Abstract
Patients affected by Attention-Deficit/Hyperactivity Disorder (ADHD) are characterized by impaired executive functioning and/or attention deficits. Our study aim is to determine whether the outcomes measured by the Attention Network Task (ANT), i.e., the reaction times (RTs) to specific target and cue conditions and alerting, orienting, and conflict (or executive control) effects are affected by cognitive training with a Dual n-back task. We considered three groups of young adult participants: ADHD patients without medication (ADHD), ADHD with medication (MADHD), and age/education-matched controls. Working memory training consisted of a daily practice of 20 blocks of Dual n-back task (approximately 30 min per day) for 20 days within one month. Participants of each group were randomly assigned into two subgroups, the first one with an adaptive mode of difficulty (adaptive training), while the second was blocked at the level 1 during the whole training phase (1-back task, baseline training). Alerting and orienting effects were not modified by working memory training. The dimensional analysis showed that after baseline training, the lesser the severity of the hyperactive-impulsive symptoms, the larger the improvement of reaction times on trials with high executive control/conflict demand (i.e., what is called Conflict Effect), irrespective of the participants’ group. In the categorical analysis, we observed the improvement in such Conflict Effect after the adaptive training in adult ADHD patients irrespective of their medication, but not in controls. The ex-Gaussian analysis of RT and RT variability showed that the improvement in the Conflict Effect correlated with a decrease in the proportion of extreme slow responses. The Dual n-back task in the adaptive mode offers as a promising candidate for a cognitive remediation of adult ADHD patients without pharmaceutical medication.
1. Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a behavioral condition caused by a complex interplay between genetic and environmental risk factors affecting brain networks and leading to a broad range of impairments that interfere with functioning and development [1,2,3,4,5]. Although ADHD was initially considered to be a childhood-onset condition with limited effect on the adult, the symptoms associated with ADHD are increasingly observed in adults [6,7,8,9]. Higher-level executive functioning and emotional control deficits are more diverse in adult than in child ADHD patients [10,11]. The current mainstay of treatment of ADHD is medication: stimulant drugs such as methylphenidate and amphetamine [12] or non-stimulant drugs such as atomoxetine [13]. Several studies have reported that stimulants induced significant improvements in core symptoms of ADHD, in daily functioning and in executive impairments [14]. The stimulants have poor adverse effect profiles and a multitude of drug interactions [15] provoking non-serious adverse events as decreased appetite, insomnia, and sleep disturbances whose etiology is still complex and unclear [16]. Therapeutic approaches avoiding drug medication attract patients who resist taking stimulants and who want to avoid the risk of side effects of drugs. In this context, cognitive training, such as working memory (WM) training, gained interest as an alternative treatment of attentional and neuropsychological deficits in ADHD patients [17,18,19,20,21].
The Attention Network Task (ANT) [22] is a computer-based task that was developed to evaluate the function of attention, which can be broken down into the three components according to the attention network theory [23,24]. These components are meant to be functionally independent and corresponding to anatomically segregated networks, each one with different yet interrelated functions of selective attention called alerting, orienting and executive attention (or conflict) network [25,26,27]. The analysis of the reaction times (RTs) in the ANT provides an estimate of the preservation of each component of the attention network. Adult ADHD patients exhibited higher intra-individual variance and longer RTs than controls, in nearly all attentional tasks [28]. However, several arguments based on typical RT distributions to neuropsychological tasks in child ADHD pointed out that characterization of clinical heterogeneity should be based rather on the analysis of parameters (i.e., mu, sigma, and tau) derived from the ex-Gaussian distributional model of RTs [29,30,31,32]. A meta-analysis investigating the RT variability in controls and ADHD children, adolescents, and adults [33] concluded that the variability in the task performance of ADHD was primarily due to a set of abnormally slow responses, rather than ubiquitous variability across all trials in the task.
In contrast to increased mean RT, the distributional parameter mu did not document a significant slowing in adult ADHD and a significant correlation was reported between tau and the number of omission errors in a Go/NoGo task bringing evidence for attentional dysfunction [34]. However, there is yet no evidence of how a cognitive training of adult ADHD patients may affect the attention network. The alerting network is related to arousal and vigilance and is meant for achieving and maintaining a state of being very sensitive to incoming stimuli combined with a readiness to react. The orienting network is defined as selecting information from sensory input and shifting attention, i.e., disengaging and re-engaging attention. The conflict network comprises mechanisms for monitoring and resolving conflict among thoughts, feelings, and responses. Children with ADHD showed acceptable criterion validity for incorrect responses, omissions, and conflict score during ANT, despite high RT variability [35,36]. In child ADHD patients, the alerting scores on the ANT after WM training with an n-back task (NBT) were found to be a good predictor on math achievement [37].
The NBT is a popular experimental paradigm for WM training [38], in which subjects are asked to monitor two sources of information simultaneously (i.e., the specification and the location of a stimulus), with increasing attentional and working memory load, and decide if the currently presented stimulus is the same as the one presented in N trials previously [39,40]. The Dual n-back task (DNBT) is a version of the task eliciting divided attention because dual, one auditory and one visual, unrelated stimuli appear simultaneously and subjects are asked to monitor both stimuli independently [41]. DNBT received notable attention for its potential to improve WM and other aspects of cognitive performance [42,43]. The present study is the first one, to our knowledge, aimed at determining whether, in ADHD, the scores for the three ANT components measured by RTs and corresponding ex-Gaussian distributional model are selectively affected by WM training with a DNBT. We tested age-matched controls, medicated, and unmedicated young ADHD adults. In addition to group comparisons, we considered the dimensional analysis [44,45] to test whether improvement through training is associated with ADHD severity [46,47] on a continuum rated by Conners’ Adult ADHD Rating Scales-Self Report subscales. We found evidence that one-month training with DNBT has an impact on the executive control of any tested group, measured by the conflict network effect in ANT.
2. Materials and Methods
2.1. Participants
We recruited 114 (64 males and 50 females) young adults between 18 and 30 years old in the three groups of study, ADHD medicated with methylphenidate hydrochloride prescription (; 30 of these patients were diagnosed as a combined (mixed) subtype of ADHD (ADHD-C), 10 as predominantly inattentive subtype (ADHD-I), and 2 patients without any precise information provided by the psychiatrist regarding the subtype of the ADHD condition), ADHD without medication (; 18 of which were diagnosed as ADHD-C patients, 13 as ADHD-I, 1 as predominantly hyperactive/impulsive subtype (ADHD-HI), and 2 patients without any precise information regarding the subtype) and controls (). All ADHD patients () were clinically referred by the Psychiatric Department of the University Hospital of Lausanne or at a psychiatrist’s practice in collaboration with the University Hospital after an initial screening appointment to ensure that they were fulfilling the criteria defined by the DSM-IV-TR for inattentive, hyperactive/impulsive, or mixed subtypes [48,49]. Under the supervision of a trained clinical psychologist, all participants underwent the Mini-International Neuropsychiatric Interview [50], a short structured diagnostic interview assessing psychiatric diseases, in order to exclude from this study those with ADHD comorbidity. We excluded from this study any individual referred taking mood and anxiety stabilizers, anti-depressants, any dopamine receptor-blocking drug and non-stimulant medications for ADHD.
The assignment of a participant to the ‘medicated ADHD group’ (MADHD) or to the ‘ADHD group without medication’ (ADHD) was decided by the psychiatrist in charge of the patients, on the exclusive basis of the patients’ therapeutic treatment. This assignment was ‘double-blind’, in the sense that the experimenters did not know which patient belonged to either ADHD group until the end of the protocol and the psychiatrist did not know which level of working memory training is assigned to a patient. Participants of the ADHD group with medication were required to stop medication 24 h prior to testing [51,52,53]. All participants underwent a short structured diagnostic interview to assess their psychiatric status. Control subjects were age/education-matched volunteer healthy controls screened prior to the experimental session to ensure that they would not report any neuropsychiatric diseases or any other exclusion criteria and none were taking any psychoactive medications. The study was carried out in accordance with the latest version of the Declaration of Helsinki [54] and approved by the mandatory Ethics Committees requested by Swiss Federal Authorities, following the constitutional article (art. 118b Cst) of 8 March 2010 and the Federal Act involving Human Beings on 30 September 2011 (revised 1 January 2014). All participants had normal or corrected-to-normal visions, none reported a history of sustained head injury. All participants were requested to fill out French versions of the adult ADHD Self-Report Scale (ASRS) and the Conners’ Adult ADHD Rating Scales-Self Report (Screening Version, CAARS-S:SV) [55,56,57] two weeks prior to the beginning of the protocol. All participants received monetary compensation following the scale approved by the mandatory Ethics Committees requested by Swiss Federal Authorities.
2.2. Experimental Protocol
The experimental procedure of this study included 3 parts. The first part was a before-training session of the ANT in the experimental laboratory (Labex, HEC-UNIL). The second part was a WM training period lasting one month with the Dual n-back task at home. The third part was an after-training session of the ANT at the same laboratory as the pre-test.
2.2.1. Attention Network Task (ANT)
This task was originally reproduced from the original ANT [22]. Each trial had a fixed duration of 3500 ms and was formed by 5 successive intervals, as described in Figure 1. At the beginning of the trial, the participants were instructed to maintain their gaze on a black fixation cross at the center of a 19-inch computer screen with white background at a viewing distance of about 70 cm. After a uniformly distributed random interval lasting from 400 to 1600 ms, a cue, represented by an asterisk having the same size of the cross, appeared for 100 ms. We considered four cue types which were randomly distributed: No Cue (NC), Center Cue (CT, a cue superimposed to the cross at the center of the screen), Double Cue (DB, a cue appearing both above and below the cross), and Spatial Cue (SP_above or SP_below, a cue appearing either above or below the cross). The participants maintained their gaze on the black fixation cross during 400 ms, after the disappearance of the cue. Then, the participants were requested to identify a target (i.e., a target arrow) and determine its direction (left or right) as quickly as possible by pressing the corresponding button (left or right) of a computer mouse. The time taken to respond was recorded as reaction time (RT). Finally, the participants maintained their gaze on the black fixation cross during the remaining time till the end of the trial.
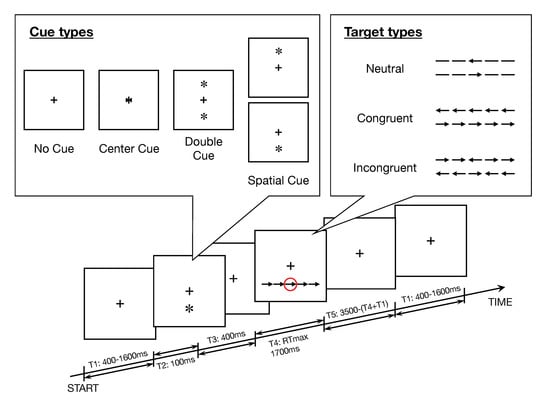
Figure 1.
Experimental procedure of the Attention Network Task (ANT). The steps in a trial are summarized as follows: (T1) The participant is requested to fix a cross at the center of the screen for a random interval in the range 400–1600 ms. (T2) A cue appears for 100 ms, in the upper window, the four cue conditions. (T3) The cue disappears. (T4) A target stimulus with flankers appears, marked in the red circle in this figure; the related panel shows the six stimuli used in the present experiment. The participant is requested to select as quickly as possible the button corresponding to the direction of the target stimulus. In this example, the correct choice is to press the right button of the computer mouse. (T5) A final interval with participant’s gaze focused on the central cross is set until the next trial is started.
The target arrow (i.e., the target) was surrounded by flankers on both sides by 2 items, such that the target was located at the center of a line of 5 items. (Figure 1). Flankers presented above or under a fixation cross and corresponding to arrows pointing to the same direction of the target were labeled Congruent (CON), Incongruent (INC, if pointing to the opposite direction), or Neutral (NTL, simple lines). Errors (trials with an incorrect answer) and no responses (trials without response) were also recorded for each participant i, in order to calculate the accuracy rate. For each participant i we computed the accuracy rate . The combinations of 5 cue types (No Cue, Center Cue, Double Cue, Spatial Cue_below, Spatial Cue_above) × 3 target types ( Neutral, Congruent, Incongruent) × 2 directions ( Left, Right) defined 30 primary patterns of trials. After 1 practice block with 24 trials pseudorandomly selected ( [No Cue, Center Cue, Double Cue, Spatial Cue_below, Spatial Cue_above] × [Neutral, Congruent, Incongruent] × [Left, Right]), the participants performed 3 experimental blocks, each one including 96 trials, that means 288 trials overall for each session and for each participant. For data analysis, all trials with Spatial Cue_below and Spatial Cue_above were pooled together and all trials with targets oriented to the left or to right side were pooled together. Hence, the task included 12 final patterns, i.e., 4 cue types (No Cue, Center Cue, Double Cue, Spatial Cue) × 3 target types (Neutral, Congruent, Incongruent).
2.2.2. Computation of the Attention Network Effects
The effectiveness of the attention networks can be estimated from the RTs measured for the cue and target conditions [22]. The distribution of the RTs are not normally distributed but skewed towards long RTs (this aspect will be treated separately in ex-Gaussian analysis). Hence, the median values () of the RTs were chosen instead of the means for the calculation of each effect, as follows: Alerting Effect (AE) , Orienting Effect (OE) and Conflict Effect (CE) . The higher the Alerting Effect and Orienting Effect scores, the more efficient the networks are. For Conflict Effect, the lower the score, the more efficient its network. Notice that the median RT for each one of the 12 final patterns is hereafter referred simply as the RT.
2.2.3. WM Training: Dual n-Back Task
The task consisted of two variants of the DNBT described in detail elsewhere [20,21,41]. The task is illustrated in Figure 2 and briefly summarized as follows. Each trial was composed of an auditory and visual stimulus presented simultaneously during 500 ms. The participants were asked to memorize the dual modality cues and to detect, by pressing a key, if any of the current stimuli correspond to the one presented in the previous trial (for 1-back). They had to press the ‘A’ keyboard letter to report the correspondence with a visual target, and to press the ‘L’ keyboard letter for matching the auditory target. The level of difficulty of the task is referred to as n-back. Then, if participants were required to detect a match with the previous trial, the mode is referred to as 1-back, called also the baseline training. In the adaptive training, the difficulty n of the task was adjusted as a function of the performance. The whole task consisted of 20 blocks of 20 + n trials with the same level of difficulty. An increase by 1 in the level of difficulty in the next block was triggered by a performance of less than 3 mistakes in each modality. With levels of difficulty higher than 1, a decrease by 1 in the level was triggered by 5 or more errors accumulated in any modality. In other cases, the level remained unchanged. The total duration of the working memory task was approximately half an hour.
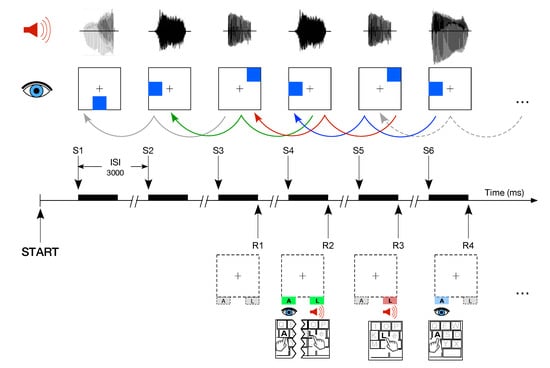
Figure 2.
Example of level of the Dual n-back task. The task consisted of 20 blocks of at least 20 trials. Each trial was composed of an auditory and visual stimulus presented simultaneously. Participants were asked to detect and to press a key if any of the current stimuli corresponded to the one presented in the previous trial. They had to press the ‘A’ keyboard letter to report the correspondence with a visual target while the auditory target required the pressing of the ‘L’ key. Modified from [21].
During the before-training session, all participants performed one session of adaptive DNBT. Participants of each group were randomly assigned to either baseline or adaptive training mode for the one-month WM training period at home. It is very important to note that the random assignment to either group of training level was done in ‘blind’ mode to the experimenter, who knew only about the recruitment of controls vs. patients. This fact is very important for the comparison of MADHD and ADHD groups. This mode of assignment explains why the structure of ADHD and MADHD groups is not balanced with respect to the diagnosed subtype of ADHD (Table 1). Participants were asked to perform each weekday one session of DNBT via a secured web page, which allowed us to monitor whether the training sessions were completed correctly. In case of problems, the participants were advised to complete the incomplete sessions during the weekends. Those who did not successfully complete at least 18 sessions were excluded from this study. At the after-training session, all participants performed again one adaptive DNBT.

Table 1.
Descriptive statistics (median, mean, and SEM) of participants’ accuracy rates of performance and DSM-IV symptom subscales. The effects of factors group and level of training were tested by a two-way analysis of variance (ANOVA). The effect size was estimated by .
2.3. Statistical Analysis
R 4.0.0 statistical software was used in all the analyses [58], with packages outliers [59], WRS2 [60], robust [61] effectsize [62], coin [63], lmtest [64], and report [62]. The non-parametric test for paired groups (Wilcoxon signed-rank test) was applied to test the effect of training before and after the working memory training (WMT) at home with the dual n-back task. The Mann–Whitney test was used for the unpaired two-groups comparisons. Factorial analysis was performed to test interactions among groups (3 ‘group’ factors: CTL, MADHD, ADHD), between sessions (2 ‘session’ factors: before-training, after-training), and between WM training modes (2 ‘level’ factors: baseline, adaptive). We assume the null hypothesis of homoscedasticity in the data samples and test it with the studentized Breusch–Pagan test. The standard ANOVA can be used for factorial analysis if the value of the test statistics is not significant and partial omega squared () is used to test the effect size. The magnitude of effect sizes is labeled as follows: statistically insignificant as “+si”: ; small as ”+s”: ; medium as “+m”: ; large as “+L”: . Notice that if the value of the observed F is less than one will be negative. In addition to being insignificant, a value of F less than one is a sign of inconsistency in the statistics. For the Student’s t-test, the effect size is assessed by Cohen’s d with magnitudes: statistically insignificant as “+si”: ; small as ”+s”: ; medium as “+m”: ; large as “+L”: .
In case homoscedasticity is rejected, we apply robust versions of ANOVA, i.e., heteroscedastic one-way ANOVA for medians and two- and three-way ANOVAs with trimmed means at level 0.2 and effect size assessed by with magnitudes: statistically insignificant as “+si”: ; small as ”+s”: ; medium as “+m”: ; large as “+L”: . For non-parametric Wilcoxon Signed-Rank and Mann–Whitney tests, the effect size is assessed by r with magnitudes: statistically insignificant as “+si”: ; small as ”+s”: ; medium as “+m”: ; large as “+L”: . Notice that there are no available tests for heteroscedastic ANOVA with unbalanced repeated measurements. The computation of the ex-Gaussian parameters (mu, sigma, and tau) were computed with the mexgauss function package retimes [65]. In general, the grouped values are reported as (median, average ±, and SEM).
3. Results
3.1. Unsupervised Exclusion of Outliers
We applied an unsupervised procedure aimed at excluding participants characterized by an outlier performance. In order to do so, we conducted a two steps procedure and we applied the scores function of the package outliers of the R statistical software throughout the study. The first step was aimed at excluding from the analysis the participants characterized by an outlying performance either before or after the WM training. For each participant i we computed the accuracy rate . We tested the normality of the distributions of using the Shapiro–Wilk test for each group, before and after training. In all groups, the distribution of was not following a normal distribution. Hence, we applied the scores function with robust estimation of the differences between each value and median, divided by median absolute deviation (“mad”). According to the size of our samples the Z score of the outliers was bounded by the value [66]. The outcome of this procedure was the removal of 5 participants (2 controls, 1 MADHD, and 2 ADHD).
In order to reduce the impact of outliers in the skewed distribution of RTs we applied a logarithmic transformation of RTs for all trials [67,68]. The second step of the procedure consisted in detecting the outlier trials using the log-transformed RTs measured for each one of the 30 primary trial patterns (see Section 2.2.1) using the scores function based on the median absolute deviation. On average we observed 18.7/288, 17.6/288, and 17.8/288 outlier trials for each participant belonging to controls, MADHD and ADHD, respectively. After removal of all outlier trials for all participants, the median RT was computed for each primary trial pattern. Then, within each group and for each such primary trial pattern, the participant outliers were detected after applying the logarithmic transformation to the series of median RTs. Any participant with more than 1 outlier median RT in the group series of any primary trial pattern was checked out as an outlier participant. The outcome of this second procedure was the removal of additional 4 participants (1 control, 1 MADHD, and 2 ADHD). At the end of both procedures for removal of outlier participants, the overall sample of the remaining 104 participants included 27,719 trials before training and 27,460 trials after training. The accuracy rates of all participants, irrespective of their groups and subgroups, were above 95% (Table 1) and all values were homoscedastic (, , and , , , before- and after-training, respectively). The outlier trials represented 6.40% and 6.98% of the total number of valid trials before and after WMT, respectively.
3.2. Clinical Assessment Scales and Subscales
A quantitative assessment of clinical symptoms fulfilling the criteria defined by the DSM-IV-TR for inattentive, hyperactive/impulsive or mixed ADHD subtypes [48] was performed using the ADHD Self-Report Scale (ASRS) [56] and Conners’ Adult ADHD Rating Scales-Self Report (Screening Version, CAARS-S:SV) [55,57]. After removal of the outliers the final sample sizes of patients were including 28 ADHD-C, 10 ADHD-I, and 2 unknown subtype and including 16 ADHD-C, 12 ADHD-I, 1 ADHD-HI, and 1 unknown subtype. We consider a model where ASRS [56] and the normalized T-score of CAARS, referred to as the ‘ADHD Index’ [55,57], depend on three factors: patients’ group × ADHD subtype × level, where level refers to the level of training assigned to each participant before WMT with the Dual n-back task.
For ASRS, the null hypothesis for homoscedasticity and homogeneity of variances were accepted (, , and Levene’s test , ), then standard ANOVA could be applied. Two main effects were significant, i.e., factor ADHD subtype (, , effect size +L) and factor group (, , +m). In both groups of patients, the ASRS score of ADHD-C patients was significantly higher than the score of the predominantly inattentive subtype ( vs. and vs. ). It is important to notice that the interaction between group and ADHD subtype is not significant (, , +si). Notice that this last F value is less than one and the lack of significant interaction mean that the effect of patients’ group is independent from the ADHD subtype. Table 1 includes also the controls and it shows the median, mean, and SEM of the clinical assessment scales and subscales for subgroups baseline and adaptive, as defined by the level of training during one month with the Dual n-back task, for each group of participants. The two-way ANOVA showed that the main effect of group, including also the group of controls, was very significant (, , +L). We observed neither a main effect of the level of training assigned to the subgroups, nor an interaction between the factors, then we considered the two groups of patients irrespective of the training level. The ASRS scores were (47.0, 47.0 ± 1.9), (65.5, 65.7 ± 1.9), and (58.5, 57.9 ± 1.9). Tukey post-hoc multiple comparisons showed that the ASRS scores of controls were different from patients’ groups (, , effect size +L and , , for MADHD and ADHD, respectively). Notice that ASRS was also different from each other patients’ group (, , +m).
One participant belonging to the MADHD group (subgroup baseline) did not complete the CAARS questionnaire. For the DSM-IV Inattentive Symptoms Subscale () the values were homoscedastic (, , ). If we test the model with two factors group×level of training we found a strong effect of group (, , +L) and a small effect of the assigned level of training (, , +s). The smallness of this effect was confirmed by the lack of significance in the difference of the DSM-IV Inattentive Symptoms between the subgroups assigned to baseline and adaptive levels of WMT irrespective of the group of participants (, , +s). Tukey post-hoc multiple comparisons showed that controls were significantly different from MADHD and ADHD (, , +L) and , , +L, respectively), but the inattentive symptoms of patients’ groups were not different from each other group (, , +s). A separate three-way ANOVA limited only to the ADHD patients diagnosed as ADHD-C and ADHD-I testing the model of with factors group×level of training×ADHD subtype showed no significant main effect (group:, , +s; ADHD subtype:, , +si; level:, , +s).
The values of the DSM-IV Hyperactive-Impulsive Symptoms Subscale () were heteroscedastic (, , ) and we used a robust two-way ANOVA for factors group×level. Table 1 shows that the outcome of the comparisons of the DSM-IV Hyperactive-Impulsive Symptoms Subscale () was similar to the Inattentive Symptoms Subscale, that is a large main effect of group (, , effect size +L) and a small effect of the assigned level of training (, , +s). The differences between the scores of the subgroups were not significant for controls and medicated ADHD (, , , +s and , , , +s, respectively), and just below the threshold (5%) for ADHD (, , , +m ). In a separate analysis limited to patients’ groups MADHD and ADHD, we observed a significant main effect of factor ADHD subtype (, , +L). In both patients’ groups, the values of the DSM-IV Hyperactive-Impulsive Symptoms Subscale of ADHD-C patients were significantly higher than the values of ADHD-I (for MADHD: vs. , Mann–Whitney test , , , +L and for ADHD: vs. ; , , , +m).
Standard ANOVA was used to analyze the model of the DSM-IV Total ADHD Symptoms Subscale () as a function of factors group×ADHD subtype×level of training because the values were homoscedastic (, , and variances were homogeneous , ). The difference in the values of scores between MADHD and ADHD was not significant (, , +si) but the main effect of ADHD subtype was significant (, , +L). The values of score of patients diagnosed with the combined subtype were larger than those diagnosed as predominantly inattentive (for MADHD: vs. , , , +L) and for ADHD: vs. ; , , +m). The ANOVA extended to the controls showed a large main effect of group (, , +L). The main effect of the assignment to the level of training was significant (, , +m) and the comparison between the baseline and adaptive subgroups, irrespective of the participant’s group was also significant , , +s). However, a more detailed analysis for patients’ groups showed no difference of score between subgroups assigned to baseline or adaptive protocol (for MADHD: , , +s and for ADHD: , , +m). It is within the control group, and not in any patients’ group, where the participants assigned to different WMT protocols showed a difference in Total ADHD symptoms (, , +m), which provoked the main effect in the factor level. Even with a small significance, any main effect associated with the assignment to the level of training should be considered carefully because it could suggest a potential bias in the outcome of the random assignments of the participants to either baseline or adaptive subgroups prior to training.
For the ‘ADHD Index’ (i.e., the normalized T-score of [55,57]), we tested again the null hypothesis for homoscedasticity and homogeneity of variances against a model with factors ADHD subtype, in addition to factors group and level. The null hypothesis was accepted for this model (, , and Levene’s test , ), then standard ANOVA could be applied. In patients’ groups comparison, the main effect of group was significant (, , +m) but the main effect of the ADHD subtype was not and no significant interaction was found between factors in the ANOVA. It is noteworthy that in ADHD patients without medication (and only in that group), the ADHD index of the patients diagnosed with a combined ADHD subtype (, ) was significantly (, , +L) larger than the ADHD index of those diagnosed with a predominantly inattentive ADHD subtype (, ). The two-way ANOVA, also including the group of controls (Table 1), shows a significant main effect of the group factor (, , +L) with differences between controls and patients (with MADHD: , , +L and with ADHD: , , +L) and between MADHD and ADHD , , +m) in a way somehow similar to what we observed with ASRS. On the contrary, the main effect of the level factor was not significant (, , +s), such that we may consider that the subgroups defined by the random assignment to either baseline and adaptive memory training are not clinically biased with respect to the ’ADHD Index’.
3.3. Dimensional Analysis of Reaction Time (RT)
The dimensional analysis assumes that a measured variable depends on the severity (i.e., intensity) of the symptoms rated on a continuous scale. In the current study, this assumption was that the reaction times should be tested against the four scores derived from the analysis of the CAARS questionnaire (i.e., , , , and ), irrespective of the participants’ groups. Figure 3 shows the corresponding scatterplots and the regression lines between the reaction times and the subscales. The correlation before training (black lines) and after the baseline training (red lines) tended to be characterized by the same slopes. The intercepts after the baseline training were smaller, thus suggesting the training produced an increased speed in the reaction time, which was the same for light or severe values of the symptoms, measured by subscales. It is interesting to note that the adaptive training tended to show lines becoming flat, thus suggesting a decrease in the reaction time, which tended to be independent of the severity of the symptoms. The only exception is illustrated by the correlations with the (i.e., the DSM-IV Hyperactive-Impulsive Symptoms Subscale, Figure 3b) with an increased speed, which tended to be the same for both training modes, irrespective of the severity of the symptoms (i.e., parallel blue and red lines). Each pattern of the ANT was tested several times for each participant, such that we should be able to use repeated measurements in statistical tests. The design of the experiment was balanced, but several trials were discarded due to the fact that either the participants did not answer within the time limit or the trial fell among the outliers. Hence, the outcome was unbalanced with respect to an ideal repeated measurements design for the number of trials in any combination of ANT patterns and training protocol.
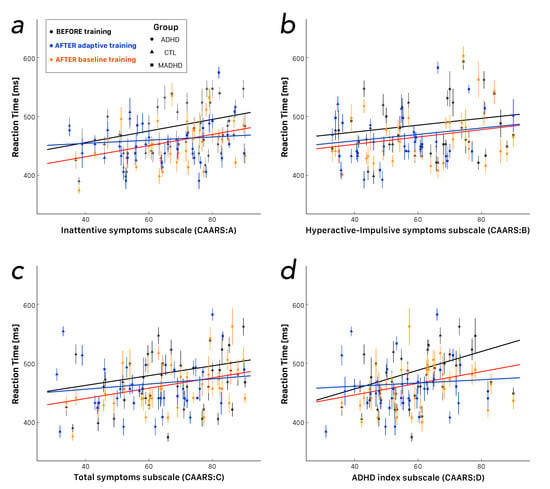
Figure 3.
Dimensional analysis of reaction times as a function of the severity of ADHD symptoms measured by Conners’ Adult ADHD Rating Scales-Self Report (Screening Version, CAARS-S:SV). (a) Scatterplot as a function of (DSM-IV Inattentive Symptoms Subscale). (b) Scatterplot as a function of (DSM-IV Hyperactive-Impulsive Symptoms Subscale). (c) Scatterplot as a function of (DSM-IV Total ADHD Symptoms Subscale). (d) Scatterplot as a function of (‘ADHD Index’, the normalized T-score of CAARS). Each point shows the median and the median absolute deviation for one participant. Participants’ groups are identified by distinct shapes, i.e., triangles for controls, circles and squares for non-medicated and medicated ADHD patients, respectively. Data points before training (all subgroups merged together) are plotted in black. Data points after training are plotted in red for the baseline level (fixed at 1-back) and in blue for the adaptive level of the Dual n-back. Color lines refer to the robust regression of the corresponding data points. Notice that the slope for participants before training and after baseline training tended to be very similar. Notice also that the slopes tended to flatten after adaptive training, irrespective of the subscale.
We tested the null hypothesis of homoskedasticity with a studentized Breusch–Pagan test for RTs as a function of each score before and after training. In all conditions, we found that the RTs were heteroscedastic, such that we should apply robust ANOVA with repeated measures. The main effects were significant for all scores, i.e., (, , +s), (, , +s), (, , +s), and (, , +m). Then, we analyzed the correlations by means of Spearman’s rank correlation and the corresponding robust linear regression between RTs and the scores (Table 2). We observed that the more severe the symptoms the longer the RT, except for most scores of the ADHD group without medication after adaptive training.

Table 2.
Descriptive statistics for robust linear regression (: intercept, : slope) and for Spearman’s rank correlation (rho and p-value) between reaction times and four CAARS scores associated with symptoms severity. Analysis is reported for all participants merged into the same sample, assuming that the correlation depends only on the scored symptoms and for each participants’ group separately. NS: not significant.
3.4. Categorical Analysis of Reaction Time (RT)
We consider at first the RTs before training to all task patterns for further assessment of a potential bias introduced by the initial assignment of a participant to one of the training protocol subgroups. Before training, the RTs of the participants assigned to the baseline mode showed only small differences among the groups (, , and ms for controls, MADHD, and ADHD, respectively) without any significant main effect of factor group by robust one-way ANOVA (, , +s). On the contrary, the main effect of factor group was significant among participants assigned to the adaptive subgroups before training (, , +s). In particular, the RTs of MADHD (511.3, 521.6 ± 6.0 ms) were significantly longer than controls (476.5, 486.5 ± 5.1 ms; Mann–Whitney test , , , +s) and longer than ADHD (453.0, 473.3 ± 5.6 ms; , , , +s). Any effect associated with the assignment of the participants to the level of baseline or adaptive protocol before WMT might reveal an initial bias in the composition of the groups. This is an important point to consider because of the limited size of our samples.
We have previously pointed out the fact that there is an unbalanced distribution of ADHD subtypes between MADHD and ADHD groups, in particular between predominantly inattentive type (ADHD-I) and combined (ADHD-C) subtypes (Table 1). For this reason we consider a model where the RTs of the patients depend on three factors: patients’ group × ADHD subtype × training level. The RTs were heteroscedastic (, , ) and variances were not homogeneous (Levene’s Test , ), hence a three-way robust ANOVA was used. The only significant main effect of this analysis was due to factor group (, , effect size +s). However, a barely significant triple interaction between group and ADHD subtype and training level (, , +si) calls for further insight. The assignment of the patients to the training level subgroups was done at random, at the very beginning of the experimental protocol. Before training, for MADHD patients assigned to the baseline subgroup, the overall (all ANT patterns pooled together) RTs of ADHD-C were shorter than the RTs of ADHD-I (484.0, 484.0 ± 5.4 ms vs. 496.2, 513.1 ± 9.8 ms; Mann–Whitney test, , , , +s). On the contrary, the RTs of ADHD patients without medication diagnosed as ADHD-C were longer than the RTs of ADHD-I (488.2, 499.9 ± 7.6 ms vs. 464.8, 469.9 ± 5.9 ms; , , , +s). In the adaptive subgroups of both ADHD and MADHD, no significant difference of RTs between ADHD-C and ADHD-I patients were observed before training. In view of these results, we may consider the interaction before training between factors ADHD subtype and training level as a spurious effect of the random sampling. For the patients’ groups, we analyzed the effect of factors group × session × ADHD subtype with all RTs to any task pattern pooled together. We found no main effect of factor ADHD subtype (, , +si), a significant main effect of factor group (, , +s), a significant main effect of factor session (, , +s), and a significant interaction group×session (, , +si). Hence, due to the smallness of the subgroups divided according to the ADHD subtype and to the fact that this factor produced neither a main effect nor a significant interaction in the previous robust ANOVAs, the following analyses discarded the factor ADHD subtype.
Figure 4 shows the RTs of all participants’ groups for any combination of the 12 final task patterns (Target × Cue) before and after training with baseline and adaptive levels. At first, we analyze the effect of factors group × session × level irrespective of any task patterns pooled together. The outcome of this robust ANOVA is a significant main effect of factor group (, , +s), a significant main effect of factor session (, ) +s), and a significant group×level two-way interaction (, , +s). Because of such interaction term, we analyzed separately the RTs for the two subgroups of training modes with two-way robust ANOVAs ( and , for baseline and adaptive, respectively). In both training modes we found a significant main effect for factor group (, , +s and , , +s, for baseline and adaptive, respectively) and a significant main effect for factor session (, , +s and , , +s, for baseline and adaptive, respectively).
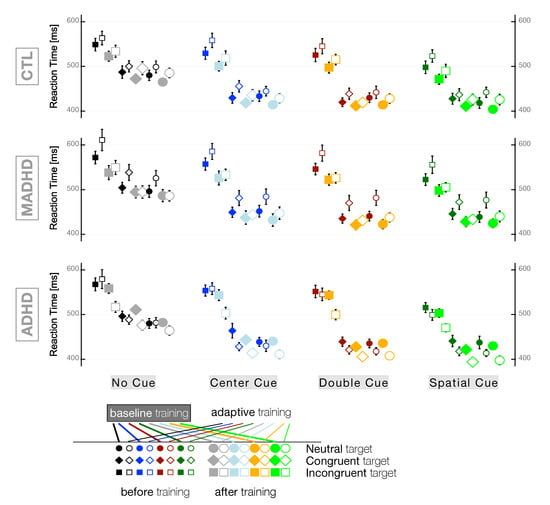
Figure 4.
Reaction Times (means ± SEM) for all conditions: Cue × Target × Group × Training level. Participants’ groups: CTL: control subjects; MADHD: ADHD patients with medication; ADHD: ADHD patients without medication.
In controls, the comparison of the RTs with their respective pre-training values by a Wilcoxon Signed-Rank test showed that both training modes produced faster reactions with a decreasing in RT of the same magnitude (after baseline training: −16.0, −18.6 ± 2.4 ms and , , , +m; after adaptive training: −16.0, −20.4 ± 3.0 ms and , , , +m). In controls, a robust one-way ANOVA to test the main effect for factor session in each training protocol showed a similar result, but to a lesser degree of significance (after baseline training: , , +s; after adaptive training: , , +s). In medicated ADHD, both training modes produced faster reactions, but after one month of adaptive training (−39.5, −44.4 ± 4.5 ms and , , , +L) the effect was larger than after baseline training (−23.0, −18.9 ± 2.9 ms and , , , +m). The factorial analysis for MADHD was also in line with this observation (after baseline training: , , +s; after baseline training: , , +m). In ADHD without medication, the decrease in overall RTs after adaptive training was even stronger than the effect observed in MADHD (−23.5, −26.5 ± 2.9 ms; , , , +L). However, in ADHD without medication, one month training in baseline mode produced faster reactions below the threshold of significance (−7.5, −6.6 ± 3.7 ms; , , , +s). The robust ANOVA showed that the main effect for factor session was neither significant for the baseline subgroup (, , +si) nor for the adaptive subgroup (, , +s). The discrepancy that we observed for the adaptive subgroup between the Wilcoxon Signed-Rank test and the robust one-way ANOVA for medians called to test the factor session also with the robust one-way ANOVA with trimmed means, which yielded a significant result after adaptive training , , +s). This may be explained by the evaluation of significance in the algorithm of robust one-way ANOVA for medians, which is biased towards the safe side and tends to underestimate the level of significance, as mentioned by the authors of the method [60].
We can observe a general pattern of RTs as a function of the cue and target types irrespective of the group and training mode: The larger information in the cue (i.e., ‘spatial cue’) the shorter the RT, the more neutral the target the shorter the RT (Figure 4). The two-way robust ANOVA showed a significant main effect of factor cue (, , +m), a significant main effect of factor target (, , +L), and a significant cue×target two-way interaction (, , +s). No significant difference was observed between RTs following congruent and neutral targets , , , +si). Because of this finding and for sake of simplicity, we skipped further analysis of the neutral targets and we focused on the differences between incongruent and congruent targets. In all groups and subgroups, we observed shorter RTs for congruent than incongruent targets (Table 3). The factorial group×training×session analysis showed main effects for factors group (, , +s) and session (, , +s) and a significant two-way interactions group×training (, , +s). Table 3 shows that the source of the interaction was mainly due to what happened in the ADHD group of participants.

Table 3.
Descriptive statistics (median, mean, and SEM) of reaction times for each group of participants and for any cue type (pooled together) as a function of Congruent and Incongruent targets. Comparisons between levels of training (Mann–Whitney test) and between before and after working memory training (WMT) within each group (Wilcoxon Signed-Rank test). For each test the corresponding p-values and effect size (r) are reported in the table.
Non-medicated ADHD patients showed a significant difference in RTs after adaptive training for both incongruent and congruent targets (, , +L and , , +m, respectively). It is worth noting that no significant difference in RTs was observed in ADHD after baseline training (, , +s) and , , +s, for incongruent and congruent targets, respectively). For controls and MADHD, the decrease in RTs was significant after either kind of training mode irrespective of the target, with a similar effect of the WMT protocols in controls (, , +s and , , +s for baseline and adaptive, respectively) and with a stronger effect after adaptive training in medicated ADHD (, , +m vs. , , +s after baseline training).
A comparison of cue types showed that RTs during the ANT depended on the amount of information contained in the cue. In the absence of information (‘No Cue’), RTs were longer and in the presence of unambiguous comprehensive information (‘Spatial Cue’), RTs were shorter. For sake of simplicity, we focus further analyses on these extreme cue conditions and skip the data obtained for ‘Center Cue’ and ‘Double Cue’ (Table 4). For both No Cue and Spatial Cue conditions, the factorial group × training × session analysis showed main effects of group (, , +s and , , +s, for No Cue and Spatial Cue, respectively) and of session (, , +s and , , +s, for No Cue and Spatial Cue, respectively). In addition, we observed significant two-way interactions group × training (, , +s and , , +s, for No Cue and Spatial Cue, respectively). This interaction effect was due to ADHD (i.e., the patients without medication), which is the only group characterized by a very strong lack of significance of baseline training on RTs in No Cue (, , , +si) and in Spatial Cue (, , , +s) conditions. In any other combination of group and training mode, the RTs were significantly shorter after one month of WMT and stronger effect after adaptive training in patients’ groups.

Table 4.
Descriptive statistics (median, mean, and SEM) of reaction times for each group of participants and for any target type (pooled together) as a function of Spatial Cue and No Cue. Comparisons between levels of training (Mann–Whitney test) and between before and after working memory training (WMT) within each group (Wilcoxon Signed-Rank test). For each test the corresponding p-values and effect size (r) are reported in the table.
3.5. Attention Network Effects
In a three-way ANOVA similar to the previous analyses, we tested whether the factor ADHD subtype affected the values of each attention network effect in the MADHD and ADHD groups and corresponding subgroups of patients randomly assigned to the baseline and adaptive levels of WMT. No heteroscedastic and no inequality of variances was observed for all network effects, thus allowing the standard parametric ANOVA to be applied for the statistical analysis. No main effect of factor ADHD subtype was found in the orienting and conflict networks (, , and (, , , respectively). On the contrary, the analysis of the Alerting Effect, showed a significant main effect of ADHD subtype (, , +m). In both patients’ groups, the value of Alerting Effect of diagnosed ADHD-C (MADHD: ; ADHD: ) was higher than the value of ADHD-I (MADHD: ADHD: ), but only in the MADHD group, this difference was significant (MADHD: , , +L; ADHD: , , +m). No interaction between the ADHD subtype and the subgroups assigned to the different training level was observed in any attention network. Hence, we considered that factor ADHD subtype plays only a marginal role in our samples and we focused the model with the values of the attention network effects as a function of factors group×training level×session×attentionnetwork. We tested the null hypothesis of homoskedasticity and no heteroscedasticity was found in our data and model (Breusch–Pagan test , , ). The four-way ANOVA corresponding to our model showed significant main effects of factors group (, , +s), session (, , +si), attentionnetwork (, , +L) and a significant session×attentionnetwork two-way interaction (, , +s).
Figure 5 illustrates how training with baseline and adaptive level affected each group for alerting, orienting, and conflict networks. Within groups comparisons were carried out with Student’s t test and corresponding Cohen’s d effect size are reported in Figure 5. No effect of training level was observed for Alerting Effect and Orienting Effect in any group. In controls, the average Conflict Effect was significantly decreased after baseline and adaptive training with moderate effect size (, , +m and , , +m, respectively). Both training modes affected the average Conflict Effect in medicated ADHD patients (, , +m and , , +m after baseline and adaptive mode, respectively). On the contrary, in the ADHD without medication only the adaptive level of training provoked a very large and significant effect (, , +L). It is important to note that a decrease in the Conflict Effect corresponds to an improvement in the executive control network. In agreement and as a confirmation of this important finding, notice that Table 3 showed only significant differences in RTs before and after adaptive level of training for ADHD without medication. This is important because the Conflict Effect is computed after the RTs following congruent and incongruent targets (Table 3).
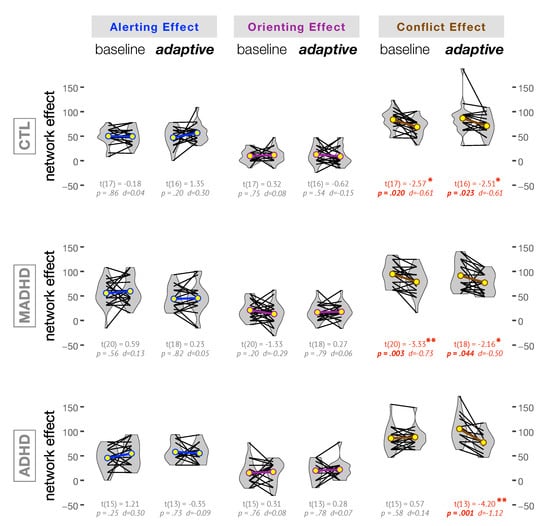
Figure 5.
Attention network effects within groups before and after training. The yellow circles in the violin plots correspond to the average values of the corresponding network effects. The p-values and Cohen’s d effect sizes are reported for paired Student’s t tests. Notice that WMT affected only the conflict network. In particular, for ADHD only the adaptive training provoked a large and significant effect. Groups: CTL: control subjects; MADHD: patients with medication; ADHD: patients without medication.
We also analyzed the changes in each attention network as a function of the severity of the symptoms measured by the Conners’ Adult ADHD Rating Scales, i.e., a dimensional analysis irrespective of the group of participants. The only significant correlation was observed between the DSM-IV Hyperactive-Impulsive Symptoms Subscale and the Conflict Effect after baseline training (Spearman’s rank correlation , and , , +m). The negative sign means that the lesser the severity of the hyperactive-impulsive symptoms the larger the improvement in the Conflict Effect.
3.6. Ex-Gaussian Distributional Model of RTs
For each participant (), we computed the three ex-Gaussian parameters mu (), sigma (), and tau () from the distribution of individual RTs. The parameters mu and sigma correspond to the estimated mean and standard deviation of the Gaussian portion of the RT distribution. Hence, parameter sigma is a good estimate of the RT variability. The parameter tau corresponds to an exponential decay parameter associated with the skewness of the tail of the RT distribution. At first, we considered a dimensional analysis of these parameters with the symptom subscales as factors in one-way ANOVAs and the corresponding correlation coefficients. Studentized Breusch–Pagan tests showed that the values were homoscedastic and we used standard ANOVAs and Pearson’s r rank correlation coefficients. Parameters mu and sigma were positively correlated with the inattentive symptoms score () (, , , +s and , , , +s, respectively). No significant correlations was observed between any parameter with the hyperactive-impulsive symptoms score (). Parameter sigma was the only one positively correlated with the total symptoms score () (, , , +s). Very significant correlations were observed between the parameters mu and sigma and the ‘ADHD Index’ (i.e., the normalized T-score of ) (, , , +m and , , , +m, respectively). Secondly, for each participant, we analyzed the differences between corresponding mu, sigma and tau values computed after and before the WM training (i.e., , and ) with the symptom subscales as factors in one-way ANOVAs and the corresponding correlation coefficients. Only one significant correlation was observed, between and the hyperactive-impulsive symptoms score () after baseline training (, , , +m). It is interesting to note that no parameters among , , and correlated with any symptom subscales after adaptive training.
We carried out the categorical analysis of the ex-Gaussian parameters in the same way as described for RT, with a model depending on three factors: patients’ group × session × training level. With this model, the parameters mu, sigma and tau were homoscedastic and variances were homogeneous on Levene’s Test. For Ex-Gaussian parameter mu, the three-way ANOVA corresponding to our model showed significant main effects of factors group (, , +s), session (, , ) and a group × training level two-way interaction just below the threshold of significance (, , +s). For ex-Gaussian parameter sigma, the three-way ANOVA yielded the same effects observed for mu, i.e., main effects for group (, , +s), session (, , +s) and a significant group × training level two-way interaction (, , +s). No significant effects were observed for parameter tau. The values of mu were always normally distributed and we used Student t-tests for within group and between-groups comparisons and Cohen’s d for the effect size. The values of tau were never normally distributed and we used the Wilcoxon Signed-Rank test for within group and the Mann–Whitney test for between groups comparisons and r value, between 0 and 1, for the effect size. For the values of sigma, most distributions were normally distributed and we used the appropriate test following the outcome of the normality test. The values of all ex-Gaussian parameters of RT distributions in all groups of participants and for all experimental conditions, are presented in Table 5. Notice that RT variability associated to parameter was significantly reduced after WMT only in the ADHD without medication in the baseline condition.

Table 5.
Descriptive statistics (median, mean, and SEM) of ex-Gaussian parameters (mu, sigma, tau) for each group of participants. Comparisons between levels of training and between before- and after-training within each group are reported with the corresponding p-values and effect sizes Cohen’s d or non-parametric r computed following the outcome of the normality tests.
For each group of participants, we have eventually analyzed the effect of the training level during WMT with any significant regression and correlation between the variation of the ex-Gaussian parameters , and and the variation of the attention network effects. Depending on the outcome of the respective normality tests we used either Pearson or Spearman rank correlations. In controls, we observed a decrease in correlated with an improvement of the Alerting Effect after adaptive training (, , , +L). In medicated ADHD participants, we observed significant correlations only after baseline training and with an improvement of the Conflict Effect, with a decrease in (, , , +L) and with a decrease in (, , , +L). In ADHD patients without medication, we observed significant positive correlations only after adaptive training. We observed that an improvement of the Conflict Effect correlated with an increase in (, , , +L) and an improvement of the Orienting Effect correlated with an increase in (, , , +L). It is important to notice that most correlations were associated with an improvement of the Conflict Effect (i.e., with a decrease in and a decrease in in MADHD after baseline training and with an increase in in ADHD without medication after adaptive training).
4. Discussion
The overall pattern of RTs to the combination of cues and targets in the Attention Network Test observed in this study showed that for any group of participants the RTs were longer after No Cue and Incongruent target conditions and the RTs were shorter after Spatial Cue and Congruent target conditions, in agreement with the well-established literature [22,28,69,70,71,72]. In ADHD patients, ANT was studied in children and adults [73,74,75,76,77,78,79,80]. In general, these studies report that RTs of ADHD patients tend to be longer than controls, but accuracy and variability characterized at several degrees those patients with inattentive symptoms and suggested dysfunctions in the coupling between alerting, orienting, and conflict (executive) networks. Several studies exist aiming at the improvement of executive functions in ADHD patients with a focus either on stimulant medication or cognitive working memory training [81,82,83,84,85], however the present study is the first one including medicated and non-medicated ADHD patients performing ANT before and after working memory training.
4.1. ADHD Diagnosed Subtypes
It is worth noting some characteristics in the composition of our patients’ samples. Our final sample of medicated ADHD () included mostly patients diagnosed with a combined inattentive/hyperactive subtype of ADHD (28 ADHD-C vs. 10 predominantly inattentive type ADHD-I and 2 undefined subtype). On the contrary, the final sample of ADHD () included 16 ADHD-C vs. 12 ADHD-I, 1 predominantly hyperactive/impulsive subtype ADHD-HI and 1 undefined subtype. The commonness of ADHD-C (overall 44 patients) with respect to ADHD-I (overall 22 patients) is in agreement with several literature reports in young adults [86,87,88,89]. We found significant differences between the values of the ADHD-C and ADHD-I patients’ score to the ADHD Self-Report Scale (ASRS) [56], in agreement with other citations [90,91]. We also found significant differences between these ADHD subtypes for the values of (DSM-IV hyperactive-impulsive symptoms) and (DSM-IV ADHD total symptoms), in agreement with previous studies [92,93]. However, the current study is definitely underpowered for a thorough ADHD subtype analysis if we consider also the subtype assignments to the subgroups of training protocol. The purpose of this subsection is to raise the attention on the potential effect of ADHD subtype diagnosis on the interpretation of the results.
The MADHD group was characterized by the prevalence of ADHD-C/ADHD-I (28/10) compared to ADHD (16/12), and it is worth noting that our MADHD group was characterized by average values higher than ADHD for ASRS ) and for the ADHD Index ). It could be argued that ADHD diagnoses exist on a continuum rather than as separate categories on the assumption of symptom ratings distributions (e.g., subscales scores) could correct some weaknesses of the DSM categorical criteria. Indeed, the dimensional approach of ADHD severity symptoms for discussion of diagnostic issues has been in the focus of DSM-V following several studies showing inconsistencies to support the discrimination of subtypes of DSM-IV ADHD, in particular ADHD-I and ADHD-C [94,95,96]. We analyzed the correlations between RTs and the four scores and we showed that the more severe the symptoms the longer the RT, thus providing arguments that ADHD diagnoses is associated with ADHD severity on a continuum. Despite the fact that the main effect of factor ADHD subtype was not significant in the RT analyses, we observed that before training MADHD reacted with RTs significantly longer than ADHD (although with a small effect size). In the literature, a study suggested that an ADHD subtype reporting effective fluctuations was characterized by slowed RTs to ANT [78], somehow similar to the finding of another study showing that combined inattentive/hyperactive (ADHD-C) patients, but not primarily inattentive (ADHD-I) patients, were also characterized by slowed RTs [73]. Hence, we cannot discard the possibility that slowed RTs in the MADHD group might be due to the prevalence (75%) of ADHD-C patients in that group, but it is also true that the MADHD group is characterized by higher scores of symptoms severity measured by the ADHD Index. It was also reported in the literature that a subset of ADHD-C patients medicated with stimulants could perform ANT nearly at the level of controls [73]. We tested also for any gender or age main effect for the ASRS and all CAARS-S:SV subscales, but none was significant.
Last but not least the double-blind assignment of patients to the subgroups following a baseline or an adaptive level of training in WMT resulted in an even representation of ADHD subtypes in the baseline and adaptive subgroups. The same reasoning might hold for the dimensional analysis. However, we observed only a couple of differences between some CAARS scores of the subgroups assigned to baseline and adaptive prior to WMT. In the non-medicated ADHD group there was a difference very close to threshold (, +m) for the DSM-IV Hyperactive-Impulsive Symptoms Subscale () dimension. In controls, a significant difference (, +m) was observed was observed for the ADHD index () dimension. We cannot rule out that these differences might have produced an impact on the final outcome of the WMT training analysis, but in both categorical factorial analysis (for the ADHD subtypes) and dimensional analysis (for the scores) the interactions due to the factor training level were not significant. We dismiss further discussion of this point, but it was worth mentioning for a thorough evaluation of this study.
4.2. RT and RT Variability
In this study, irrespective of the assignment subgroup of training and for median RTs for all stimulus patterns merged together, we observed that controls performed faster than ADHD patients of both patients’ groups together (before training with a small effect size ; after training with a large effect size ). However, this observation could be misleading for several reasons. Firstly, RTs of MADHD were longer than ADHD possibly because our MADHD sample was characterized by a different composition of ADHD subtypes and by a different intensity in the severity of symptoms assessed by CAARS. Secondly, the computations based on the median RTs for each stimulus pattern eliminate the effect of the biased distribution of RTs, which is usually characterized by long tails towards long RTs. ADHD patients are characterized as ubiquitously slower and with greater RT variability relative to controls [33,34]. Stimulant medication of ADHD in a Go/NoGo task slowed RT and increased RT variability was attenuated, but remained unaffected by non-stimulant medical and psychosocial interventions [31,33]. A meta-analysis review [33] showed also that slower average processing speed in ADHD was not confirmed after accounting for RT variability, whereas large magnitude RT variability deficits remained after accounting for mean RT.
The ex-Gaussian distribution model was used to model RT and RT variability in ADHD performing several tasks [29,30,31,32], but never yet in the attention network task. In contrast to increased mean RT, the distributional parameter (derived from the mean of the Gaussian component of the distribution) did not document a significant slowing in adult ADHD patients. Several studies showed that ADHD were characterized by smaller values of parameter than controls [30,32,97,98]. In the dimensional analysis of our study, we found a positive correlation between parameter and the intensity of inattentive symptoms and ADHD index rated by the Conners’ Adult ADHD Rating Scales-Self Report subscales ( and ). Adult ADHD, with minimal differences across the ADHD subtypes, were characterized by increased intra-individual variability throughout the entire RT distribution as indicated by the parameters (derived from the standard deviation of the RT distribution) and by a greater proportion of abnormally slow responses associated with parameter (i.e., the exponential component which reflects the extreme values) [29,34,36,97,99]. We found that parameter correlated positively with the severity of the symptoms rated by all subscales, with the notable exception of hyperactive-impulsive symptoms (). We did not find any significant correlation between the ratings and parameter before WMT. In our categorical analysis, the ANOVA did not reveal any main effect of factor group with either ex-Gaussian parameter, in agreement with another study using choice RT tasks that did not demonstrate a group difference without taking into account the comorbities [100].
4.3. Working Memory Training
For controls and MADHD, the outcome of the WMT during one month was significant shorter RTs to ANT with either baseline or adaptive mode in the Dual n-back task. In the case of non-medicated ADHD, the significant effect of WMT was observed only if the training was done in the most cognitive demanding version of the task, that is the adaptive Dual n-back task. The effect of WMT on RTs was already reported for healthy adults [101], but our study is the first one showing a significant effect in adult ADHD. After training, the analysis of network effects showed that there were no significant changes in both Alerting Effect and Orienting Effect for all participant groups. The literature reports that Alerting Effect is improved by the stimulant medication in patients diagnosed as ADHD-C subtype, but not in ADHD-I subtype [73]. In both patients’ groups, we observed improved Alerting Effect in ADHD-C vs. ADHD-I subgroups, but this difference was statistically significant only in MADHD. However, there was no significant difference between the two patients’ groups neither before nor after training. Differences between medicated and non-medicated ADHD patients in the Alerting Effect might depend on the kind of medication. In the case of dextroamphetamine, extracellular norepinephrine is much more increased than after dopamine [102,103]. Dextroamphetamine was prescribed to the majority (9/14) of the patients of the study reported in the literature [73]. In our MADHD group, methylphenidate, which affects mainly the dopamine system, was prescribed to all patients. These drugs have a very different mechanism of action [104,105,106,107] and the alerting network involves brain areas activated by the norepinephrine system [26]. Therefore, it is likely that the patients of our MADHD group are less affected by the medication with respect to the values of Alerting Effect reported elsewhere [73].
In our WMT, the baseline mode of the Dual n-back task corresponds to the 1-back, i.e., when participants were required to detect a match with the immediately previous trial. This means that the baseline mode is characterized by a rather moderate attentional and cognitive load. Then, it is interesting to notice that WMT produced a little improvement, if any, in the conflict (or executive control) network of the ADHD group without medication. The outcome of the ex-Gaussian analysis was in the same direction, with a much stronger correlation of with Conflict Effect after adaptive () than after baseline training (). Both controls and MADHD showed an improvement of Conflict Effect by WMT (Figure 5). In agreement with this finding, the dimensional analysis after baseline training showed a significant correlation such that the lesser the severity of the hyperactive-impulsive symptoms () the larger the improvement in the Conflict Effect. In addition, the lesser the severity of these symptoms the smaller the variation in the tail of RT distributions (i.e., ) and the smaller the the larger the improvement in the Conflict Effect. These findings are in agreement with the ex-Gaussian analysis reported for a Stroop task showing that the response conflicts mainly affected the Gaussian components, whereas the task conflicts were more prominent in the exponential component [108].
The different outcome of baseline mode on Conflict Effect between ADHD groups with and without medication might be explained by the effect of the stimulants [73,109]. The conflict (or executive control) network is mainly modulated by the dopamine system and involves brain structures in the prefrontal and anterior cingulate cortex, the anterior insula, and the basal ganglia [110,111,112]. It has been suggested that WMT might change the density of cortical dopamine receptors in the prefrontal cortex [113,114,115]. It is known that the activity of the prefrontal cortex, especially in the right hemisphere, is impaired in adult ADHD patients [116,117,118]. This impairment is likely to be responsible of the deficits in response inhibition and working memory [119,120,121], as suggested in previous studies of ANT with ADHD children [74,122]. Medication by methylphenidate is meant to block the reuptake of dopamine and noradrenaline in the central nervous system and result in increased concentrations of dopamine at the synaptic cleft [123,124]. This may explain why WMT in the baseline mode of training did not improve Conflict Effect in our participants belonging to the non-medicated ADHD group.
On the contrary, the adaptive mode of training produced an unexpected and significant () improvement of Conflict Effect in non-medicated ADHD patients. These patients demonstrated a poor functioning of the conflict network when resolving the conflict generated by the Incongruent target stimuli (Figure 4). However, after adaptive training, the corresponding ADHD subgroup showed that RTs became significantly shorter even with Incongruent stimuli (Table 3). Other studies reported that WMT contributed to reducing ADHD symptoms and reinforcing inhibitory control after computerized WMT [83] and n-back training in ADHD children [125]. Therefore, we raise the hypothesis that WMT with high demanding attentional and cognitive load may contribute to improving conflict network performance by means of activation of the dopaminergic pathway in the prefrontal cortex. Then, a training with Dual n-back task in the adaptive mode carries the potential to reduce adults’ ADHD symptoms. It is important to consider also the role played by motivation. Impairment in response inhibition [126] and motivational dysfunction with a serious sensitivity for immediate rewards [127] are among the most important deficits associated with patients suffering of ADHD. The elevated need of reinforcement in these patients may result in motivational problems during executive tasks and cognitive training when the subject has to repeat the same response over and over again for many trials, making most cognitive training tedious and boring [128]. Cognitive-motivational deficits associated with ADHD are a factor of treatment adherence especially regarding the degree of interest and stimulation of tasks [129]. For these motivational reasons, WMT in adaptative condition could trigger subject’s engagement and might be considered more rewarding than in baseline condition, especially for the ADHD group without medication.
4.4. Limitations and Future Investigations
The results presented here should be considered in light of some limitations. The current study, as any other one with ADHD patients, is influenced by the limited size of the samples and by the heterogeneity in symptoms and executive function deficits observed in the groups of ADHD and MADHD patients. The design of this study corresponds to the clinical practice combining medication and other interventions. The combination between clinical and pharmacological interventions is usually considered as first-line treatment for ADHD [130]. Nevertheless, there is a need of future studies aimed at cost and time-effective multimodal treatments with more effective and adjunctive interventions for ADHD [131] and the mechanisms underlying cognitive and symptoms enhancement [84].
We have focused our study on the effects of WMT on ANT, which led us to give priority to the double-blind assignment of patients to the subgroups trained either with the baseline or adaptive mode of the Dual n-back task. The consequence is that the subgroups were not balanced with respect to patients’ diagnosis as ADHD-C or ADHD-I, but the cofactor ADHD subtype did not appear to play a major role in the statistical effect of the interactions computed in our analyses. A theoretical optimal design of the study should include a group of medication-naive participants receiving a placebo, which would allow us to better separate the intrinsic effects of medication. However, such a design is not allowed by ethics committees. Additional randomization of the patients’ assignment in subgroups taking into account motivational factors and ADHD developmental factors in patients’ life course would certainly contribute to better identification of the attention network components liable to be influenced by WMT [36,125,132,133]. Furthermore, patients with ADHD are particularly sensitive to immediate reinforcement [36,134], which makes measurements of attention processes by ANT after WMT difficult to interpret given a lack of ecological validity on the daily functioning of adult ADHD patients [135,136].
The completeness of this study could also be improved. The dimensional analysis has shown that longer RTs were associated with more severe symptoms scored in any of the Conners’ Adult ADHD Rating Scales-Self Report (Screening Version, CAARS-S:SV) subscales, with the notable exception of the ADHD group without medication after adaptive training. This result is interesting because it suggests that, in ADHD, the overall decrease in RTs after adaptive training (by ms, with large effect size ) is likely to be associated with cognitive processes not tested thoroughly by CAARS. Recent studies of adult ADHD patients medicated with atomoxetine [137,138] pointed out that executive functioning in everyday life may be better assessed by means of the questionnaire Behavior Rating Inventory of Executive Function-Adult Version [139,140]. We recommend to include both CAARS and BRIEF in future studies.
5. Conclusions
The Attentional Network Task allows testing the plasticity of brain circuits in ADHD patients in a notable way. The present study demonstrates that working memory training for one month using the Dual n-back task training in the adaptive mode produced a significant improvement in such Conflict Effect of adult ADHD patients irrespective of their medication. The baseline mode was insufficient to produce measurable effects in the non-medicated ADHD patients, which may explain previous contradictory reports in the literature with respect to the usefulness of working memory training. Hence, the Dual n-back task in the adaptive mode offers as a promising candidate for a cognitive remediation of adult ADHD patients without pharmaceutical medication.
Author Contributions
Conceptualization, A.E.P.V., M.B., and A.L.; formal analysis, M.D., S.K.M., and A.E.P.V.; funding acquisition, A.E.P.V., M.B., and Y.A.; clinical investigation, S.K.M., A.L., and M.B.; methodology, M.D., S.K.M., A.E.P.V., M.B., and A.L.; project administration, A.E.P.V.; supervision, A.E.P.V., M.B., and Y.A.; validation, A.L. and A.E.P.V.; visualization, M.D., A.E.P.V., and A.L.; writing—original draft, M.D. and M.B.; writing—review and editing, A.E.P.V. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Swiss National Science Foundation, grant number CR13I1-138032, by “TOBITATE! Young Ambassador Program” of the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and by the “Self-Development Course” of the Faculty of Medicine and Health Sciences of Yamaguchi University.
Acknowledgments
The authors are grateful to the ADHD patients and their families who kindly agreed to be part of the project. They also wish to thank Takeshi Abe for his advice to statistical design and analysis, Maria Soares Duarte for the psychological screening, and Damiano Cereghetti for the technical assistance in the development of the Dual n-back task.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barkley, R.A.; Fischer, M.; Smallish, L.; Fletcher, K. Young adult outcome of hyperactive children: Adaptive functioning in major life activities. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Instanes, J.T.; Haavik, J.; Halmøy, A. Personality Traits and Comorbidity in Adults With ADHD. J. Atten. Disord. 2016, 20, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Mesrobian, S.K.; Villa, A.E.P.; Bader, M.; Götte, L.; Lintas, A. Event-Related Potentials during a Gambling Task in Young Adults with Attention-Deficit/Hyperactivity Disorder. Front. Hum. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef]
- Shoham, R.; Sonuga-Barke, E.; Yaniv, I.; Pollak, Y. ADHD Is Associated With a Widespread Pattern of Risky Behavior Across Activity Domains. J. Atten. Disord. 2019, e1087054719875786. [Google Scholar] [CrossRef]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Asherson, P.; Buitelaar, J.; Faraone, S.V.; Rohde, L.A. Adult attention-deficit hyperactivity disorder: Key conceptual issues. Lancet Psychiatry 2016, 3, 568–578. [Google Scholar] [CrossRef]
- Zalsman, G.; Shilton, T. Adult ADHD: A new disease? Int. J. Psychiatry Clin. Pract. 2016, 20, 70–76. [Google Scholar] [CrossRef]
- Katzman, M.A.; Bilkey, T.S.; Chokka, P.R.; Fallu, A.; Klassen, L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry 2017, 17, 302. [Google Scholar] [CrossRef]
- Adler, L.A.; Faraone, S.V.; Spencer, T.J.; Berglund, P.; Alperin, S.; Kessler, R.C. The structure of adult ADHD. Int. J. Methods Psychiatr. Res. 2017, 26, e1555. [Google Scholar]
- Chung, W.; Jiang, S.F.; Paksarian, D.; Nikolaidis, A.; Castellanos, F.X.; Merikangas, K.R.; Milham, M.P. Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder Among Adults and Children of Different Racial and Ethnic Groups. JAMA Netw. Open 2019, 2, e1914344. [Google Scholar] [CrossRef] [PubMed]
- Hodgkins, P.; Shaw, M.; Coghill, D.; Hechtman, L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: Complementary treatment options. Eur. Child Adolesc. Psychiatry 2012, 21, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P.; Keating, G.M. Atomoxetine: A review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr. Drugs 2009, 11, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.J.; Brown, A.; Seidman, L.J.; Valera, E.M.; Makris, N.; Lomedico, A.; Faraone, S.V.; Biederman, J. Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of MRI-based neuroimaging studies. J. Clin. Psychiatry 2013, 74, 902. [Google Scholar] [CrossRef]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Cortese, S.; Adamo, N.; Janiri, L. Pharmacological and non-pharmacological treatment of adults with ADHD: A meta-review. Evid. Based Ment. Health 2017, 20, 4–11. [Google Scholar] [CrossRef]
- Melby-Lervåg, M.; Hulme, C. Is working memory training effective? A meta-analytic review. Dev. Psychol. 2013, 49, 270–291. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.; Brandeis, D.; Holtmann, M.; Cortese, S. Computer-based cognitive training for ADHD: A review of current evidence. Child Adolesc. Psychiatr. Clin. 2014, 23, 807–824. [Google Scholar] [CrossRef]
- Au, J.; Buschkuehl, M.; Duncan, G.J.; Jaeggi, S.M. There is no convincing evidence that working memory training is NOT effective: A reply to Melby-Lervåg and Hulme (2015). Psychon. Bull. Rev. 2016, 23, 331–337. [Google Scholar] [CrossRef]
- Mesrobian, S.K.; Lintas, A.; Jacquerod, M.; Bader, M.; Götte, L.; Villa, A.E. An ERP Study Reveals How Training with Dual N-Back Task Affects Risky Decision Making in a Gambling Task in ADHD Patients. In Advances in Cognitive Neurodynamics (VI); Delgado-García, J.M., Pan, X., Sánchez-Campusano, R., Wang, R., Eds.; Springer: Singapore, 2018; Chapter 34; pp. 271–277. [Google Scholar]
- Jaquerod, M.E.; Mesrobian, S.K.; Villa, A.E.P.; Bader, M.; Lintas, A. Early Attentional Modulation by Working Memory Training in Young Adult ADHD Patients during a Risky Decision-Making Task. Brain Sci. 2020, 10, 38. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the efficiency and independence of attentional networks. J. Cognit. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Duque, D.; Posner, M.I. Relating the mechanisms of orienting and alerting. Neuropsychologia 1997, 35, 477–486. [Google Scholar] [CrossRef]
- Posner, M.I.; Rothbart, M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007, 58, 1–23. [Google Scholar] [CrossRef]
- Fuentes, L.J.; Campoy, G. The time course of alerting effect over orienting in the attention network test. Exp. Brain Res. 2008, 185, 667–672. [Google Scholar] [CrossRef]
- Lampe, K.; Konrad, K.; Kroener, S.; Fast, K.; Kunert, H.J.; Herpertz, S.C. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychol. Med. 2007, 37, 1717–1729. [Google Scholar] [CrossRef]
- Leth-Steensen, C.; Elbaz, Z.K.; Douglas, V.I. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychol. (Amst.) 2000, 104, 167–190. [Google Scholar] [CrossRef]
- Hervey, A.S.; Epstein, J.N.; Curry, J.F.; Tonev, S.; Eugene Arnold, L.; Keith Conners, C.; Hinshaw, S.P.; Swanson, J.M.; Hechtman, L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006, 12, 125–140. [Google Scholar] [CrossRef]
- Epstein, J.N.; Conners, C.K.; Hervey, A.S.; Tonev, S.T.; Arnold, L.E.; Abikoff, H.B.; Elliott, G.; Greenhill, L.L.; Hechtman, L.; Hoagwood, K.; et al. Assessing medication effects in the MTA study using neuropsychological outcomes. J. Child Psychol. Psychiatry 2006, 47, 446–456. [Google Scholar] [CrossRef]
- Hwang-Gu, S.L.; Chen, Y.C.; Liang, S.H.Y.; Ni, H.C.; Lin, H.Y.; Lin, C.F.; Gau, S.S.F. Exploring the Variability in Reaction Times of Preschoolers at Risk of Attention-Deficit/Hyperactivity Disorder: An ex-Gaussian Analysis. J. Abnorm. Child Psychol. 2019, 47, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.J.; Rapport, M.D.; Sarver, D.E.; Raiker, J.S.; Orban, S.A.; Friedman, L.M.; Kolomeyer, E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013, 33, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Gmehlin, D.; Fuermaier, A.B.M.; Walther, S.; Debelak, R.; Rentrop, M.; Westermann, C.; Sharma, A.; Tucha, L.; Koerts, J.; Tucha, O.; et al. Intraindividual variability in inhibitory function in adults with ADHD–an ex-Gaussian approach. PLoS ONE 2014, 9, e112298. [Google Scholar] [CrossRef] [PubMed]
- Forns, J.; Esnaola, M.; López-Vicente, M.; Suades-González, E.; Alvarez-Pedrerol, M.; Julvez, J.; Grellier, J.; Sebastián-Gallés, N.; Sunyer, J. The n-back test and the attentional network task as measures of child neuropsychological development in epidemiological studies. Neuropsychology 2014, 28, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.N.; Langberg, J.M.; Rosen, P.J.; Graham, A.; Narad, M.E.; Antonini, T.N.; Brinkman, W.B.; Froehlich, T.; Simon, J.O.; Altaye, M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology 2011, 25, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Antonini, T.N.; Kingery, K.M.; Narad, M.E.; Langberg, J.M.; Tamm, L.; Epstein, J.N. Neurocognitive and Behavioral Predictors of Math Performance in Children With and Without ADHD. J. Atten. Disord. 2016, 20, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.J.; Conway, A.R.A.; Miura, T.K.; Colflesh, G.J.H. Working memory, attention control, and the N-back task: A question of construct validity. J. Exp. Psychol. Learn. Mem. Cognit. 2007, 33, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Perrig, W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. USA 2008, 105, 6829–6833. [Google Scholar] [CrossRef] [PubMed]
- Au, J.; Sheehan, E.; Tsai, N.; Duncan, G.J.; Buschkuehl, M.; Jaeggi, S.M. Improving fluid intelligence with training on working memory: A meta-analysis. Psychon. Bull. Rev. 2015, 22, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Soveri, A.; Antfolk, J.; Karlsson, L.; Salo, B.; Laine, M. Working memory training revisited: A multi-level meta-analysis of n-back training studies. Psychon. Bull. Rev. 2017, 24, 1077–1096. [Google Scholar] [CrossRef] [PubMed]
- Helzer, J.E.; Kraemer, H.C.; Krueger, R.F.; Wittchen, H.U.; Sirovatka, P.J.; Regier, D.A. (Eds.) Dimensional Approaches in Diagnostic Classification: Refining the Research Agenda for DSM-V; American Psychiatric Association: Washington, DC, USA, 2009. [Google Scholar]
- Swanson, J.M.; Wigal, T.; Lakes, K. DSM-V and the future diagnosis of attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 2009, 11, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.; Goodman, R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 400–403. [Google Scholar] [CrossRef]
- Lubke, G.H.; Hudziak, J.J.; Derks, E.M.; van Bijsterveldt, T.C.E.M.; Boomsma, D.I. Maternal ratings of attention problems in ADHD: Evidence for the existence of a continuum. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1085–1093. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Text Rev., ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Haavik, J.; Halmøy, A.; Lundervold, A.J.; Fasmer, O.B. Clinical assessment and diagnosis of adults with attention-deficit/hyperactivity disorder. Expert Rev. Neurother. 2010, 10, 1569–1580. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33. [Google Scholar]
- Cross-Villasana, F.; Finke, K.; Hennig-Fast, K.; Kilian, B.; Wiegand, I.; Müller, H.J.; Möller, H.J.; Töllner, T. The Speed of Visual Attention and Motor-Response Decisions in Adult Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2015, 78, 107–115. [Google Scholar] [CrossRef]
- Groom, M.J.; Bates, A.T.; Jackson, G.M.; Calton, T.G.; Liddle, P.F.; Hollis, C. Event-related potentials in adolescents with schizophrenia and their siblings: A comparison with Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2008, 63, 784–792. [Google Scholar] [CrossRef]
- Mazaheri, A.; Fassbender, C.; Coffey-Corina, S.; Hartanto, T.A.; Schweitzer, J.B.; Mangun, G.R. Differential oscillatory electroencephalogram between Attention-Deficit/Hyperactivity Disorder subtypes and typically developing adolescents. Biol. Psychiatry 2014, 76, 422–429. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2000, 284, 3043–3045. [Google Scholar] [CrossRef]
- Conners, C.K.; Erhardt, D.; Sparrow, E. Conner’s Adult ADHD Rating Scales: Technical Manual; Multi-Health Systems Incorporated (MHS): North Tonawanda, NY, USA, 1999. [Google Scholar]
- Kessler, R.C.; Adler, L.; Ames, M.; Demler, O.; Faraone, S.; Hiripi, E.; Howes, M.J.; Jin, R.; Secnik, K.; Spencer, T.; et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol. Med. 2005, 35, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Fumeaux, P.; Mercier, C.; Roche, S.; Iwaz, J.; Bader, M.; Stéphan, P.; Ecochard, R.; Revol, O. Validation of the French Version of Conners’ Parent Rating Scale Revised, Short Version: Factorial Structure and Reliability. Can. J. Psychiatry 2016, 61, 236–242. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Komsta, L. Outliers: Tests for Outliers; R Package Version 0.14; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zamar, R.; Marazzi, A.; Yohai, V.; Salibian-Barrera, M.; Maronna, R.; Zivot, E.; Rocke, D.; Martin, D.; Maechler, M.; et al. Robust: Port of the S+ “Robust Library”; R Package Version 0.5-0.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Lüdecke, D. The easystats collection of R packages. GitHub 2020. Available online: https://github.com/easystats/easystats (accessed on 7 October 2020).
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a class of permutation tests: The coin package. J. Stat. Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Massidda, D. Retimes: Reaction Time Analysis, R Package Version 0.1-2 ed.; CRAN; 2013. Available online: https://CRAN.R-project.org/package=retimes (accessed on 7 October 2020).
- Shiffler, R.E. Maximum Z Scores and Outliers. Am. Stat. 1988, 42, 79–80. [Google Scholar] [CrossRef]
- Ratcliff, R. Methods for dealing with reaction time outliers. Psychol. Bull. 1993, 114, 510–532. [Google Scholar] [CrossRef]
- Robinson, M.D. Lives lived in milliseconds: Using cognitive methods in personality research. In Handbook of Research Methods in Personality Psychology; Robins, R.W., Fraley, R.C., Krueger, R.F., Eds.; The Guilford Press: New York, NY, USA, 2009; Chapter 20; pp. 345–359. [Google Scholar]
- Redick, T.S.; Engle, R.W. Working memory capacity and attention network test performance. Appl. Cognit. Psychol. 2006, 20, 713–721. [Google Scholar] [CrossRef]
- Jennings, J.M.; Dagenbach, D.; Engle, C.M.; Funke, L.J. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Neuropsychol. Dev. Cognit. B Aging Neuropsychol. Cognit. 2007, 14, 353–369. [Google Scholar] [CrossRef]
- Macleod, J.W.; Lawrence, M.A.; McConnell, M.M.; Eskes, G.A.; Klein, R.M.; Shore, D.I. Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test. Neuropsychology 2010, 24, 637–651. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.M.; Shore, D.I. Mixing measures: Testing an assumption of the Attention Network Test. Atten. Percept. Psychophys. 2011, 73, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, B.G.; Alford, J.L.; Marrocco, R.T. Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behav. Brain Res. 2005, 165, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Robertson, I.H.; Barry, E.; Mulligan, A.; Dáibhis, A.; Daly, M.; Watchorn, A.; Gill, M.; Bellgrove, M.A. Impaired conflict resolution and alerting in children with ADHD: Evidence from the Attention Network Task (ANT). J. Child Psychol. Psychiatry 2008, 49, 1339–1347. [Google Scholar] [CrossRef]
- Adólfsdóttir, S.; Sørensen, L.; Lundervold, A.J. The attention network test: A characteristic pattern of deficits in children with ADHD. Behav. Brain Funct. 2008, 4, 9. [Google Scholar] [CrossRef]
- Gupta, R.; Kar, B.R. Development of attentional processes in ADHD and normal children. Prog. Brain Res. 2009, 176, 259–276. [Google Scholar] [CrossRef]
- Bush, G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology 2010, 35, 278–300. [Google Scholar] [CrossRef]
- Lundervold, A.J.; Adolfsdottir, S.; Halleland, H.; Halmøy, A.; Plessen, K.; Haavik, J. Attention Network Test in adults with ADHD–the impact of affective fluctuations. Behav. Brain Funct. 2011, 7, 27. [Google Scholar] [CrossRef]
- Sripada, C.; Kessler, D.; Fang, Y.; Welsh, R.C.; Prem Kumar, K.; Angstadt, M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014, 35, 4693–4705. [Google Scholar] [CrossRef]
- Hasler, R.; Perroud, N.; Meziane, H.B.; Herrmann, F.; Prada, P.; Giannakopoulos, P.; Deiber, M.P. Attention-related EEG markers in adult ADHD. Neuropsychologia 2016, 87, 120–133. [Google Scholar] [CrossRef]
- Beck, S.J.; Hanson, C.A.; Puffenberger, S.S.; Benninger, K.L.; Benninger, W.B. A controlled trial of working memory training for children and adolescents with ADHD. J. Clin. Child Adolesc. Psychol. 2010, 39, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Chaban, P.; Martinussen, R.; Goldberg, R.; Gotlieb, H.; Kronitz, R.; Hockenberry, M.; Tannock, R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: A randomized controlled trial. J. Child Psychol. Psychiatry 2012, 53, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Gropper, R.J.; Gotlieb, H.; Kronitz, R.; Tannock, R. Working memory training in college students with ADHD or LD. J. Atten. Disord. 2014, 18, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Van der Donk, M.; Hiemstra-Beernink, A.C.; Tjeenk-Kalff, A.; Van Der Leij, A.; Lindauer, R. Cognitive training for children with ADHD: A randomized controlled trial of cogmed working memory training and ‘paying attention in class’. Front. Psychol. 2015, 6, 1081. [Google Scholar] [CrossRef]
- Holmes, J.; Woolgar, F.; Hampshire, A.; Gathercole, S.E. Are working memory training effects paradigm-specific? Front. Psychol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Sobanski, E.; Brüggemann, D.; Alm, B.; Kern, S.; Philipsen, A.; Schmalzried, H.; Hesslinger, B.; Waschkowski, H.; Rietschel, M. Subtype differences in adults with attention-deficit/hyperactivity disorder (ADHD) with regard to ADHD-symptoms, psychiatric comorbidity and psychosocial adjustment. Eur. Psychiatry 2008, 23, 142–149. [Google Scholar] [CrossRef]
- Johansson, S.; Halleland, H.; Halmøy, A.; Jacobsen, K.K.; Landaas, E.T.; Dramsdahl, M.; Fasmer, O.B.; Bergsholm, P.; Lundervold, A.J.; Gillberg, C.; et al. Genetic analyses of dopamine related genes in adult ADHD patients suggest an association with the DRD5-microsatellite repeat, but not with DRD4 or SLC6A3 VNTRs. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147B, 1470–1475. [Google Scholar] [CrossRef]
- Salvi, V.; Migliarese, G.; Venturi, V.; Rossi, F.; Torriero, S.; Viganò, V.; Cerveri, G.; Mencacci, C. ADHD in adults: Clinical subtypes and associated characteristics. Riv. Psichiatr. 2019, 54, 84–89. [Google Scholar] [CrossRef]
- Weibel, S.; Menard, O.; Ionita, A.; Boumendjel, M.; Cabelguen, C.; Kraemer, C.; Micoulaud-Franchi, J.A.; Bioulac, S.; Perroud, N.; Sauvaget, A.; et al. Practical considerations for the evaluation and management of Attention Deficit Hyperactivity Disorder (ADHD) in adults. Encephale 2020, 46, 30–40. [Google Scholar] [CrossRef]
- Lis, S.; Baer, N.; Stein-en Nosse, C.; Gallhofer, B.; Sammer, G.; Kirsch, P. Objective measurement of motor activity during cognitive performance in adults with attention-deficit/hyperactivity disorder. Acta Psychiatr. Scand. 2010, 122, 285–294. [Google Scholar] [CrossRef]
- Weissenberger, S.; Děchtěrenko, F.; Klicperova-Baker, M.; Vňuková, M.; Zimbardo, P.; Raboch, J.; Anders, M.; Braaten, E.; Ptáček, R. ADHD Symptoms in Adults and Time Perspectives - Findings From a Czech National Sample. Front. Psychol. 2020, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Solanto, M.V.; Etefia, K.; Marks, D.J. The utility of self-report measures and the continuous performance test in the diagnosis of ADHD in adults. CNS Spectr. 2004, 9, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.R.; Jain, U.R.; Shapiro, C.M. Sleep and daytime function in adults with attention-deficit/hyperactivity disorder: Subtype differences. Sleep Med. 2013, 14, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Nikolas, M.A.; Nigg, J.T. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology 2013, 27, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G.; Chhabildas, N.; Kinnear, M.; DeFries, J.C.; Olson, R.K.; Leopold, D.R.; Keenan, J.M.; Pennington, B.F. The internal and external validity of sluggish cognitive tempo and its relation with DSM-IV ADHD. J. Abnorm. Child Psychol. 2014, 42, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Parke, E.M.; Mayfield, A.R.; Barchard, K.A.; Thaler, N.S.; Etcoff, L.M.; Allen, D.N. Factor structure of symptom dimensions in attention-deficit/hyperactivity disorder (ADHD). Psychol. Assess. 2015, 27, 1427–1437. [Google Scholar] [CrossRef]
- Buzy, W.M.; Medoff, D.R.; Schweitzer, J.B. Intra-individual variability among children with ADHD on a working memory task: An ex-Gaussian approach. Child Neuropsychol. 2009, 15, 441–459. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hwang-Gu, S.L.; Gau, S.S.F. Intra-individual reaction time variability based on ex-Gaussian distribution as a potential endophenotype for attention-deficit/hyperactivity disorder. Acta Psychiatr. Scand. 2015, 132, 39–50. [Google Scholar] [CrossRef]
- Karalunas, S.L.; Geurts, H.M.; Konrad, K.; Bender, S.; Nigg, J.T. Annual research review: Reaction time variability in ADHD and autism spectrum disorders: Measurement and mechanisms of a proposed trans-diagnostic phenotype. J. Child Psychol. Psychiatry 2014, 55, 685–710. [Google Scholar] [CrossRef]
- Geurts, H.M.; Grasman, R.P.P.P.; Verté, S.; Oosterlaan, J.; Roeyers, H.; van Kammen, S.M.; Sergeant, J.A. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia 2008, 46, 3030–3041. [Google Scholar] [CrossRef]
- Oelhafen, S.; Nikolaidis, A.; Padovani, T.; Blaser, D.; Koenig, T.; Perrig, W.J. Increased parietal activity after training of interference control. Neuropsychologia 2013, 51, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H.; Dersch, C.M.; Romero, D.V.; Rice, K.C.; Carroll, F.I.; Partilla, J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001, 39, 32–41. [Google Scholar] [CrossRef]
- Kuczenski, R.; Segal, D.S. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J. Neurochem. 1997, 68, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Madras, B. Anti-hyperactivity medication: Methylphenidate and amphetamine. Mol. Psychiatry 1998, 3, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Ding, Y.S. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1410–1415. [Google Scholar] [CrossRef]
- Wilens, T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008, 28, S46–S53. [Google Scholar] [CrossRef]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Steinhauser, M.; Hübner, R. Distinguishing response conflict and task conflict in the Stroop task: Evidence from ex-Gaussian distribution analysis. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 1398–1412. [Google Scholar] [CrossRef]
- Kratz, O.; Studer, P.; Baack, J.; Malcherek, S.; Erbe, K.; Moll, G.H.; Heinrich, H. Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: An event-related potential study using the Attention Network Test. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 37, 81–89. [Google Scholar] [CrossRef]
- Swanson, J.M.; Flodman, P.; Kennedy, J.; Spence, M.A.; Moyzis, R.; Schuck, S.; Murias, M.; Moriarity, J.; Barr, C.; Smith, M.; et al. Dopamine genes and ADHD. Neurosci. Biobehav. Rev. 2000, 24, 21–25. [Google Scholar] [CrossRef]
- Li, D.; Sham, P.C.; Owen, M.J.; He, L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum. Mol. Genet. 2006, 15, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, W.; Ma, K.; Wei, J.; Zhong, P.; Cho, K.; Yan, Z. The ADHD-linked human dopamine D4 receptor variant D4.7 induces over-suppression of NMDA receptor function in prefrontal cortex. Neurobiol. Dis. 2016, 95, 194–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McNab, F.; Varrone, A.; Farde, L.; Jucaite, A.; Bystritsky, P.; Forssberg, H.; Klingberg, T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 2009, 323, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Li, Y.C.; Gao, W.J. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016, 1641, 217–233. [Google Scholar] [CrossRef]
- Lai, T.K.Y.; Su, P.; Zhang, H.; Liu, F. Development of a peptide targeting dopamine transporter to improve ADHD-like deficits. Mol. Brain 2018, 11, 66. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Murray, K.E.; Seidman, L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2007, 61, 1361–1369. [Google Scholar] [CrossRef]
- Amen, D.G.; Hanks, C.; Prunella, J. Preliminary evidence differentiating ADHD using brain SPECT imaging in older patients. J Psychoact. Drugs 2008, 40, 139–146. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. The Emerging Neurobiology of Attention Deficit Hyperactivity Disorder: The Key Role of the Prefrontal Association Cortex. J. Pediatr. 2009, 154, I-S43. [Google Scholar] [CrossRef]
- Clark, L.; Blackwell, A.D.; Aron, A.R.; Turner, D.C.; Dowson, J.; Robbins, T.W.; Sahakian, B.J. Association between response inhibition and working memory in adult ADHD: A link to right frontal cortex pathology? Biol. Psychiatry 2007, 61, 1395–1401. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Kolb, B.; Forssberg, H. Motor inhibitory role of dopamine D1 receptors: Implications for ADHD. Physiol. Behav. 2007, 92, 155–160. [Google Scholar] [CrossRef]
- Morein-Zamir, S.; Dodds, C.; van Hartevelt, T.J.; Schwarzkopf, W.; Sahakian, B.; Müller, U.; Robbins, T. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Hum. Brain Mapp. 2014, 35, 5141–5152. [Google Scholar] [CrossRef] [PubMed]
- Fabio, R.A.; Urso, M.F. The analysis of Attention Network in ADHD, attention problems and typically developing subjects. Life Span Disabil. 2014, 17, 199–221. [Google Scholar]
- Scahill, L.; Carroll, D.; Burke, K. Methylphenidate: Mechanism of action and clinical update. J. Child Adolesc. Psychiatr. Nurs. 2004, 17, 85. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Gerasimov, M.; Maynard, L.; Ding, Y.S.; Gatley, S.J.; Gifford, A.; Franceschi, D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 2001, 21, RC121. [Google Scholar] [CrossRef]
- Jones, M.R.; Katz, B.; Buschkuehl, M.; Jaeggi, S.M.; Shah, P. Exploring N-Back Cognitive Training for Children With ADHD. J. Atten. Disord. 2020, 24, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci. Biobehav. Rev. 2003, 27, 593–604. [Google Scholar] [CrossRef]
- Prins, P.J.M.; Brink, E.T.; Dovis, S.; Ponsioen, A.; Geurts, H.M.; de Vries, M.; van der Oord, S. “Braingame Brian”: Toward an Executive Function Training Program with Game Elements for Children with ADHD and Cognitive Control Problems. Games Health J. 2013, 2, 44–49. [Google Scholar] [CrossRef]
- Houtepen, J.A.B.M.; Sijtsema, J.J.; Van der Lem, R.; Scheres, A.; Bogaerts, S. Cognitive-motivational, interpersonal, and behavioral functioning in relationship to treatment and research engagement in forensic patients with ADHD. J. Clin. Psychol. 2020. [Google Scholar] [CrossRef]
- Chacko, A.; Feirsen, N.; Bedard, A.C.; Marks, D.; Uderman, J.Z.; Chimiklis, A. Cogmed Working Memory Training for youth with ADHD: A closer examination of efficacy utilizing evidence-based criteria. J. Clin. Child Adolesc. Psychol. 2013, 42, 769–783. [Google Scholar] [CrossRef]
- Rutledge, K.J.; van den Bos, W.; McClure, S.M.; Schweitzer, J.B. Training cognition in ADHD: Current findings, borrowed concepts, and future directions. Neurotherapeutics 2012, 9, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Stimulants and the developing brain. Trends Pharmacol. Sci. 2005, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Schrantee, A.; Tamminga, H.G.H.; Bouziane, C.; Bottelier, M.A.; Bron, E.E.; Mutsaerts, H.J.M.M.; Zwinderman, A.H.; Groote, I.R.; Rombouts, S.A.R.B.; Lindauer, R.J.L.; et al. Age-Dependent Effects of Methylphenidate on the Human Dopaminergic System in Young vs Adult Patients With Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.A.; Kerns, K.A.; Mateer, C.A. The effect of reinforcement variables on inhibition in children with ADHD. Child Neuropsychol. 2005, 11, 295–302. [Google Scholar] [CrossRef]
- Mullane, J.C.; Corkum, P.V.; Klein, R.M.; McLaughlin, E.N.; Lawrence, M.A. Alerting, orienting, and executive attention in children with ADHD. J. Atten. Disord. 2011, 15, 310–320. [Google Scholar] [CrossRef]
- Lawrence, V.; Houghton, S.; Douglas, G.; Durkin, K.; Whiting, K.; Tannock, R. Executive function and ADHD: A comparison of children’s performance during neuropsychological testing and real-world activities. J. Atten. Disord. 2004, 7, 137–149. [Google Scholar] [CrossRef]
- De Bruyckere, K.; Bushe, C.; Bartel, C.; Berggren, L.; Kan, C.C.; Dittmann, R.W. Relationships Between Functional Outcomes and Symptomatic Improvement in Atomoxetine-Treated Adult Patients with Attention-Deficit/Hyperactivity Disorder: Post Hoc Analysis of an Integrated Database. CNS Drugs 2016, 30, 541–558. [Google Scholar] [CrossRef]
- Adler, L.A.; Solanto, M.; Escobar, R.; Lipsius, S.; Upadhyaya, H. Executive Functioning Outcomes Over 6 Months of Atomoxetine for Adults With ADHD: Relationship to Maintenance of Response and Relapse Over the Subsequent 6 Months After Treatment. J. Atten. Disord. 2020, 24, 363–372. [Google Scholar] [CrossRef]
- Roth, R.M.; Lance, C.E.; Isquith, P.K.; Fischer, A.S.; Giancola, P.R. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function-Adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch. Clin. Neuropsychol. 2013, 28, 425–434. [Google Scholar] [CrossRef]
- Løvstad, M.; Sigurdardottir, S.; Andersson, S.; Grane, V.A.; Moberget, T.; Stubberud, J.; Solbakk, A.K. Behavior Rating Inventory of Executive Function Adult Version in Patients with Neurological and Neuropsychiatric Conditions: Symptom Levels and Relationship to Emotional Distress. J. Int. Neuropsychol. Soc. 2016, 22, 682–694. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).