Effects of Biochar on the C Use Efficiency of Soil Microbial Communities: Components and Mechanisms
Abstract
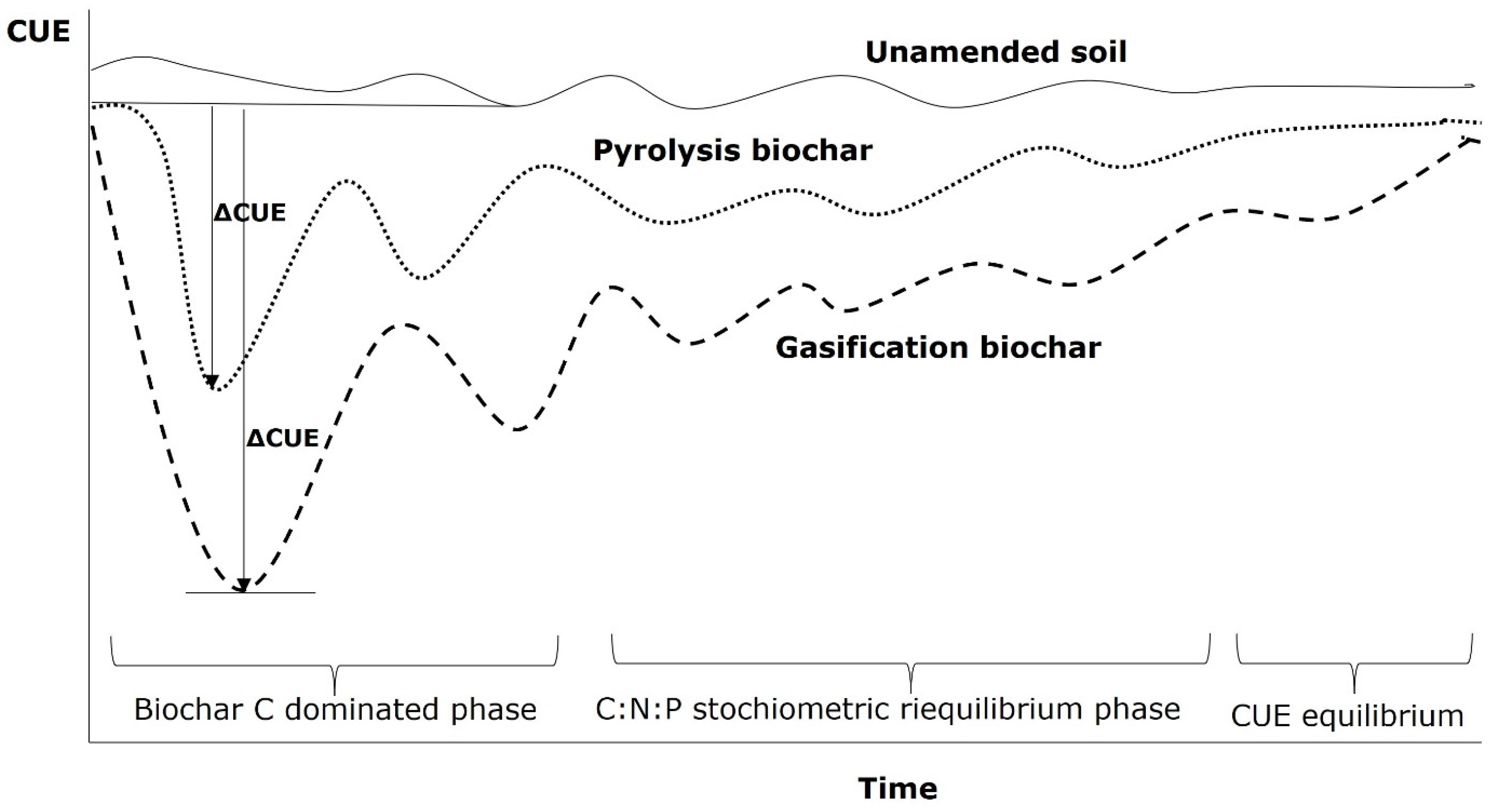
:1. Biochar: Chemical Stability Influences the Carbon Use Efficiency (CUE) in Soil
2. Microbial CUE: Definition for Soil Systems and Changes in Biochar-Amended Soils
3. Biochar-Induced Temperature and Moisture Effects on Soil CUE
4. Biochar Effects on C Availability, Nutrient Stoichiometry and CUE in Soil
4.1. Biochar Changes the Soil N and P Contents and the C:N:P Stoichiometry
4.2. Determination of Soil Microbial Biomass Homeostatic C:N:P Ratios in Biochar-Amended Soils
5. Microbial Community Composition and CUE in Biochar-Amended Soils
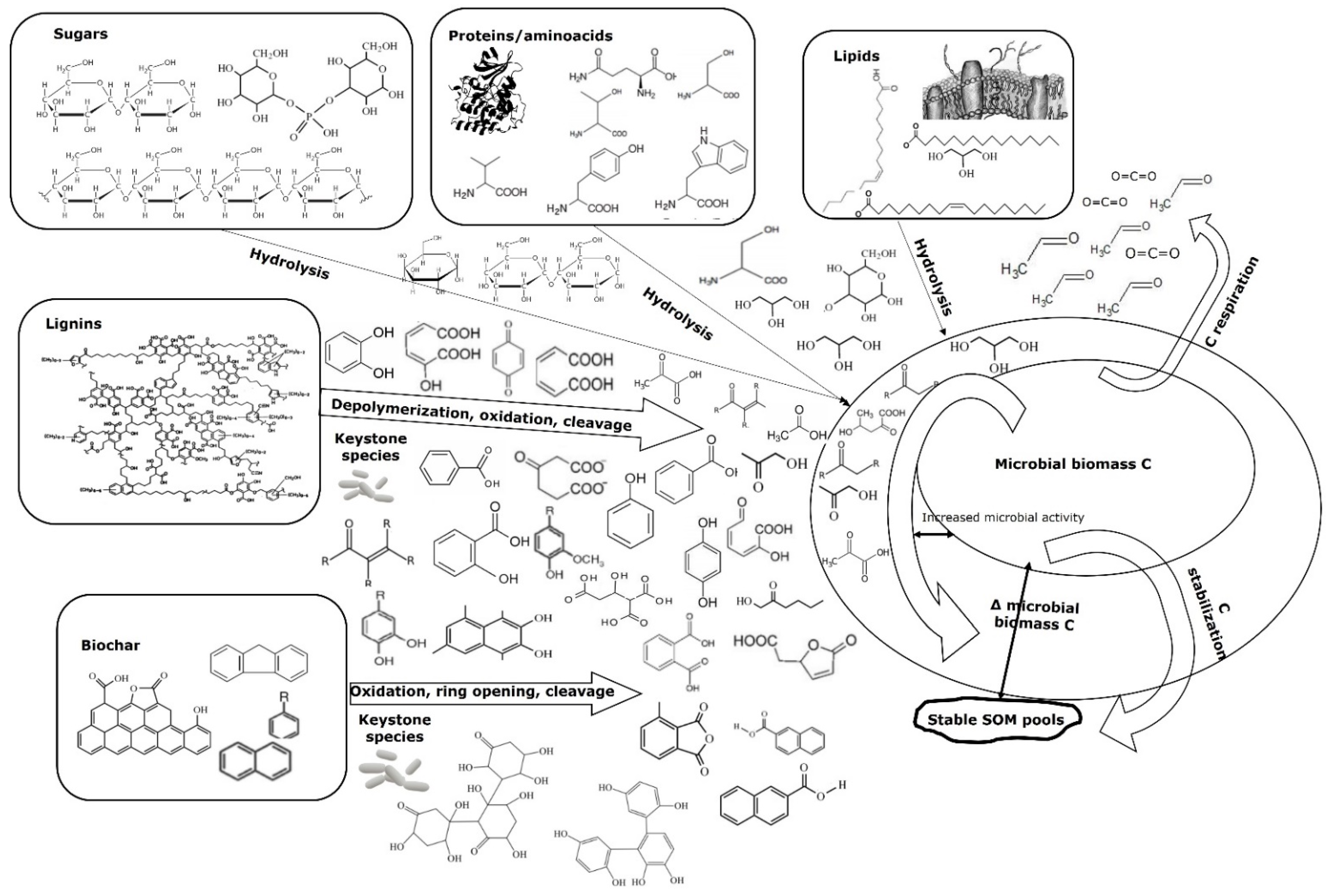
5.1. Polycyclic Aromatic Hydrocarbon Degraders: The Chemical Gate Operators
5.2. Enzyme Activity: The Toolbox
5.3. P and N Mineralizing Microorganisms: The Helpers
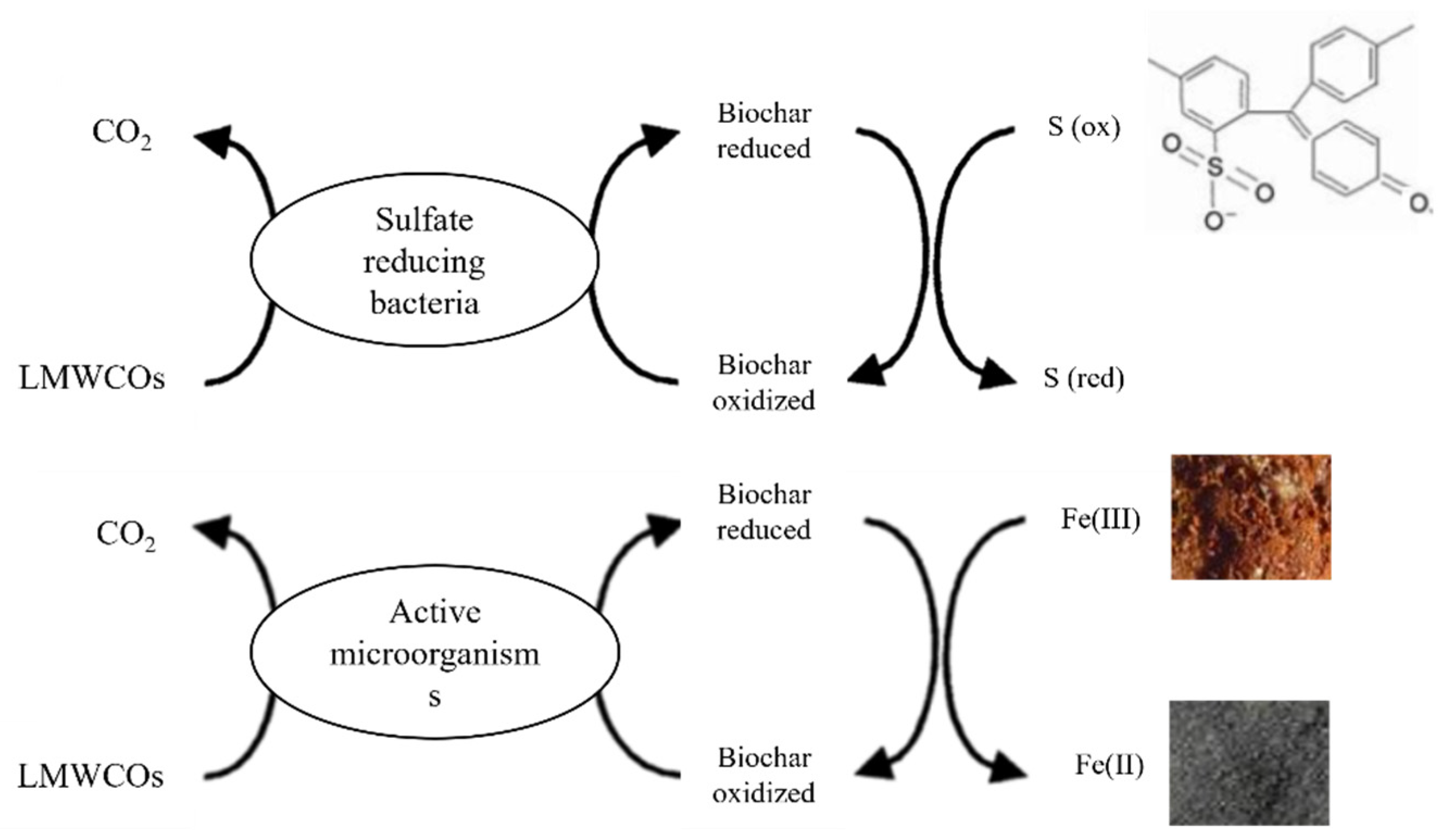
5.4. Sulfur Reducing Bacteria: The Stone Guest?
6. Conclusions and Research Needs
- i.
- Determine the theoretical CUE of biochar-amended soils and under different environmental conditions, soil types and management. Determination of the thermodynamic maxima of different biochar types can also allow the improved determination the limiting or unlocking effects of increased nutrient availability;
- ii.
- Models operating on finer time scales (days to seasons) should consider the effects of changing SOM molecular composition, multi-element stoichiometric constraints, and microbial community physiology. In addition, environmental drivers should be implemented on data from long-term field trials to predict the CUE of biochar-amended soils under different management. The effects of larger N and P availability as driving forces, and changes in enzymatic activity, should be tested, particularly in arable soils amended with biochar and under chemical fertilization, in order to determine the stability and MRT of the biochar-borne C. An interesting technique that could be tested to assess the extent of biochar stability in soil could be the reverse stable isotope labelling [154];
- iii.
- Perform hypothesis-driven metagenomic research to reveal presence, activity, and evolution of microbial metabolic pathways in long-term biochar-amended soils. Complementary proteomic and metabolomic studies may help to elucidate the hypothesized pathway of release of LMWOCs from biochar, and better estimate the C partition in CO2, microbial and SOM pools, also analyzing the changes of the 13C signature of biochar and SOM;
- iv.
- Adopt imaginative direct observation approaches to observe the surface of weathered biochar, also extracted from amended soils, to describe the formation of the ‘charrosphere’ and its relations with biochar biodegradation and the release of LMWOCs into the soil solution.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Earthscan: London, UK, 2015. [Google Scholar]
- Laird, A.D. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central Amazon Basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Wong, J.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.; Bolan, N.; Wang, H.; Ok, Y. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar]
- Jaiswal, A.; Alkan, N.; Elad, Y.; Sela, N.; Philosoph, A.M.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020, 10, 13934. [Google Scholar] [CrossRef]
- Sambroek, W.G. Amazonian soils: A reconnaissance of the soils of the Brazilian Amazon Valley. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, February 1966. [Google Scholar]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O: C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Budai, A.; Zimmerman, A.R.; Cowie, A.L.; Webber, J.B.W.; Singh, B.P.; Glaser, B.; Masiello, C.A.; Andersson, D.; Shields, F.; Lehmann, J.; et al. Biochar Carbon Stability Test Method: An Assessment of Methods to Determine Biochar Carbon Stability; Technical Report for International Biochar Initiative: Canandaigua, NY, USA, September 2013. [Google Scholar]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- EBC. European Biochar Foundation—European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012. [Google Scholar]
- Wiedemeier, D.B.; Brodowski, S.; Wiesenberg, G.L.B. Pyrogenic molecular markers: Linking PAH with BPCA analysis. Chemosphere 2015, 119, 432–437. [Google Scholar] [CrossRef] [Green Version]
- McBeath, A.V.; Wurster, C.M.; Bird, M.I. Influence of feedstock properties and pyrolysis conditions on biochar carbon stability as determined by hydrogen pyrolysis. Biomass Bioenergy 2015, 73, 155–173. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. A method for screening the relative long-term stability of biochar. GCB Bioenergy 2013, 5, 215–220. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Gommers, P.J.F.; van Schie, B.J.; van Dijken, J.P.; Kuenen, J.G. Biochemical limits to microbial growth yields: An analysis of mixed substrate utilization. Biotechnol. Bioeng. 1998, 82, 86–94. [Google Scholar]
- Gunina, A.; Smith, A.R.; Kuzyakov, Y.; Jones, D.L. Microbial uptake and utilization of low molecular weight organic substrates in soil depend on carbon oxidation state. Biogeochemistry 2017, 133, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M. Biochemical ecology of soil microorganisms. Annu. Rev. Microbiol. 1964, 18, 217–250. [Google Scholar] [CrossRef]
- O’Malley, M.A.; Walsh, D.A. Rethinking microbial infallibility in the metagenomics era. FEMS Microbiol. Ecol. 2021, 97, fiab092. [Google Scholar] [CrossRef]
- Gale, E.F. The Chemical Activities of Bacteria, 3rd ed.; Academic Press Inc.: Cambridge, MA, USA, 1951. [Google Scholar]
- Tiedje, J.M.; Cho, J.C.; Murray, A.; Treves, D.; Xia, B.; Zhou, J. Soil teeming with life: New frontiers for soil science. In Sustainable management of Soil Organic Matter; Rees, R.M., Ball, B.C., Campbell, C.D., Watson, C.A., Eds.; CABI: Wallingford, UK, 2001. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Soil carbon and nitrogen mineralization: Theory and models across scales. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contribution to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Pirt, S.J. Principles of Microbe and Cell Cultivation; Wiley: Hoboken, NJ, USA, 1985. [Google Scholar]
- Payne, W.J.; Wiebe, W.J. Growth yield and efficiency in chemosynthetic microorganisms. Ann. Rev. Microbiol. 1978, 32, 155–183. [Google Scholar] [CrossRef]
- Narang, A. The steady states of microbial growth on mixtures of substitutable substrates in a chemostat. J. Theor. 1998, 190, 241–261. [Google Scholar] [CrossRef]
- Wang, G.S.; Post, W.M. A theoretical reassessment of microbial maintenance and implications for microbial ecology modeling. FEMS Microbiol. Ecol. 2012, 81, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Bölscher, T.; Wadsö, L.; Börjesson, G.; Herrmann, A.M. Differences in substrate use efficiency: Impacts of microbial community composition, land use management, and substrate complexity. Biol. Fertil. Soils 2016, 52, 547–559. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Wagai, R.; Kishimoto-Mo, A.W.; Yonemura, S.; Shirato, Y.; Hiradate, S.; Yagasaki, Y. Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob. Change Biol. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zhan, L.; Xu, X.; Bi, R.; Xiong, Z. Biochar addition stabilized soil carbon sequestration by reducing temperature sensitivity of mineralization and altering the microbial community in a greenhouse vegetable field. J. Environ. Manag. 2022, 313, 114972. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Wang, P.; Liu, X.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Crowley, D.; et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A Meta-analysis. Agric. Ecosyst. Environ. 2017, 239, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Button, D.K. Nutrient-limited microbial-growth kinetics—Overview and recent advances. Antonie Van Leeuwenhoek 1993, 63, 225–235. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Hobbie, E.A. Amino acid cycling in planktonic and soil microbes studied with radioisotopes: Measured amino acids in soil do not reflect bioavailability. Biogeochemistry 2012, 107, 339–360. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Integrating resource utilization and temperature in metabolic scaling of riverine bacterial production. Ecology 2010, 91, 1455–1465. [Google Scholar] [CrossRef]
- Dijkstra, P.; Thomas, S.C.; Heinrich, P.L.; Koch, G.W.; Schwartz, E.; Hungate, B.A. Effect of temperature on metabolic activity of intact microbial communities: Evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol. Biochem. 2011, 43, 2023–2031. [Google Scholar] [CrossRef]
- Spohn, M.; Klaus, K.; Wanek, W.; Richter, A. Microbial carbon use efficiency and biomass turnover times depending on soil depth—implications for carbon cycling. Soil Biol. Biochem. 2016, 96, 74–81. [Google Scholar] [CrossRef]
- Robinson, C. Heterotrophic bacterial respiration. In Microbial Ecology of the Oceans; Kirchman, D.L., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 299–334. [Google Scholar]
- Vetter, Y.A.; Deming, J.W.; Jumars, P.A.; Krieger-Grockett, B.B. A predictive model of bacterial foraging by means of freely released extracellular enzymes. Microb. Ecol. 1998, 36, 75–92. [Google Scholar] [CrossRef]
- Hazen, T.C. Cometabolic Bioremediation. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2505–2514. [Google Scholar]
- Mahendra, S.; Alvarez-Cohen, L. Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ. Sci. Technol. 2006, 40, 5435–5442. [Google Scholar] [CrossRef]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change 2013, 3, 395–398. [Google Scholar]
- Genesio, L.; Miglietta, F.; Lugato, E.; Baronti, S.; Pieri, M.; Vaccari, F.P. Surface albedo following biochar application in durum wheat. Environ. Res. Lett. 2012, 70, 14025. [Google Scholar] [CrossRef]
- Baumgardner, M.F.; Sylva, L.F.; Biehl, L.L.; Stoner, E.R. Reflectance Properties of Soils. Adv. Agron. 1985, 38, 1–44. [Google Scholar]
- Brutsaert, W. Evaporation into the Atmosphere; D. Reidel Publishing Company: Dordrecht, Holland, 1982. [Google Scholar]
- Boot, C.M.; Schaeffer, S.M.; Schimel, J.P. Static osmolyte concentrations in microbial biomass during seasonal drought in a California grassland. Soil Biol. Biochem. 2013, 57, 356–361. [Google Scholar] [CrossRef]
- Uhlırova, E.; Elhottova, D.; Trıska, J.; Santruckova, H. Physiology and microbial community structure in soil at extreme water content. Folia Microbiol. 2005, 50, 161–166. [Google Scholar] [CrossRef]
- Herron, P.M.; Stark, J.M.; Holt, C.; Hooker, T.; Cardon, Z.G. Microbial growth efficiencies across a soil moisture gradient assessed using 13C-acetic acid vapor and 15N-ammonia gas. Soil Biol. Biochem. 2009, 41, 1262–1269. [Google Scholar] [CrossRef]
- von Stockar, U.; Marison, I.W. The definition of energetic growth efficiencies for aerobic and anerobic microbial growth and their determination by calorimetry and other means. Thermochim. Acta 1993, 229, 157–172. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Bosatta, E.; Ågren, G.I. Soil organic matter quality interpreted thermodynamically. Soil Biol. Biochem. 1999, 31, 1889–1891. [Google Scholar] [CrossRef]
- Öquist, M.G.; Erhagen, B.; Haei, M.; Sparrman, T.; Ilstedt, U.; Schleuche, J.; Nilsson, M.B. The effect of temperature and substrate quality on the carbon use efficiency of saprotrophic decomposition. Plant Soil 2017, 414, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Ågren, G.I.; Wetterstedt, J.A.M. What determines the temperature response of soil organic matter decomposition? Soil Biol. Biochem. 2007, 39, 1794–1798. [Google Scholar] [CrossRef] [Green Version]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Resat, H.; Bailey, V.; McCue, L.A.; Konopka, A. Modeling microbial dynamics in environments: Growth on soil carbon sources. Microb 2012, 63, 883–897. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Zheng, J.; Han, J.; Liu, Z.; Xia, W.; Zhang, X.; Li, L.; Liu, X.; Bian, R.; Cheng, K.; Zheng, J.; et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 2017, 241, 70–78. [Google Scholar] [CrossRef]
- Chen, K.; Peng, J.; Li, J.; Yang, Q.; Zhan, X.; Liu, N.; Han, X. Stabilization of soil aggregate and organic matter under the application of three organic resources and biochar-based compound fertilizer. J. Soils Sediments 2020, 20, 3633–3643. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 45–55. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Long-term aging of biochar: A molecular understanding with agricultural and environmental implications. Adv. Agron. 2017, 141, 1–51. [Google Scholar]
- Yang, K.; Jiang, Y.; Wang, J.; Cai, X.; Wen, Z.; Qiu, Z.; Qiao, G. Tobacco straw biochar improved the growth of Chinese cherry (Prunus pseudocerasus) via altering plant physiology and shifting the rhizosphere bacterial community. Sci. Hortic. 2022, 303, 111244. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Singh, B.P.; Wei, L.; Huang, L.; Huang, Y.; Huang, Q.; Chen, X.; Su, Y.; Liu, Z.; et al. Biochar protects hydrophilic dissolved organic matter against mineralization and enhances its microbial carbon use efficiency. Sci. Total Environ. 2021, 795, 148793. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Wang, J.; Liu, X.; Guo, W.; Zheng, J.; Bian, R.; Wang, G.; Zhang, X.; Cheng, K.; et al. The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. GCB Bioenergy 2019, 11, 1408–1420. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Li, J.; Mia, S.; Singh, B.; Wu, J.; Dijkstra, F.A. Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma 2021, 382, 114710. [Google Scholar] [CrossRef]
- Singh, B.; Sarkar, S.; Churchman, J.; Bolan, N.; Mandal, S.; Menon, M.; Purakayastha, T.J.; Beerling, D.J. Stabilization of soil organic carbon as influenced by clay mineralogy. Adv. Agron. 2018, 148, 33–84. [Google Scholar]
- Cheng, H.; Hill, P.W.; Bastami, M.S.; Jones, D.L. Biochar stimulates the decomposition of simple organic matter and suppresses the decomposition of complex organic matter in a sandy loam soil. GCB Bioenergy 2017, 9, 1110–1121. [Google Scholar] [CrossRef]
- Silva-Sánchez, A.; Soares, M.; Rousk, J. Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol. Biochem. 2019, 134, 25–35. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2013, 117, 101–113. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau, China. Soil Tillage Res. 2020, 200, 104605. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–342. [Google Scholar] [CrossRef] [Green Version]
- Lasota, J.; Babiak, T.; Błońsk, E. C:N:P stoichiometry associated with biochar in forest soils at historical charcoal production sites in Poland. Geoderma. Reg. 2022, 28, e00482. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef]
- Hong, C.; Lu, S. Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ. Sci. Pollut. Res. 2018, 25, 8725–8734. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Cherif, M.; Loreau, M. Stoichiometric constraints on resource use, competitive interactions, and elemental cycling in microbial decomposers. Am. Nat. 2007, 169, 709–724. [Google Scholar] [CrossRef]
- Ahmed, I.U.; Mengistie, H.K.; Godbold, D.L.; Sanden, H. Soil moisture integrates the influence of land-use and season on soil microbial community composition in the Ethiopian highlands. Appl. Soil Ecol. 2019, 135, 85–90. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef] [Green Version]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2400. [Google Scholar] [CrossRef]
- Zhou, C.; Heal, K.; Tigabu, M.; Xia, L.; Hu, H.; Yin, D.; Ma, X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. For. Ecol. Manag. 2020, 455, 117635. [Google Scholar] [CrossRef]
- Mahendra, S.; Alvarez-Hong, C.; Lu, S. Biochar, Ochre, and Manure Maturation in an Acidic Technosol Helps Stabilize As and Pb in Soil and Allows Its Vegetation by Salix triandra. Environment 2022, 9, 87. [Google Scholar]
- Frost, P.C.; Benstead, J.P.; Cross, W.F.; Hillebrand, H.; Larson, J.H.; Xenopoulos, M.A.; Yoshida, T. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol. Lett. 2006, 9, 774–779. [Google Scholar] [CrossRef]
- Doi, H.; Cherif, M.; Iwabuchi, T.; Katano, I.; Stegen, J.C.; Striebel, M. Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 2010, 119, 752–765. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extra cellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Shen, J.; Bartha, R. Metabolic efficiency and turnover of soil microbial communities in biodegradation tests. Appl. Environ. Microbiol. 1996, 62, 2411–2415. [Google Scholar] [CrossRef] [Green Version]
- Newell, S.Y.; Fallon, R.D. Toward a method for measuring instantaneous fungal growth rates in field samples. Ecology 1991, 72, 1547–1559. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hammerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef]
- Zhang, J.; Elser, J.J. Carbon:Nitrogen:Phosphorus stoichiometry in fungi: A meta-analysis. Front. Microbiol. 2017, 8, 1281. [Google Scholar] [CrossRef]
- Gao, W.; Gao, K.; Guo, Z.; Liu, Y.; Jiang, L.; Liu, C.; Liu, X.; Wang, G. Different responses of soil bacterial and fungal communities to 3 years of biochar amendment in an alkaline soybean soil. Front. Microbiol. 2021, 12, 630418. [Google Scholar] [CrossRef]
- Scott, J.T.; Cotner, J.B.; LaPara, T.M. Variable stoichiometry and homeostatic regulation of bacterial biomass elemental composition. Front. Microbiol. 2012, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Miao, R.; Guo, M.; Shang, X.; Zhou, Y.; Zhu, J. Biochar enhanced polycyclic aromatic hydrocarbons degradation in soil planted with ryegrass: Bacterial community and degradation gene expression mechanisms. Sci. Total Environ. 2022, 838, 156076. [Google Scholar] [CrossRef]
- Hagemann, N.; Harter, J.; Behrens, S. Elucidating the impacts of biochar applications on nitrogen cycling microbial communities. In Biochar Application; Ralebitso-Senior, T.K., Orr, C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–198. [Google Scholar]
- He, X.; Xie, H.; Gao, D.; Khashi, M.; Rahman, U.; Zhou, X.; Wu, F. Biochar and intercropping with potato–onion enhanced the growth and yield advantages of tomato by regulating the soil properties, nutrient uptake, and soil microbial community. Front. Microbiol. 2021, 12, 695447. [Google Scholar] [CrossRef] [PubMed]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting efects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Niu, X.; Zhang, N.; Li, T. Effect of biochar-immobilized Sphingomonas sp. PJ2 on bioremediation of PAHs and bacterial community composition in saline soil. Chemosphere 2021, 279, 130427. [Google Scholar] [CrossRef]
- Tsai, S.; O’neill, B.; Cannavan, F.S.; Saito, D.; Falcao, N.P.S.; Kern, D.C.; Grossman, J.; Thies, J. The Microbial World of Terra Preta. In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., Winkler Prins, A., Rebellato, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Glaser, B. Prehistorically modified soils of central Amazonia: A model for sustainable agriculture in the twenty-first century. Philos. Trans. R. Soc. B 2007, 362, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-S.; Sparovek, G.; Longo, R.M.; De Melo, W.J.; Crowley, D. Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol. Biochem. 2007, 39, 684–690. [Google Scholar] [CrossRef]
- de Lima Brossi, M.J.; Mendes, L.W.; Gomes Germano, M.; Barbosa Lima, A.; Tsai, S.M. Assessment of Bacterial bph Gene in Amazonian Dark Earth and Their Adjacent Soils. PLoS ONE 2014, 9, e99597. [Google Scholar]
- Germano, M.G.; Cannavan, F.S.; Mendes, L.W.; Lima, A.B.; Teixeira, W.G.; Pellizzari, V.H.; Tsai, S.M. Functional diversity of bacterial genes associated with aromatic hydrocarbon degradation in anthropogenic dark earth of Amazonia. Pesqui. Agropecu. Bras. 2012, 47, 654–664. [Google Scholar] [CrossRef]
- Sierra, C.A. Temperature sensitivity of organic matter decomposition in the Arrhenius equation: Some theoretical considerations. Biogeochemistry 2012, 108, 1–15. [Google Scholar] [CrossRef]
- Leifeld, J.; von Lützow, M. Chemical and microbial activation energies of soil organic matter decomposition. Biol. Fertil. Soils 2014, 50, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Ding, G.C.; Heuer, H.; Zuhlke, S.; Spiteller, M.; Pronk, J.G.; Heister, K.; Kögel-Knabner, I.; Smalla, K. Soil type-dependent responses to phenanthrene as revealed by determining the diversity and abundance of polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenase genes by using a novel PCR detection system. Appl. Environ. Microbiol. 2010, 76, 4765–4771. [Google Scholar] [CrossRef] [Green Version]
- Storey, S.; Ashaari, M.M.; McCabe, G.; Harty, M.; Dempsey, R.; Doyle, O.; Clipson, N.; Doyle, E.M. Microbial community structure during fluoranthene degradation in the presence of plants. J. Appl. Microbiol. 2014, 117, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Storey, S.; Ashaari, M.M.; Clipson, N.; Doyle, E. Benzo(a)pyrene degradation and microbial community responses in composted soil. Environ. Sci. Pollut. Res. 2016, 24, 5404–5414. [Google Scholar] [CrossRef] [PubMed]
- Louvel, B.; Cébron, A.; Leyval, C. Root exudates affect phenanthrene biodegradation bacterial community and functional gene expression in sand microcosms. Int. Biodeterior. 2011, 65, 947–953. [Google Scholar] [CrossRef]
- Ahmad, M.; Yang, Q.S.; Zhang, Y.Y.; Ling, J.; Sajjad, W.; Qi, S.H.; Zhou, W.G.; Zhang, Y.; Lin, X.C.; Zhang, Y.H.; et al. The distinct response of phenanthrene enriched bacterial consortia to different PAHs and their degradation potential: A mangrove sediment microcosm study. J. Hazard. Mater. 2019, 380, 120863. [Google Scholar] [CrossRef] [PubMed]
- Maienza, A.; Baronti, S.; Cincinelli, A.; Martellini, T.; Grisolia, A.; Miglietta, F.; Renella, G.; Stazi, S.R.; Vaccari, F.P.; Genesio, L. Biochar improves the fertility of a Mediterranean vineyard without toxic impact on the microbial community. Agron. Sustain. Dev. 2017, 37, 47. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, B.; Kilpeläinen, P.; Kitunen, V.; Smolander, A. Potential activities of enzymes involved in N, C, P and S cycling in boreal forest soil under different tree species. Pedobiologia 2014, 57, 97–102. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leiros, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- van Hees, P.A.W.; Jones, D.L.; Finlay, R.; Godbold, D.L.; Lundström, U.S. The carbon we do not see—The impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biol. Biochem. 2005, 37, 1–13. [Google Scholar] [CrossRef]
- Song, X.; Razavi, B.S.; Ludwig, B.; Zamanian, K.; Gunina, A. Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci. Total Environ. 2020, 735, 139393. [Google Scholar] [CrossRef]
- Giagnoni, L.; Maienza, A.; Baronti, S.; Vaccari, F.P.; Genesio, L.; Taiti, C.; Martellini, T.; Scodellini, R.; Cincinelli, A.; Costa, C.; et al. Long-term soil biological fertility, volatile organic compounds and chemical properties in a vineyard soil after biochar amendment. Geoderma 2019, 344, 127–136. [Google Scholar] [CrossRef]
- Takriti, M.; Wild, B.; Schnecker, J.; Mooshammer, M.; Knoltsch, A.; Lashchinskiy, N.; Eloy Alves, R.J.; Gentsch, N.; Gittel, A.; Mikutta, R.; et al. Soil organic matter quality exerts a stronger control than stoichiometry on microbial substrate use efficiency along a latitudinal transect. Soil Biol. Biochem. 2018, 21, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.K.; Jones, D.L.; Sanders, H.R.; Benitez, G.V.; Plante, A.F. Effects of 7 years of field weathering on biochar recalcitrance and solubility. Biochar 2019, 1, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, M.; Glenn, J.K.; Morgan, M.A.; Gold, M.H. Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 1984, 169, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2008, 27, 185–194. [Google Scholar] [CrossRef]
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C. Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS ONE 2014, 9, e86388. [Google Scholar]
- Hou, L.; Zhang, L.; Chen, X.; Li, X.; Zhang, Z.; Lin, Y.B. The ben efits of biochar: Enhanced cadmium remediation, inhibited precursor production of nitrous oxide and a short-term disturbance on rhizosphere microbial community. Environ. Pollut. 2021, 272, 116040. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Huérfano, X.; Vega-Mas, I.; Torralbo, F.; Menéndez, S.; Ippolito, J.A.; Kammann, C.; Wrage-Mönnig, N.; Cayuela, M.L.; Borchard, N.; et al. Biochar reduces the efficiency of nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) mitigating N2O emissions. Sci. Rep. 2019, 9, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Li, X.; Hao, X.; Luo, X.; Chen, W.; Huang, Q. Ammonia level influences the assembly of dissimilatory nitrate reduction to ammonia bacterial community in soils under different heavy metal remediation treatments. Sci. Total Environ. 2022, 838, 156393. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Li, S.; Geng, Y.; Miao, Y.; Zou, J. Differential responses of soil N2O to biochar depend on the predominant microbial pathway. Appl. Soil Ecol. 2019, 145, 103348. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fertil. Soils 2014, 50, 321–332. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Zhang, Z.; Shao, H.; Xu, G. Temperature and moisture responses to carbon mineralization in the biochar-amended saline soil. Sci. Total Environ. 2016, 569–570, 390–394. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, J.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Zhang, H.; Guo, L.; Chen, Y.; Heiling, M.; Zhou, B.; Mao, Y. Biochar interaction with chemical fertilizer regulates soil organic carbon mineralization and the abundance of key C-cycling-related bacteria in rhizosphere soil. Eur. J. Soil Biol. 2021, 106, 103350. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Van Zwieten, L.; Wallace, H.M. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil. Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Klimkowicz-Pawlas, A.; Gondek, K. Influence of poultry litter and poultry litter biochar on soil microbial respiration and nitrifying bacteria activity. Waste Biomass Valorization 2018, 9, 379–389. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Feng, Y.; Du, H.; Wulandari, T.; Poinern, G.E.J.; Jiang, Z.-T.; Fawcett, D.; Hassan, N.; Xue, L.; Yang, L. Hydrochar amendments stimulate soil nitrous oxide emission by increasing production of hydroxyl radicals and shifting nitrogen functional genes in the short term: A culture experiment. Chemosphere 2022, 302, 134771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Song, M.; Dong, M.; Xiong, Z. N2O and NO production and functional microbes responding to biochar aging process in an intensified vegetable soil. Environ. Pollut. 2022, 307, 119491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Hu, W.; Wang, Y.; Zhang, H.; Zhou, X.; Fei, J.; Luo, G. Understanding how reed-biochar application mitigates nitrogen losses in paddy soil: Insight into microbially-driven nitrogen dynamics. Chemosphere 2022, 295, 133904. [Google Scholar] [CrossRef] [PubMed]
- Kuever, J.; Konneke, M.; Galushko, A.; Drzyzga, O. Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov and description of strain Sax (T) as Desulfotignum balticum gen. nov., sp nov. Int. J. Syst. Evol. Microbiol. 2001, 51, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhou, Y.; Liang, M.; Lu, X.; Chen, G.; Zan, F. Insights into the role of biochar on the acidogenic process and microbial pathways in a granular sulfate-reducing up-flow sludge bed reactor. Bioresour. Technol. 2022, 355, 127254. [Google Scholar] [CrossRef]
- Pannekens, M.; Kroll, L.; Müller, H.; Mbow, F.T.; Meckenstock, R.U. Oil reservoirs, an exceptional habitat for microorganisms. New Biotechnol. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic biodegradation of hydrocarbon contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The role of sulfur- and phosphorus-mobilizing bacteria in biochar-induced growth promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zheng, X.; Cheng, W. Biochar combined with ferrous sulfate reduces nitrogen and carbon losses during agricultural waste composting and enhances microbial diversity. Process Saf. Environ. 2021, 162, 531–542. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe (III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Schulte, S.; Köster, D.; Jochmann, M.; Meckenstock, R. Applying reverse stable isotope labeling analysis by mid-infrared laser spectroscopy to monitor BDOC in recycled wastewater. Sci. Total Environ. 2019, 665, 1064–1072. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giagnoni, L.; Renella, G. Effects of Biochar on the C Use Efficiency of Soil Microbial Communities: Components and Mechanisms. Environments 2022, 9, 138. https://doi.org/10.3390/environments9110138
Giagnoni L, Renella G. Effects of Biochar on the C Use Efficiency of Soil Microbial Communities: Components and Mechanisms. Environments. 2022; 9(11):138. https://doi.org/10.3390/environments9110138
Chicago/Turabian StyleGiagnoni, Laura, and Giancarlo Renella. 2022. "Effects of Biochar on the C Use Efficiency of Soil Microbial Communities: Components and Mechanisms" Environments 9, no. 11: 138. https://doi.org/10.3390/environments9110138
APA StyleGiagnoni, L., & Renella, G. (2022). Effects of Biochar on the C Use Efficiency of Soil Microbial Communities: Components and Mechanisms. Environments, 9(11), 138. https://doi.org/10.3390/environments9110138








