Chemical Stabilization Used to Reduce Geogenic Selenium, Molybdenum, Sulfates and Fluorides Mobility in Rocks and Soils from the Parisian Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Excavated Rocks and Soils
- A calcareous sample (CS) mainly composed of carbonates from the Eocene period (Lutetian inferior, (depth. 24–27 m)) was collected by a mechanical excavator in Courbevoie (Hauts-de-Seine, France) in March 2018.
- A marly limestone sample (MLS-A) was extracted in Vitry-Sur-Seine (Val-de-Marne, France) in March 2018 and corresponded to sulfate rich carbonates from the Eocene period (Lutetian superior, (depth. 13–14 m)).
- A loamy sample (LS) was collected in Clamart (Hauts de Seine, France) in November 2018. It was mainly composed of clayey minerals from the Eocene period (Ypresian inferior, (depth. C.a. 15 m)).
- A tunnel muck sample I was extracted by a tunnel-boring machine below the city of Vitry-sur-Seine (Val-de-Marne, France). Its digging horizon was at the border between CS and MLS-A geological formations (depth. 30–40 m). This material followed several steps of treatment carried out directly in a slurry treatment plan located on the construction site. In particular, lime was added to the slurry to facilitate subsequent de-watering operations.
2.2. Reagents
2.3. Stabilization Protocol
2.4. Standardized Batch Leaching Tests
3. Results
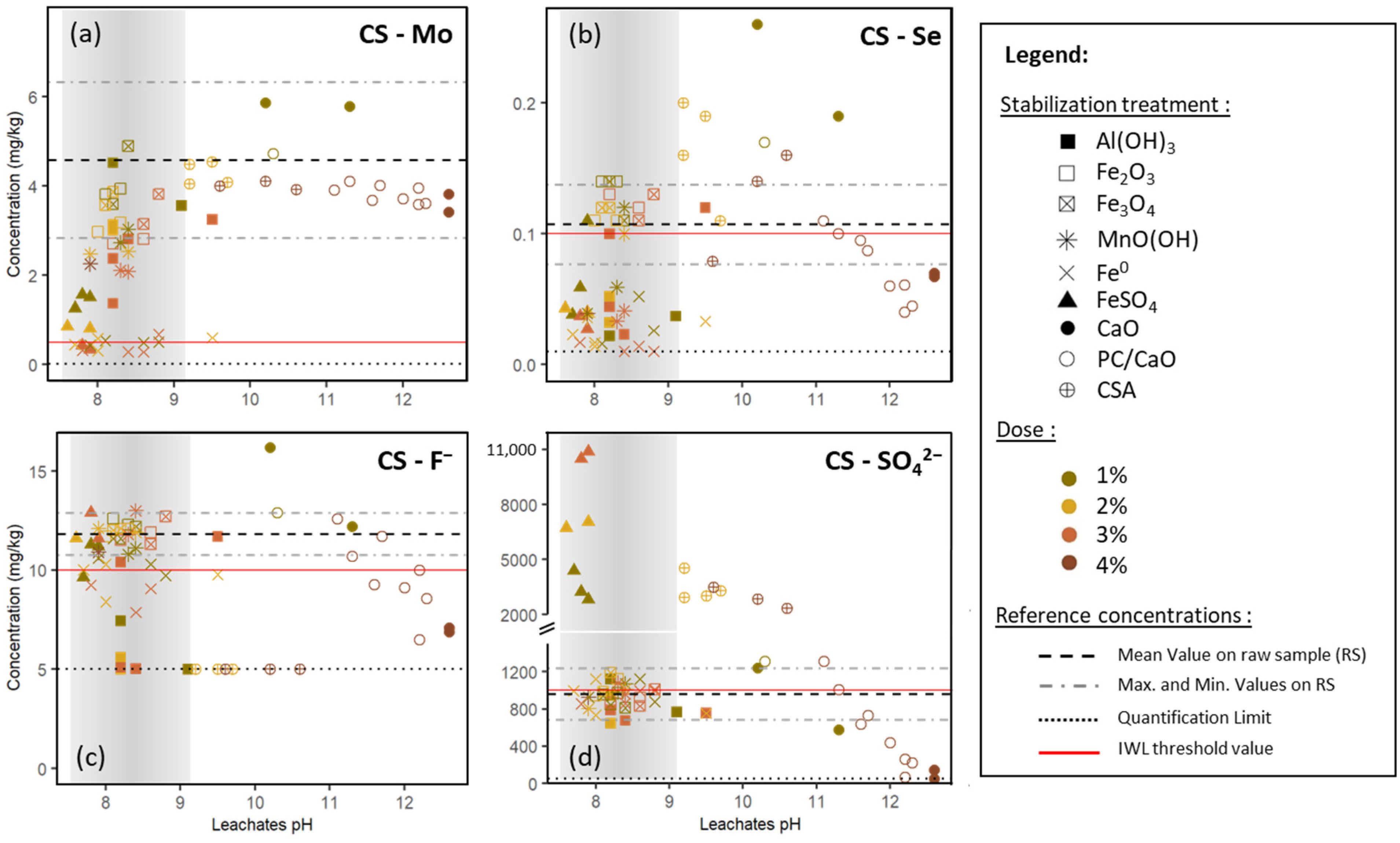
3.1. Calcareous Sample
3.2. Loamy Sample
3.3. Marly Limestone Sample
3.4. Tunnel Muck
4. Discussion
4.1. Effect of Metal Based Reagents
4.2. Effects of Alkaline Reagents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- SGP. Le défi de la Valorisation des déblais. The Challenge of Managing the Excavated Soils. Société du Grand Paris. 2019. Available online: https://www.societedugrandparis.fr/info/gestion-et-valorisation-des-d%C3%A9blais (accessed on 1 June 2020).
- OJEC. European Decision 2003/33/EC. Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003D0033&qid=1656310258356 (accessed on 1 June 2020).
- OJEC. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste. 1999. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31999L0031 (accessed on 1 June 2020).
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. Spec. Sect. Symp. Post-Combust. Carbon Dioxide Capture 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar] [CrossRef] [Green Version]
- Neunhäuserer, C.; Berreck, M.; Insam, H. Remediation of Soils Contaminated with Molybdenum using Soil Amendments and Phytoremediation. Water Air Soil Pollut. 2001, 128, 85–96. [Google Scholar] [CrossRef]
- Brandely, M.; Coussy, S.; Blanc-Biscarat, D.; Gourdon, R. Assessment of Molybdenum and Antimony speciation in excavated rocks and soils from the Parisian basin using mineralogical and chemical analyses coupled to geochemical modelling. Appl. Geochem. 2021, 136, 105129. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Bulteel, D.; Waligora, J.; Landrot, G.; Fonda, E.; Olard, F. Chemical, mineralogical, and environmental characterization of tunnel boring muds for their valorization in road construction: A focus on molybdenum characterization. Environ. Sci. Pollut. Res. 2020, 27, 44314–44324. [Google Scholar] [CrossRef] [PubMed]
- Caselles, L.D.; Roosz, C.; Hot, J.; Blotevogel, S.; Cyr, M. Immobilization of molybdenum by alternative cementitious binders and synthetic C-S-H: An experimental and numerical study. Sci. Total Environ. 2021, 789, 148069. [Google Scholar] [CrossRef]
- Gourcy, L.; Winckel, A.; Brugeron, A. RP-57344-FR–Origine du Sélénium et Compréhension des Processus dans les Eaux du Bassin Seine-Normandie; Bureau de Recherches Géologiques et Minières: Orléans, France, 2011. [Google Scholar]
- Vernoux, J.F. Les anomalies en Sélénium dans les eaux des captages d’lle-de-France (Essonne, Seine-et-Marne); Bureau de Recherches Géologiques et Minières: Orléans, France, 1998. [Google Scholar]
- Tabelin, C.; Sasaki, R.; Igarashi, T.; Park, I.; Tamoto, S.; Arima, T.; Ito, M.; Hiroyoshi, N. Simultaneous leaching of arsenite, arsenate, selenite and selenate, and their migration in tunnel-excavated sedimentary rocks: I. Column experiments under intermittent and unsaturated flow. Chemosphere 2017, 186, 558–569. [Google Scholar] [CrossRef]
- Caselles, L.D.; Hot, J.; Roosz, C.; Cyr, M. Stabilization of soils containing sulfates by using alternative hydraulic binders. Appl. Geochem. 2020, 113, 104494. [Google Scholar] [CrossRef]
- SGP. Le Catalogue des Fiches Formations Géologiques. Société du Grand Paris. 2017. Available online: https://mediatheque.societedugrandparis.fr/sgp/media?mediaTitle=title%20Catalogue+des+fiches+formations+g%26eacute%3Bologiques&mediaId=75804 (accessed on 1 June 2020).
- Lions, J.; Mauffret, A.; Devau, N. Evaluation des Concentrations de Référence des Fonds Hydrogéochimiques des Eaux Souterraines par Lithologie des Aquifères (No. RP-65594-FR); Bureau de Recherches Géologiques et Minières: Orléans, France, 2016. [Google Scholar]
- Mohapatra, M.; Anand, S.; Mishra, B.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Sandy, T.; DiSante, C. Review of Available Technologies for the Removal of Selenium from Water; CH2M: Corvallis, OR, USA, 2010. [Google Scholar]
- Runtti, H.; Tolonen, E.-T.; Tuomikoski, S.; Luukkonen, T.; Lassi, U. How to tackle the stringent sulfate removal requirements in mine water treatment—A review of potential methods. Environ. Res. 2018, 167, 207–222. [Google Scholar] [CrossRef]
- Verbinnen, B.; Block, C.; Lievens, P.; Van Brecht, A.; Vandecasteele, C. Simultaneous Removal of Molybdenum, Antimony and Selenium Oxyanions from Wastewater by Adsorption on Supported Magnetite. Waste Biomass-Valorization 2013, 4, 635–645. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Antelo, J.; Brännvall, E.; Carabante, I.; Ek, K.; Komárek, M.; Söderberg, C.; Wårell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. Appl. Geochem. 2018, 100, 335–351. [Google Scholar] [CrossRef]
- Kamei, T.; Ahmed, A.; Horai, H.; Ugai, K. A novel solidification technique for fluorine-contaminated bassanite using waste materials in ground improvement applications. J. Mater. Cycles Waste Manag. 2014, 17, 380–390. [Google Scholar] [CrossRef]
- Moon, D.H.; Grubb, D.G.; Reilly, T.L. Stabilization/solidification of selenium-impacted soils using Portland cement and cement kiln dust. J. Hazard. Mater. 2009, 168, 944–951. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H.; Godfrey, C. Molybdenum Adsorption on Oxides, Clay Minerals, and Soils. Soil Sci. Soc. Am. J. 1996, 60, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S. Competitive Adsorption of Molybdenum in the Presence of Phosphorus or Sulfur on Gibbsite. Soil Sci. 2010, 175, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Geng, C.; Jian, X.; Su, Y.; Hu, Q. Assessing Molybdenum Adsorption onto an Industrial Soil and Iron Minerals. Water Air Soil Pollut. 2013, 224, 1743. [Google Scholar] [CrossRef]
- Duc, M.; Lefèvre, G.; Fédoroff, M. Sorption of selenite ions on hematite. J. Colloid Interface Sci. 2006, 298, 556–563. [Google Scholar] [CrossRef]
- Loffredo, N.; Mounier, S.J.L.; Thiry, Y.; Coppin, F. Sorption of selenate on soils and pure phases: Kinetic parameters and stabilisation. J. Environ. Radioact. 2011, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.A.; Burau, R.G. Selenium Immobilization in Evaporation Pond Sediments by in Situ Precipitation of Ferric Oxyhydroxide. Environ. Sci. Technol. 1995, 29, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kang, Y.; Sakurai, K. Selenium in amorphous iron (hydr)oxide-applied soil as affected by air-drying and pH. Soil Sci. Plant Nutr. 2002, 48, 243–250. [Google Scholar] [CrossRef]
- Mancini, G.; Palmeri, F.; Luciano, A.; Viotti, P.; Fino, D. Partial Stabilization of Mo-Containing Hazardous Wastes Using a Ferrous Sulfate-Based Additive as a Redox Agent. Waste Biomass-Valorization 2020, 11, 5493–5502. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, B.; Chuaicham, C.; Sasaki, K. Mechanism analysis of selenium (VI) immobilization using alkaline-earth metal oxides and ferrous salt. Chemosphere 2020, 248, 126123. [Google Scholar] [CrossRef]
- Wang, X.; Brunetti, G.; Tian, W.; Owens, G.; Qu, Y.; Jin, C.; Lombi, E. Effect of soil amendments on molybdenum availability in mine affected agricultural soils. Environ. Pollut. 2020, 269, 116132. [Google Scholar] [CrossRef]
- Essington, M.E.; Stewart, M.A. Adsorption of Antimonate, Sulfate, and Phosphate by Goethite: Reversibility and Competitive Effects. Soil Sci. Soc. Am. J. 2018, 82, 803–814. [Google Scholar] [CrossRef]
- NF EN 12457-2–AFNOR; Characterization of Waste–Leaching–Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 4 mm (without or With Size Reduction). AFNOR: Paris, France, 2002.
- NF T90-004–AFNOR; Water Quality–Determination of Fluoride Ion–Potentiometric Method. AFNOR: Paris, France, 2002.
- NF ISO 15923-1–AFNOR; Water Quality–Determination of Selected Parameters by Discrete Analysis Systems—Part 1: Ammonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection. AFNOR: Paris, France, 2014.
- Komarek, M.; Vanek, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2014, 13, 49–58. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Xu, N.; Christodoulatos, C.; Braida, W. Modeling the competitive effect of phosphate, sulfate, silicate, and tungstate anions on the adsorption of molybdate onto goethite. Chemosphere 2006, 64, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- van Geen, A.; Robertson, A.P.; O Leckie, J. Complexation of carbonate species at the goethite surface: Implications for adsorption of metal ions in natural waters. Geochim. Cosmochim. Acta 1994, 58, 2073–2086. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.; Dixon, J.B.; Weed, S.B. Iron Oxides. In Minerals in Soil Environments; John Wiley & Sons: Hoboken, NJ, USA, 1989; Volume 1, pp. 379–438. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Y.H.; Zeng, H.; Zhang, Z. Reductive removal of selenate by zero-valent iron: The roles of aqueous Fe2+ and corrosion products, and selenate removal mechanisms. Water Res. 2014, 67, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Balistrieri, L.S.; Chao, T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta 1990, 54, 739–751. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Bang, S.; Kim, K.-W.; Kim, M.G.; Park, S.Y.; Choi, W.-K. Selenate removal by zero-valent iron in oxic condition: The role of Fe(II) and selenate removal mechanism. Environ. Sci. Pollut. Res. 2015, 23, 1081–1090. [Google Scholar] [CrossRef]
- Kumpiene, J. Trace Element Immobilization in Soil Using Amendments. In Trace Elements in Soils; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 353–379. [Google Scholar] [CrossRef]
- Dou, W.; Zhou, Z.; Jiang, L.-M.; Jiang, A.; Huang, R.; Tian, X.; Zhang, W.; Chen, D. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization. J. Environ. Manag. 2017, 196, 518–526. [Google Scholar] [CrossRef]
- Pickering, W. The mobility of soluble fluoride in soils. Environ. Pollut. Ser. B Chem. Phys. 1985, 9, 281–308. [Google Scholar] [CrossRef]
- Henry, R.L. Low Temperature Aqueous Solubility of Fluorite at Temperatures of 5, 25, and 50 °C and Ionic Strengths up to 0.72m–ProQuest; Colorado School of Mines: Golden, CO, USA, 2018. [Google Scholar]




| Sample | Geological Formation | Excavation Site | Main Characteristics | Natural pH | (oxy-)anions Mobility | Trace Elements Speciation |
|---|---|---|---|---|---|---|
| CS | Lutetian Limestone (Eocene, Lutetian inferior) | Courbevoie | Almost exclusively composed of carbonated minerals (mainly calcite) | 8.8 | Mo +++ Se + SO42− − F− + | Mo mainly exchangeable and presenting associations with pyrites and carbonated minerals Se exchangeable fraction at pHnat only represent 5% of total Se |
| MLS-A | Lutetian Marly Limestones (Eocene, Lutetian superior) | Vitry-Sur-Seine | Mainly carbonated (calcite, dolomite) with high content of sulfates (gypsum, celestite) | 7.6 | Mo ++ Se + SO42− +++ F− + | Mo and Se mainly associated with celestite TE exchangeable fractions represent 10%wt of total TE |
| LS | Ypresian Clays (Eocene, Ypresian inferior) | Clamart | Mainly composed of clay minerals (smectite and kalonite) Presence of natural oxides (Ti, Fe) | 8.0 | Mo − Se +++ SO42− − F− +++ | Mo almost exclusively insoluble Se mainly exchangeable with equal repartition between SeVI and SeIV, the former being retained at oxides’ surfaces at pHnat |
| TM | Lutetian Limestone and Lutetian Marly Limestones | Vitry-Sur-Seine | Mainly carbonated (calcite, dolomite) Presence of sulfate (gypsum, celestite) Composition impacted by liming carried out after excavation (precipitation of ettringite, partial dissolution of celestite, Mo impoverishment) | 12.2 | Mo +++ Se − SO42− ++ F− + | Mo highly exchangeable with small incorporation in ettringite and associations with celestite, carbonated minerals and pyrite Se mostly insoluble with similar associations |
| Treatment | CS | LS | MLS-A | TM |
|---|---|---|---|---|
| Raw Sample | 9 | 7 | 6 | 6 |
| Zero valent iron (ZVI) | ||||
| 1% | 4 | 2 | 2 | 1 |
| 2% | 4 | - | 1 | 1 |
| 3% | 4 | 2 | 1 | 1 |
| Iron sulfate (FeSO4) | ||||
| 1% | 3 | 2 | - | - |
| 2% | 2 | - | - | - |
| 3% | 2 | 2 | - | - |
| Aluminum hydroxide (Al(OH)3) | ||||
| 1% | 2 | 2 | 1 | 1 |
| 2% | 2 | - | - | 1 |
| 3% | 4 | 2 | - | 1 |
| Magnetite (Fe3O4) | ||||
| 1% | 2 | 2 | 2 | - |
| 2% | 2 | - | 2 | - |
| 3% | 2 | 2 | - | - |
| Hematite (Fe2O3) | ||||
| 1% | 2 | 2 | 2 | - |
| 2% | 2 | - | 2 | - |
| 3% | 2 | 2 | - | - |
| Manganite (MnOOH) | ||||
| 1% | 2 | 2 | 2 | - |
| 2% | 2 | - | 2 | - |
| 3% | 2 | 2 | - | - |
| 4% | 1 | - | - | - |
| Lime (CaO) | ||||
| 1% | 2 | - | - | 1 |
| 2% | - | 2 | 1 | 1 |
| 4% | 2 | 2 | 1 | 1 |
| Calcium sulfoaluminate clinker (CSA) | ||||
| 2% | 4 | 4 | - | - |
| 3% | - | - | - | 2 |
| 4% | 3 | 4 | - | - |
| Portland cement + lime (PC/CaO) | ||||
| 1% | 1 | - | 2 | 1 |
| 2% | - | 5 | - | 1 |
| 4% | 8 | 5 | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandely, M.; Coussy, S.; Blanc-Biscarat, D.; Gourdon, R.; Blanck, G. Chemical Stabilization Used to Reduce Geogenic Selenium, Molybdenum, Sulfates and Fluorides Mobility in Rocks and Soils from the Parisian Basin. Environments 2022, 9, 78. https://doi.org/10.3390/environments9070078
Brandely M, Coussy S, Blanc-Biscarat D, Gourdon R, Blanck G. Chemical Stabilization Used to Reduce Geogenic Selenium, Molybdenum, Sulfates and Fluorides Mobility in Rocks and Soils from the Parisian Basin. Environments. 2022; 9(7):78. https://doi.org/10.3390/environments9070078
Chicago/Turabian StyleBrandely, Maxime, Samuel Coussy, Denise Blanc-Biscarat, Remy Gourdon, and Gaëtan Blanck. 2022. "Chemical Stabilization Used to Reduce Geogenic Selenium, Molybdenum, Sulfates and Fluorides Mobility in Rocks and Soils from the Parisian Basin" Environments 9, no. 7: 78. https://doi.org/10.3390/environments9070078






