environ. Manag. 2016, 167, 130–138. [Google Scholar] [CrossRef]
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health B 2019, 54, 728–735. [Google Scholar] [CrossRef]
- Petter, F.A.; Ferreira, T.S.; Sinhorin, A.P.; Lima, L.B.; Almeida, F.A.; Pacheco, L.P.; Silva, A.F. Biochar increases diuron sorption and reduces the potential contamination of subsurface water with diuron in a sandy soil. Pedosphere 2019, 29, 801–809. [Google Scholar] [CrossRef]
- García-Jaramillo, M.; Trippe, K.M.; Helmus, R.; Knicker, H.E.; Cox, L.; Hermosín, M.C.; Kalbitz, K. An examination of the role of biochar and biochar water-extractable substances on the sorption of ionizable herbicides in rice paddy soils. Sci. Total Environ. 2020, 706, 135682. [Google Scholar] [CrossRef]
- Khan, S.U. Pesticides in the Soil Environment; Elsevier: New, York, NY, USA, 2016. [Google Scholar]
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci. Total Environ. 2019, 658, 87–94. [Google Scholar] [CrossRef]
- Cox, L.; Cecchi, A.; Celis, R.; Hermosín, M.; Koskinen, W.; Cornejo, J. Effect of exogenous carbon on movement of simazine and 2,4–D in soils. Soil Sci. Soc. Am. J. 2001, 65, 1688–1695. [Google Scholar] [CrossRef]
- Plaza, C.; Polo, A.; Brunetti, G.; Garcia-Gil, J.; D’Orazio, V. Soil fulvic acid properties as a means to assess the use of pig amendment. Soil Till. Res. 2003, 74, 179–190. [Google Scholar] [CrossRef]
- Thorstensen, C.; Lode, O.; Eklo, O.; Christianse, A. Sorption of bentazone, dichlorprop, MCPA, and propiconazole in references soils from Norway. J. Environ. Qual. 2001, 30, 2046–2052. [Google Scholar] [CrossRef]
- Cambier, P.; Pot, V.; Mercier, V.; Michaud, A.; Benoit, P.; Revallier, A.; Houot, S. Impact of long–term organic residue recycling in agriculture on soil solution composition and trace metal leaching in soils. Sci. Total Environ. 2014, 499, 560–573. [Google Scholar] [CrossRef]
- Barriuso, E.; Andrades, M.S.; Benoit, P.; Houot, S. Pesticide desorption from soils facilitated by dissolved organic matter coming from composts: Experimental data and modelling approach. Biogeochemistry 2011, 106, 117–133. [Google Scholar] [CrossRef]
- Huang, X.; Lee, S. Effects of dissolved organic matter from animal waste effluent on chlorpyrifos sorption by soils. J. Environ. Qual. 2001, 30, 1258–1265. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Ordax, J.M.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Leaching of two fungicides in spent mushroom substrate amended soil: Influence of amendment rate; fungicide ageing and flow condition. Sci. Total Environ. 2017, 584–585, 828–837. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Peña, A. Adsorption-desorption of dimethenamid and fenarimol onto three agricultural soils as affected by treated wastewater and fresh sewage sludge-derived dissolved organic carbon. J. Environ. Manag. 2018, 217, 592–599. [Google Scholar] [CrossRef]
- Wanner, U.; Führ, F.; Burauel, P. Influence of the amendment of corn straw on the degradation behaviour of the fungicide dithianon in soil. Environ. Pollut. 2005, 133, 63–70. [Google Scholar] [CrossRef]
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J. Soils Sediments 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Thevenot, M.; Dousset, S. Compost effect on diuron retention and transport in structured vineyard soils. Pedosphere 2015, 25, 25–36. [Google Scholar] [CrossRef]
- Li, K.; Xing, B.S.; William, A.T. Effect of organic fertilizers derived dissolved organic matter on they sorption and leaching. Environ. Pollut. 2005, 134, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Spark, K.M.; Swift, R.S. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161. [Google Scholar] [CrossRef]
- Parlavecchia, M.; Orazio, V.D.; Loffredo, E. Wood biochars and vermicomposts from digestate modulate the extent of adsorption-desorption of the fungicide metalaxyl-m in a silty soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 35924–35934. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Feng, D.; He, J.; Li, F.; Yu, H.; Ge, C. Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Mendes, K.F.; Hall, K.E.; Takeshita, V.; Rossi, M.L.; Tornisielo, V.L. Animal bonechar increases sorption and decreases leaching potential of aminocyclopyrachlor and mesotrione in a tropical soil. Geoderma 2018, 316, 11–18. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Arienzo, M.; Sánchez-Martín, M.J. Study of processes influencing bioavailability of pesticides in wood-soil systems: Effect of different factors. Ecotoxicol. Environ. Saf. 2017, 139, 454–462. [Google Scholar] [CrossRef]
- Mendes, K.F.; Alonso, F.G.; Mertens, T.B.; Inoue, M.; Oliveira, M.G.D.; Tornisielo, V.L. Aminocyclopyrachlor and mesotrione sorption-desorption in municipal sewage sludge-amended soil. Soil Plant Nutr. 2019, 78, 131–140. [Google Scholar] [CrossRef]
- Duhan, A.; Oliver, D.P.; Rashti, M.R.; Du, J.; Kookana, R.S. Organic waste from sugar mills as a potential soil ameliorant to minimize herbicide runoff to the Great Barrier Reef. Sci. Total Environ. 2020, 713, 136640. [Google Scholar] [CrossRef]
- Di Marsico, A.; Scrano, L.; Amato, M.; Gàmiz, B.; Real, M.; Cox, L. Mucilage from seeds of chia (Salvia hispanica L.) used as soil conditioner, effects on the sorption-desorption of four herbicides in three different soils. Sci. Total Environ. 2018, 625, 531–538. [Google Scholar] [CrossRef]
- Xing, B. Sorption of naphthalene and phenanthrene by soil humic acids. Environ. Pollut. 2001, 111, 303–309. [Google Scholar] [CrossRef]
- Gaonkar, O.D.; Nambi, I.M.; Govindarajan, S.K. Soil organic amendments: Impacts on sorption of organophosphate pesticides on an alluvial soil. J. Soils Sediments 2019, 19, 566–578. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Zhang, H.; Zhong, X.; Liu, Y.; Liao, S.; Yuea, X.; Zhouc, G. Sorption of carbendazim on activated carbons derived from rape straw and its mechanism. RSC Adv. 2019, 9, 41745–41754. [Google Scholar] [CrossRef]
- García-Delgado, C.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Organic carbon nature determines the capacity of organic amendments to adsorb pesticides in soil. J. Hazard. Mater. 2020, 390, 122162. [Google Scholar] [CrossRef]
- Bear, J.; Cheng, A.H.D. Modeling Groundwater Flow and Contaminant Transport; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Carter, A. Herbicide movement in soils: Principles, pathways and processes. Weed Res. 2000, 40, 113–122. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Navarro, G. An overview on the environmental behavior of pesticide residues in soils. Span. J. Agric. Res. 2007, 5, 357–375. [Google Scholar] [CrossRef]
- Kerle, E.A.; Jenkins, J.J.; Vogue, P.A. Understanding Pesticide Persistence and Mobility for Groundwater and Surface Water Protection; Extension & Station Communications Oregon State University: Corvallis, OR, USA, 2007. [Google Scholar]
- Reichenberger, S.; Bach, M.; Skitschak, A.; Frede, H.G. Mitigation strategies to reduce pesticide inputs into ground– and surface water and their effectiveness, A review. Sci. Total Environ. 2007, 384, 1–35. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Effect of spent mushroom substrate amendment of vineyard oils on the behavior of fungicides: 2. Mobility of penconazole and metalaxyl in undisturbed soil cores. J. Agric. Food Chem. 2009, 57, 9643–9650. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Mamy, L. Modeling fungicides mobility in undisturbed vineyard soil cores unamended and amended with spent mushroom substrates. Chemosphere 2015, 134, 408–416. [Google Scholar] [CrossRef]
- López-Piñeiro, J.A.; Peña, D.; Albarrán, A.; Sánchez-Llerena, J.; Becerra, D. Behavior of MCPA in four intensive cropping soils amended with fresh, composted, and aged olive mill waste. J. Contam. Hydrol. 2013, 152, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Aharonov-Nadborny, R.; Raviv, M.; Graber, E.R. Soil spreading of liquid olive mill processing wastes impacts leaching of adsorbed terbuthylazine. Chemosphere 2016, 156, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Vela, N.; Navarro, G.; Perez-Lucas, G.; Navarro, S. Assessment of agro-industrial and composted organic wastes for reducing the potential leaching of triazine herbicide residues through the soil. Sci. Total Environ. 2014, 493, 124132. [Google Scholar] [CrossRef]
- Larsbo, M.; Löfstrand, E.; de Veer, D.V.; Ulén, B. Pesticide leaching from two Swedish top soils of contrasting texture amended with biochar. J. Contam. Hydrol. 2013, 147, 73–81. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Hermosín, M.C.; Cox, L. Biochar soil additions affect herbicide fate: Importance of application timing and feedstock species. J. Agric. Food Chem. 2017, 65, 3109–3117. [Google Scholar] [CrossRef]
- Gámiz, B.; Cox, L.; Hermosín, M.C.; Spokas, K.; Celis, R. Assessing the effect of organoclays and biochar on the fate of abscisic acid in soil. J. Agric. Food Chem. 2017, 65, 29–38. [Google Scholar] [CrossRef]
- Majumdar, K.; Singh, N. Effect of soil amendments on sorption and mobility of metribuzin in soils. Chemosphere 2007, 66, 630–637. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Singh, N. Managing metolachlor and atrazine leaching losses using lignite fly ash. Ecotoxicol. Environ. Saf. 2012, 84, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bayo, J.D.; Nogales, R.; Romero, E. Winery vermicomposts to control the leaching of diuron, imidacloprid and their metabolites: Role of dissolved organic carbon content. J. Environ. Sci. Health B 2015, 50, 190–200. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Hellin, P.; Flores, P.; Vela, N.; Navarro, S. Use of different organic wastes as strategy to mitigate the leaching potential of phenylurea herbicides through the soil. Environ. Sci. Pollut. Res. 2015, 22, 4336–4349. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Transport of 14C–prosulfocarb through soil columns under different amendment, herbicide incubation and irrigation regimes. Sci. Total Environ. 2020, 701, 1–8. [Google Scholar] [CrossRef]
- Delwiche, K.B.; Lehmann, J.; Walter, M.T. Atrazine leaching from biochar-amended soils. Chemosphere 2014, 95, 346–352. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agric. Food. Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, Q.; Yang, Y.; Zhu, X.; Fu, H. Organochlorine pesticides in the lower reaches of Yangtze River: Occurrence, ecological risk and temporal trends. Ecotoxicol. Environ. Saf. 2013, 87, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Xiong, Z.; Liu, P.; Pan, G. Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol. Fertil. Soils. 2011, 47, 887–896. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Vela, N.; Escudero, J.A.; Navarro, G.; Navarro, S. Valorization of organic wastes to reduce the movement of priority substances through a semiarid soil. Water Air Soil Pollut 2017, 228, 119. [Google Scholar] [CrossRef]
- Samarendra-Singh, N.; Irani, S.; Shaon, M.; Das, K. Leaching of Clothianidin in Two Different Indian Soils: Effect of Organic Amendment. Bull. Environ. Contam. Toxicol. 2018, 100, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, H.; Börjesson, E.; Stenström, J. Effects of a wood-based biochar on the leaching of pesticides chlorpyrifos, diuron, glyphosate and MCPA. J. Environ. Manag. 2017, 191, 28–34. [Google Scholar] [CrossRef]
- Ling, W.; Xu, J.; Gao, Y. Dissolved organic matter enhances the sorption of atrazine by soils. Biol. Fertil. Soils 2006, 42, 418–425. [Google Scholar] [CrossRef]
- Chabauty, F.; Pot, V.; Bourdat-Deschamps, M.; Bernet, N.; Labat, C.; Benoit, P. Transport of organic contaminants in subsoil horizons and effects of dissolved organic matter related to organic waste recycling practices. Environ. Sci. Pollut. Res. Int. 2016, 23, 6907–6918. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Alexander, M. How toxic are toxic chemicals in soil. Environ. Sci. Technol. 1995, 29, 2713–2717. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, G.; Singh, S.B.; Lal, S.P.; Yaduraju, N.T. Effect of long-term field application of pendimethalin: Enhanced degradation in soil. Pest Manag. Sci. 2000, 56, 202–206. [Google Scholar] [CrossRef]
- Luo, Y.; Atashgahi, S.; Rijnaarts, H.H.M.; Comans, R.N.J.; Sutton, N.B. Influence of different redox conditions and dissolved organic matter on pesticide biodegradation in simulated groundwater systems. Sci. Total Environ. 2019, 677, 692–699. [Google Scholar] [CrossRef]
- Yavari, S.; Sapari, N.B.; Malakahmad, A.; Yavari, S. Degradation of imazapic and imazapyr herbicides in the presence of optimized oil palm empty fruit bunch and rice husk biochars in soil. J. Hazard. Mater. 2019, 366, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.E.; Estrada, I.B.; Martínez, O.; Martín-Villacorta, J.; Aller, A.; Morán, A. Influence of the application of sewage sludge on the degradation of pesticides in the soil. Chemosphere 2004, 57, 673–679. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Field versus laboratory experiments to evaluate the fate of azoxystrobin in an amended vineyard soil. J. Environ Manag. 2015, 163, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Arshad, M.; Springael, D.; SøRensen, S.R.; Bending, G.D.; Devers-Lamrani, M.; Maqbool, Z.; Martin-Laurent, F. Abiotic and biotic processes governing the fate of phenylurea herbicides in soils: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1947–1998. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Pose-Juan, E.; Rodríguez-Cruz, M.S. Effect of different rates of spent mushroom substrate on the dissipation and bioavailability of cymoxanil and tebuconazole in an agricultural soil. Sci. Total Environ. 2016, 550, 495–503. [Google Scholar] [CrossRef]
- Flores-Céspedes, F.; Fernández-Pérez, M.; Villafranca-Sánchez, M.; González-Pradas, E. Co-sorption study of organic pollutants and dissolved organic matter in a soil. Environ. Pollut. 2006, 142, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Liébana, J.A.; ElGouzi, S.; Peña, A. Laboratory persistence in soil of thiacloprid, pendimethalin and fenarimol incubated with treated wastewater and dissolved organic matter solutions. Contribution of soil biota. Chemosphere 2017, 181, 508–517. [Google Scholar] [CrossRef]
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Ehaliotis, C. Degradation and adsorption of pesticides in compost–based biomixtures as potential substrates for biobeds in southern Europe. J. Agric. Food Chem. 2010, 58, 9147–9156. [Google Scholar] [CrossRef]
- Karanasios, E.; Tsiropoulos, N.G.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U. Novel biomixtures based on local Mediterranean lignocellulosic materials: Evaluation for use in biobed systems. Chemosphere 2010, 80, 914–921. [Google Scholar] [CrossRef] [PubMed]
- García-Delgado, C.; D’Annibale, A.; Pesciaroli, L.; Yunta, F.; Crognale, S.; Petruccioli, M.; Eymar, E. Implications of polluted soil biostimulation and bioaugmentation with spent mushroom substrate (Agaricus bisporus) on the microbial community and polycyclic aromatic hydrocarbons biodegradation. Sci. Total Environ. 2015, 508, 20–28. [Google Scholar] [CrossRef]
- Castillo, M.P.; Torstensson, L. Effect of biobed composition, moisture and temperature on the degradation of pesticides. J. Agric. Food Chem. 2007, 55, 5725–5733. [Google Scholar] [CrossRef]
- Tang, F.; Xu, Z.; Gao, M.; Li, L.; Li, H.; Cheng, H.; Tian, G. The dissipation of cyazofamid and its main metabolite in soil response oppositely to biochar application. Chemosphere 2019, 218, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Hao, H.; Ding, M.; Wu, R.; Xu, H.; Xue, F.; Lu, C. Adsorption and degradation of imazapic in soils under different environmental conditions. PLoS ONE 2019, 14, e0219462. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Previous degradation study of two herbicides to simulate their fate in a sandy loam soil: Effect of the temperature and the organic amendments. Sci. Total Environ. 2019, 653, 1301–1310. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; García-Delgado, C.; Sánchez-Martín, M.J.; Rodríguez–Cruz, M.S. Assessment of 14C–prosulfocarb dissipation mechanism in soil after amendment and its impact on the microbial community. Ecotoxicol. Environ. Saf. 2019, 182, 109395. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Lian, J.; Wang, H.; Cai, L.; Yu, Y. Exploring bacterial community structure and function associated with atrazine biodegradation in repeatedly treated soils. J. Hazard. Mater. 2015, 286, 457–465. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Wang, X.; Wang, J.; Wang, J. Impact of repeated applications of metalaxyl on its dissipation and microbial community in soil. Water Air Soil Pollut. 2015, 226, 1–14. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Dong, F.; Liu, X.; Wu, X.; Zheng, Y. Effects of repeated applications of chlorimuron–ethyl on the soil microbial biomass, activity and microbial community in the greenhouse. Bull. Environ. Contam. Toxicol. 2014, 92, 175–182. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Igual, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Influence of herbicide triasulfuron on soil microbial community in an unamended soil and a soil amended with organic residues. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of herbicides after repeated application in soils amended with green compost and sewage sludge. J. Environ. Manag. 2018, 223, 1068–1077. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Barba, V.; Ordax, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Application of green compost as amendment in an agricultural soil: Effect on the behaviour of triasulfuron and prosulfocarb under field conditions. J. Environ. Manag. 2018, 207, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Barba, V.; Ordax, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Recycling organic residues in soils as amendments: Effect on the mobility of two herbicides under different management practices. J. Environ. Manag. 2018, 224, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.J.; Rodríguez-Cruz, M.S.; García-Delgado, C.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Mobility monitoring of two herbicides in amended soils: A field study for modeling applications. J. Environ. Manag. 2020, 260, 110161. [Google Scholar] [CrossRef]
- FOCUS (Forum for Coordination of Pesticide Fate Models and Their Use). FOCUS Groundwater Scenarios in the EU Review of Active Substances; Report of the FOCUS Groundwater Scenarios Workgroup EC; Document Reference Sanco/321/2000 Rev.2; European Commission: Luxembourg, 2000; p. 202. [Google Scholar]
- Marín–Benito, J.M.; Mamy, L.; Carpio, M.J.; Sánchez–Martín, M.J.; Rodríguez–Cruz, M.S. Sci. Total Environ. Modelling herbicides mobility in amended soils: Calibration and test of PRZM and MACRO. Sci. Total Environ. 2020, 717, 137019. [Google Scholar] [CrossRef]
- Marín–Benito, J.M.; Carpio, M.J.; Mamy, L.; Andrades, M.S.; Sánchez–Martín, M.J.; Rodríguez–Cruz, M.S. Field measurement and modelling of chlorotoluron and flufenacet persistence in unamended and amended soils. Sci. Total Environ. 2020, 725, 138374. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | Soil Characteristics | Organic Amendment/Dose | Experimental Design | Results | Reference |
|---|---|---|---|---|---|
| Metalaxyl-M | Silt loam soil (pH 6.70, OC 2.90%) | Biochar from grape vine pruning residues (BC–G) (pH 9.9, OC 75.1%) and spruce wood (BC–S) (pH 9.1, OC 83.8%). Vermicomposts (VC) from manure and olive mill wastewater (VC–M) (pH 7.9, OC 31.6%) and buffalo manure (VC–B) (pH 7.8, OC 36.6%) Biochar/soil: 2% (w w–1) | Sorbent/Solution: 25 mg biochar/5 mL or 3 g soil/8 mL water solution Herbicide concentration: 1–20 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC | Metalaxyl sorption order: non–amended soil < soil–VC–M ≤ soil–VC–B < soil–BC–S < soil–BC–G Much higher sorption efficiency by BC than by VC and a lower extent of metalaxyl desorption due to composition and structural differences of the organic matter of BC. | Parlavecchia et al. [103] |
| Oxyfluorfen | Loamy clay soil (pH 4.85, OC 0.84%) Sandy loam soil (pH 7.55, OC 0.98%) Clay loam soil (pH 6.59, OC 2.23%) | Biochar from peanut (BCP) (pH, 7.05, C 49.17%), chestnut (BCC) (pH 6.08, C 58.07%), bamboo (BCB) (pH 7.45, C 63.25%), maize straw (BCM) (pH 6.83, C 43.36%), rice hull (BCR) (pH 6.96, C 33.60%) BCR/soil: 0.5%, 1%, or 2% (w w–1) | Sorbent/Solution: 0.1 g biochar/40 mL or 2 g soil/200 mL 0.01 M CaCl2 Herbicide concentration: 0.05–10 mg L−1 Shaken: 6 days, T: 25 °C Aging time of BCR-soil: 1, 3, 6 months Analytical determination: GC/MS | BC sorption capacities followed the order: BCR > BCB > BCM > BCC > BCP owing to differences in physicochemical properties. BCR sorption capacity decreased with aging time. | Wu et al. [89] |
| Atrazine | Krasnozem soil (pH 7.05, OC 0.89%, clay 28.2%, silt 37.8%) | Biochar from cassava wastes (pH 9.55, C 62.38%) obtained at 750 °C (MS750). SSA: 430.4 m2/g, MP: 0.144 m3/g Biochar/soil: 0%, 0.1%, 0.5%, 1%, 3% and 5% (w w–1) | Sorbent/Solution: 0.2–2 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.5–20 mg L−1 Shaken: 24 h T: 15, 25, 35 °C, pH: 3,5, 7, 9 Analytical determination: HPLC | Great sorption capacity for atrazine of MS750 in soil due to high surface area and micropore volume. High degrees of aromaticity and hydrophobicity (H/C: 0.02, N + O/C: 0.09) of MS750 supplied numerous sorption sites. | Deng et al. [104] |
| Hexazinone Metribuzin Quinclorac | Sandy loam soil (pH 6.9, OC 0.52%, clay 15.1%, silt 3.3%) | Bone char (BC) (pH 9.72, C 11%) BC/soil: 5% (w w–1) or 60 t ha−1 | Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.63–3.13 mgL−1, 1.60–8 mgL−1, 0.31–1.56 mgL−1 Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation | High sorption of herbicides by BC, regardless of the application form of the material (topsoil or incorporated in the surface layer in leaching columns). | Mendes et al. [105] |
| Aminocyclopyrachlor Mesotrione | Clay soil (pH 6.44, OC 2.73%), clay 50.9%, silt 19.6%) | Bone Char (BC) (pH 9.72, C 11%) BC/soil: 0%, 1%, 5%, 10%, and 100% (w w−1) or 0, 12, 60, 120, and 1200 t ha−1 BC particle size groups: 0.3–0.6 and 0.15–0.3 mm | Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.051 mg L−1 (0.32 Bq L−1) aminocyclopyrachlor 5.0 mg L−1 (1.13 Bq L−1) mesotrione Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation | Higher BC rates (regardless of the particle size) increased both herbicides adsorption and decreased their desorption. | Mendes et al. [106] |
| Linuron Alachlor Metalaxyl | Sandy loam soil (pH 6.3, OC 0.51%, clay 11.8, silt 13.6%), Sandy clay soil (pH 6.9, OC 1.04%, clay 38.1%, silt 5.8%) | Pine Wood (OC 41.6%, DOM 1.62%, lignin 24.4%), oak wood (OC 38.5%, DOM 6.86%, lignin 18.2%) Wood/soil: 5% and 50% (w w–1) (40 and 400 t C ha–1) | Sorbent/Solution: 5 g/10 mL water solution Herbicide concentration: 1–25 mg L−1 (100 kBq L−1) Shaken: 24 h, T: 20 °C Incubation times: 0, 5 and 12 months Analytical determination: Liquid scintillation | Pesticide adsorption increased with high wood dose but OC nature was not relevant. Adsorption did not change after incubation times. The adsorption irreversibility decreased in presence of wood for alachlor and increased that of linuron and metalaxyl. | Marín–Benito et al. [107] |
| Aminocyclopyrachlor Mesotrione | Clay soil (pH 6.0, OC 2.21%, clay 60.5%, silt 11.3%) | Sewage sludge (SS) (pH 6.8, OC 16.64%) SS/soil: 0.1%, 1%, and 10% (w∙w–1) or 1.2, 12, and 120 t∙ha–1 | Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.08–0.64 Bq·L−1 (aminocyclopyrachlor) 0.28–2.27 Bq·L−1 (mesotrione) Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation | SS slightly affected sorption–desorption of both herbicides (lowest Kd at soil-SS1%). Kd for mesotrione was ~3.5–fold higher than for aminocyclopyrachlor (higher water solubility). | Mendes et al. [108] |
| Imazapic Atrazine Hexazinone Diuron Metribuzin | Red Ferrusol (pH 7.1, OC 2.1%, clay 41%, silt 23%), Grey Dermosol (pH 5.7, OC 0.9%, clay 30%, silt 22%), Red Kandosol (pH 6.5, OC 3.5%, clay 22%, silt 8%) | Eleven mill muds/ash from different sugar mills (pH 6.04–7.26, OC 27.7–37.8%) Mill muds/soil: 5–25% (w w–1) | Sorbent/Solution: 1 g/5 mL 0.01 M CaCl2 Herbicide concentration: 0.5 mg L−1 Shaken: 24 h, T: 25 °C Analytical determination: Q-TOF | Sorption order: diuron > atrazine = metribuzin > hexazinone = imazapic (consistent with herbicide properties). Mill muds at 5% dose increased herbicide retention up to tenfold. Amendments reduced desorption of mobile herbicides in low OC soils. | Duhan et al. [109] |
| MCPA Diuron Clomazone Terbuthylazine | Sandy loam soil (pH 7.93, OC 0. 54%, clay 6.7%, silt 16.8%) Loam soil (pH 6.77, OC 1.77%, clay 22.1%, silt 34.2%) Clay loam soil (pH 8.14, OC 1.38%, clay 31.1%, silt 26.8%) | Mucilage extracted from chia seeds (Salvia hispanica L.) Organic residue/soil: 10% (w w–1) | Sorbent/Solution: 0.5 g unamended or amended soil/8 mL water solution Herbicide concentration: 1 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC | Soil porosity decreased by mucilage amendment. Sorption of herbicides increased in amended soils (sandy–loam < loam < clay–loam). Diuron recorded the highest Kd value and desorption was observed only for terbuthylazine. | Marsico et al. [110] |
| Dichlorvos Chlorpyrifos | Sandy soil (pH 8.52, OC 0.7%, clay + silt 9.3%) | Compost (C) from mixed wastes (pH 6.61, OC 29.5%, DOM 354 mg L−1), and dried goat organic manure (OM) (pH 8.67, OC 14.4%, DOM 620 mg L−1) Organic residues/soil: 2.5 and 5% (w w–1) | Sorbent/Solution: 5 g soil/100 mL in C-DOM or 0.01 M CaCl2 Herbicide concentration: 0.1–10 mg L−1 (chlorpyrifos) 0.25–100 mg L−1 (dichlorvos) Shaken: 24 h, T: 25 °C Analytical determination: GC | C–and OM–DOM increased dichlorvos sorption (S < S–OM–DOM< S–C–DOM) and decreased chlorpyrifos sorption (S > S–C–DOM> S–OM–DOM). Humified and aromatic nature of DOM determines the interactions with pesticides with different hydrophobic character. | Gaonkar et al. [112] |
| Triasulfuron Prosulfocarb Chlorotoluron Flufenacet | Sandy loam soil (pH 7.36, OC 1.20%, clay 17%, silt 25%) Loamy sand soil (pH 7.61, OC 0.9%, clay 13%, silt 6%) | Spent mushroom substrate (pH 7.9, C 26.4%, DOM 1.29%), green compost (pH 7.2, C 23.6%, DOM 0.69%), manure (C 18.5%, DOM 1.32%), sewage sludge (pH 7.6, C 28.9%, DOM 1.18%) Organic residues/soils: 10% (w w–1) | Sorbent/Solution: 5 g soil or 0.1 g organic residues/10 mL 0.01 M CaCl2 Herbicide concentration: 1–25 mg L−1 (TSF, CTL, FNC) 0.25–10 mg L−1 (100 Bq mL−1) (PSC) Shaken: 24 h, T: 20 °C Analytical determination: HPLC/MS and Liquid scintillation | Highest adsorption for prosulfocarb (lowest water solubility and highest Kow) in all materials. Aliphatic and aromatic structures optimize adsorption and O-alkyl and N-alkyl groups enhance desorption hysteresis. | García–Delgado et al. [114] |
| Pesticide/Dose | Soil Characteristics | Organic Amendment/Dose | Experimental Design | Results | Reference |
|---|---|---|---|---|---|
| Alachlor Chlorfenvinphos Chlorpyrifos C 150 μg mL–1 | Hypercalcic calcisol (pH 7.9, OC 0.9%, clay 29.1%, silt 33.4%) | Composted sheep manure (EC) (pH 8.3, OC 264.9 g kg–1) and Coir (CR) (pH 7.5, OC 442 g kg–1) Organic residues/soils 1% | Packed columns (5 cm i.d. × 30 cm length) of S, S + EC and S + CR. Saturation with CaCl2 solution (0.01 M) at maximal WHC. Drainage for 24 h. Determination of PVs (mL) of soil columns. Application of pesticide (1 mL). Leaching volume 750 mL of CaCl2 solution (0.01 M) for 10 days. Leached volume/day: 50 mL Analytical determination: GC/MS | Highest leaching for alachlor. Chlorfenvinphos and chlorpyrifos had low leachability through soil columns (related with their low water solubility). Both compounds were recovered in higher proportions from the soil column than alachlor. | Pérez–Lucas et al. [137] |
| Penconazole C 500 μg mL–1 | Sandy soil (pH 6.20, OC 0.56%, clay 14.92%, silt 33.44%) | Sugarcane bagasse compost (SBC) (pH 6.32, OC 56.16%), chicken manure compost (CMC) (pH 6.27, OC 27.41%). Organic residues/soil: 2.5% and 5.0% (w w–1) | Glass columns (4.8 cm i.d. × 32 cm length). Pre-saturation with CaCl2 solution (0.01 M) (500 mL) for 16 h. Application of pesticide (1 mL). Leaching volume 2500 mL of CaCl2 solution (0.01 M) for 50 h. Leached volume 50 mL fractions. Extraction of pesticide from soil column (each 5 cm). Analytical determination: UPLC/TUV | SBC and CMC reduced penconazole leaching by decreased soil porosity and increased adsorption by amended soils. Inhibition of leaching by CMC was lower than by SBC (due to differences in sorption capacity). On the contrary, its content was higher in SBC-soil than in CMC-soil columns. | Jiang et al. [84] |
| Clothianidin C 10 µg mL–1 | Clay loam S1 (pH 5.06, OC 0.95%, clay 30.4%, silt 36.5%) Sandy loam S2 (pH 8.41, OC 0.29%, clay 10.4%, silt 18.1%) | Farm yard manure (FYM) (pH 6.6, OC 23.7%) Organic residue/soil: 2.5% (w w–1) | Packed soil columns (2.1 cm i.d. × 50 cm length) (200 g S or S + FYM). Pre-saturation overnight in water. Leaching flow: 400 mL of water (1156 mm of rainfall) as continuous flow, or amounts of 20, 40, 80 and 160 mL of water (51.92, 103.85, 207.71 and 415.42 mm of rainfall) as discontinuous flow). Extraction of pesticide from column soil (each 5 cm). Analytical determination: HPLC/PDA | Clothianidin leaching was minimized in S1 compared to S2 after FYM application. Both soils concentrated maximum residue with or without FYM in 0–20 cm soil depth. Clothianidin did not leach under different and discontinuous flow conditions. | Samarendra–Singh et al. [138] |
| 14C-Tebuconazole 14C -Cymoxanil C 1 mg and 10 kBq mL−1 | Sandy clay loam soil (pH 7.52, OC 0.67%, clay 21.1%, silt 11.9%) | Spent mushroom substrate (SMS) (pH 6.97, OC 24.5%, DOM 1.91%) Organic residue/soil: 5% and 50% (w w–1) | Packed soil columns (3 cm i.d. × 25 cm length) (100 g S, S + SMS5 and S + SMS50). Chloride as an ion tracer. Leaching flow: 500 mL of CaCl2 solution (0.01 M) (12 PV). Washing flow regimes: saturated (continuously pumped for ≈8 h) and saturated–non saturated (20 days, 25 mL/day). Columns nonincubated and incubated over 30 days. Analytical determination: Liquid scintillation | Amendments decreased leaching of tebuconazole under different flow conditions, and decreased leaching of cymoxanil under saturated-unsaturated flow. Ageing favored retention decreasing tebuconazole leaching or cymoxanil mineralization. | Álvarez–Martín et al. [96] |
| 14C-Prosulfocarb C 1 mg and 10 kBq mL−1 (2.5 times the agronomic dose) | Sandy clay loam soil (pH 7.35, OC 1.30, clay 17%, silt 25%) | Green compost (GC) (pH 7.20, OC 24.1%, DOM 0.703%) Organic residue/soil: 20% w w–1 (180 t ha–1) | Packed soil columns (3 cm i.d. × 25 cm length) (100 g S and S + GC). Chloride as an ion tracer. Leaching flow: 500 mL of CaCl2 (0.01 M) (12 PV) under saturated flow and under saturated–unsaturated flow (20 mL/ day). Columns nonincubated and incubated over 28 days. Analytical determination: Liquid scintillation | Leached amounts decreased in S and S + GC columns after incubation. Retained amounts were lower in S than in S + GC columns under saturated flow. Prosulfocarb was retained in the first segment of columns under all conditions. Herbicide incubation increased the mineralized amount under saturated flow. | Barba et al. [132] |
| Isoproturon C 47–59 µg L–1 Epoxiconazole C 89.6–117 µgL–1 Ibuprofen C 64.7–94.4 µg L–1 Sulfamethoxazole C 39.0–51.9 µg L–1 | Loamy soil Ap (0–28 cm) (pH 7.0, OC 0.23%, clay 14.6%, silt 79%) Bt (60–90 cm) (pH 7.5, OC 1.02%, clay 31.1%, silt 64.6%) | Combined compost of sewage sludges and green wastes (SWG) (pH 6.9, OC 15.8 g kg–1) | Undisturbed soil cores (14 cm i.d. × 30 cm length) with 5300 cm3 volume. Bromide as ion tracer. Leaching experiments with synthetic water, DOM of soil or soil + SWG. Unsaturated steady–state flow regime of two consecutive rainfalls of 1.76 mm h−1 intensity and separated by a 1–week flow interruption on triplicated cores for 28 days. Analytical determination: UHPLC/MS/MS | DOM increased mobility of Br- and all pollutants. The mobility increase was greater for more hydrophobic compounds (epoxiconazole and ibuprofen). DOM can also enhance the transport of anionic molecules but for these compounds also depend on their affinity for the soil matrix including soil solution composition and its pH. | Chabauty et al. [141] |
| Pesticide | Soil Characteristics | Organic Amendment/Dose | Experimental Design | Results | Reference |
|---|---|---|---|---|---|
| Imazapic Imazapyr mixture of them (Onduty®) | Clay loam soil (pH 6.36, OC 0.99%, clay 37.9%, silt 21.58%) | Biochar from fruit bunch of oil palm (EFB) (pH 6.13, C 58.60%) and rice husk (RH) (pH 6.32, C 48.26%) Biochar/soil: 1.0% (w w–1) | Hydrolysis: Solution/herbicide concentration 50 mL/10 mg L−1, pH 3, 7 and 9 Photodegradation: Soil/herbicide 30 g/0.2 µg g−1. Biodegradation: Soil/herbicide 10 g/0.2 µg g−1. T: 25 °C. Relative humidity: 85% Time up to 70 days. Analytical determination: HPLC | All herbicides were resistant to hydrolysis degradation. Photolysis rates of herbicides were reduced by use of biochar, particularly EFB. Biodegradation of herbicides accelerated significantly by the use of biochars. | Yavari et al. [146] |
| Cyazofamid Metabolite CCIM (4-chloro-5-p-tolylimidazole-2-carbonitrile) | Silty soil (pH 7.14, OC 0.71%, clay 8.25%, silt 85.55%) | Biochars from rice straw (RS) (pH 9.87, C 36.58%), corn straw (CS) (pH 9.97, C 57.33%), chicken manure (CM) (pH 8.16, C 27.73%) and tire rubber (TR) (pH 8.82, C 74.60%) Biochar/soil: 3% (w w–1) | Soil samples: 20.6 g Pesticide applied: 2.5 mg/kg of dry soil Soil moisture content: 40% WHC Incubation T: 25 °C Sampling times up to 40 days Analytical determination: HPLC/MS/MS | Cyazofamid dissipation order: CS > RS > CM TR depressed cyazofamid dissipation. Adsorption, hydrolysis and microbial degradation were all involved in its dissipation. CM and CS enhanced the cyazofamid dissipation by biodegradation. CCIM residual increased by 8–15 times after biochar application, regardless of biochar type. | Tang et al. [158] |
| Imazapic | Silty soil (pH 8.1, OM 0.55%, clay 9.1%, silt 69.1%) | Chicken manure (pH 7.1, OM 21.5%) biogas slurry (pH 7.0, OM 20.1%) CM/soil: 2.1–16%; BS/soil: 3.6–0.9% | Soil samples: 1000 g Pesticide applied: 20 mL (50 mg L−1) Incubation T: 15, 25 and 35 °C Soil moisture contents: 15%, 40%, 60%, and 90% pH values: 6.0, 7.0, and 8.0 Sampling times up to 150 days Analytical determination: HPLC/MS/MS | Imazapic degradation rate increased with temperature, soil pH, and soil moisture, and it decreased with OM content. Biogas slurry accelerated imazapic degradation (significant microbial contribution to its degradation). | Su et al. [159] |
| Chlorotoluron Flufenacet | Sandy loam soil (pH 6.34, OC 0.77, clay 14.9%, silt 4.7%) | Spent mushroom substrate (SMS) (pH 7.9, OM 59.4%, DOM 0.8%) Green compost (GC) (pH 7.2, OM 46.0%, DOM 0.7%). SMS and GC/soil: 140 and 85 t ha−1 | Soil samples: 600 g Pesticide applied: 14 mg (chlorotoluron) and 5.5 mg (flufenacet)/kg of dry soil Soil moisture content: 40% WHC Incubation T: 6 and 16 °C Sampling times up to 67 or 273 days Analytical determination: HPLC/MS | Flufenacet degradation was slower than that of chlorotoluron. Amendments increased DT50 values for both herbicides incubated at both temperatures, especially at 16 °C due to the higher microbiological activity. | Marín–Benito et al. [160] |
| 14C-Prosulfocarb | Sandy clay loam soil (pH 7.35, OC 1.30, clay 17%, silt 25%) | Green compost (GC) (pH 7.20, OC 24.1%, DOM 0.703%) GC/soil: 20% w w–1 (180 t ha–1) | Soil samples: 500–700 g Pesticide applied: 4 and 10 mg kg−1 of dry soil and 100 Bq g−1 Soil moisture content: 40% WHC Incubation T: 20 °C Sampling times up to 50 days Analytical determination: HPLC/MS and Liquid scintillation | Highest DT50 values in amended soil. They increased with the herbicide concentration in unamended soil but decreased in amended soil. Lost through volatilization of herbicide was consistent with the total 14C mass balance close to 70% at the end of dissipation period. | Barba et al. [161] |
| Mesotrione Pethoxamid Triasulfuron | Sandy loam soil (pH 6.3, OC 0.49%, clay 10.7%, silt 5.9%) | Sewage sludge (SS) (pH 7.08, OC 8.06%, DOM 0.102%) Green compost (GC) (pH 6.73, OC 27.0%, DOM 2.17%) SS or GC/soil: 50 t ha−1 | Soil samples: 800 g Pesticide applied: 2 mg kg−1 of dry soil Soil moisture content: 40% WHC Incubation T: 20 °C Sampling times up to 99, 43 and 144 days Analytical determination: HPLC/MS | Repeated application of pesticides decreased (mesotrione) or increased (petoxamide) its dissipation rate in all treatments. For triasulfuron, it increased only in amended soils. Highest DT50 values for pethoxamid and triasulfuron in S + GC, and for mesotriona in S + SS. | Pose et al. [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments 2021, 8, 32. https://doi.org/10.3390/environments8040032
Carpio MJ, Sánchez-Martín MJ, Rodríguez-Cruz MS, Marín-Benito JM. Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments. 2021; 8(4):32. https://doi.org/10.3390/environments8040032
Chicago/Turabian StyleCarpio, María J., María J. Sánchez-Martín, M. Sonia Rodríguez-Cruz, and Jesús M. Marín-Benito. 2021. "Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research" Environments 8, no. 4: 32. https://doi.org/10.3390/environments8040032
APA StyleCarpio, M. J., Sánchez-Martín, M. J., Rodríguez-Cruz, M. S., & Marín-Benito, J. M. (2021). Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments, 8(4), 32. https://doi.org/10.3390/environments8040032








